Abstract
Context: Norepinephrine and serotonin are two important neurotransmitters whose variations in brain are reported to be associated with many common neuropsychiatric disorders. Yet, relevant literature on estimation of monoamines in biological samples using HPLC-UV is limited.
Objective: The present study involves the development of a simultaneous HPLC-UV method for estimation of norepinephrine and serotonin along with optimization of the sample preparation technique.
Materials and methods: Chromatographic separation was achieved by injecting 20 µL of the sample after extraction into Quaternary pump HPLC equipped with C18 column using 0.05% formic acid and acetonitrile (90:10, v/v) as the mobile phase with 1 mL min−1 flow rate. The developed method was validated as per the ICH guidelines in terms of linearity, accuracy, repeatability, precision, and robustness.
Results and discussion: The method showed a wide range of linearity (50–4000 and 31.25–4000 ng mL−1 for norepinephrine and serotonin, respectively). The recovery was found to be in the range of 86.04–89.01% and 86.43–89.61% for norepinephrine and serotonin, respectively. The results showed low value of %RSD for repeatability, intra and inter-day precision, and robustness studies. Four different methods were used for the extraction of these neurotransmitters and the best one with maximum recovery was ascertained.
Conclusion: Here, we developed and validated a simple, accurate, and reliable method for the estimation of norepinephrine and serotonin in mice brain samples using HPLC-UV. The method was successfully applied to quantify these neurotransmitters in mice brain extracted by optimized sample preparation technique.
Introduction
Norepinephrine (NE) and serotonin (5-hydroxytryptamine or 5-HT) are the most important monoamine neurotransmitters in the brain and the changes in concentration of these monoamines are involved in many common neuropsychiatric disorders (Hows et al., Citation2004; Manini et al., Citation2000). They are also important in the regulation of immune function (Bergquist & Silberring, Citation1998). Noradrenaline () is a catecholamine neurotransmitter associated with arousal. Serotonin () is important in mood, behavior, movement, pain appreciation, sexual activity, appetite, endocrine secretions, cardiac functions, sleep, and dreaming. The role of changes in serotonin level is particularly implicated in depression and some forms of epilepsy (Pitchot et al., Citation2005; Svenningson et al., Citation2006). Antidepressant drugs of selective serotonin reuptake inhibitors (SSRI) group like fluoxetine work through the serotonergic system. Norepinephrine is involved in maintaining alertness, focus, and motivation. Tricyclic antidepressants (TCAs) having secondary amine structure preferentially affect the norepinephric system and serotonin norepinephrine reuptake inhibitors (SNRIs) act by both serotonergic and norepinephric systems in brain (Koenig et al., Citation2009; Samanidou et al., Citation2012).
Many experimental methods having been developed for the analysis of these brain monoamines like fluorometric detection, chemiluminiscence detection, gas chromatography (GC), capillary zone electrophoresis, liquid chromatography with mass spectrometry (LCMS), and high performance liquid chromatography (HPLC) with electrochemical detector (ECD) (Lunte & O’Shea, Citation1994; Saha et al., Citation2002; Semerdjian-Rouquier et al., Citation1981). The estimation of monoamines by HPLC with ECD is the most widely used method (Cooper et al., Citation1994; Kulkarni & Dhir, Citation2008), but due to sudden noise problems, it is not preferable for routine analysis (Yoshitake et al., Citation2004; Carrera et al., Citation2007).
Although HPLC with the UV detector is widely available and easy to handle, still there is lack of literatures on the estimation of monoamines in biological samples by this method. Norepinephrine, serotonin, dopamine, and its derivatives were estimated in brain samples by HPLC-UV by Komura and Sakamoto (Citation1990), but they used ion pair reagents with a complicated procedure. Thus, there is a need to develop a cost-effective and routinely performable method for the analysis of monoamines in biological samples. Therefore, the present study was designed with an aim to develop a simple and cost-effective HPLC-UV method for simultaneous estimation of norepinephrine and serotonin in brain samples. The developed method was used to optimize the sample preparation method.
Materials and methods
Chemicals
Norepinephrine and serotonin were purchased from Sigma Aldrich, Mumbai, India. Methanol, formic acid, acetonitrile, acetic acid, and phosphoric acid (HPLC grade) were purchased from Merck (Mumbai, India). Perchloric acid, EDTA, sodium bisulfite, chloroform, and isopropanol were also purchased from Merck (Mumbai, India).
Instruments
Chromatography was performed with Quaternary pump HPLC (SHIMADZU: SPD-10A VP, Shimadzu, Kyoto, Japan), equipped with 25 cm length, 4.6 mm internal diameter, and Cosmosil C18 Column (particle size 5 µm) (Phenomenex, Torrance, CA). Rheodyne syringe injector (25 µL) was used and the sample loop had a capacity of 20 µL. For sample preparation, cooling Centrifuge (Sigma 3-30K, Osterode, Germany) was used.
Animals
All the experiments were carried out using Swiss albino mice (5–7 weeks, body wt 30–35 g), procured from the Central Animal House Facility, Jamia Hamdard, Hamdard University, New Delhi, India. The animals had free access to standard laboratory food and water ad libitum, and they were housed in a natural light-dark cycle (12 h each). The animals were acclimatized to the laboratory conditions for at least 7 d before experiments. The experimental protocol was approved by the Institutional Animal Ethical Committee (743/CPCSEA, 2011) and the care of laboratory animal was taken as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Optimization of chromatographic conditions
Various mobile phases were tried referring to previous literatures on HPLC and LC-MS like acetic acid (0.01–0.1%):methanol, phosphoric acid (0.01–0.1%):methanol, and formic acid (0.01–0.1%):acetonitrile. The wavelength was selected as 280 nm from previous the literature (Komura & Sakamoto, Citation1990) and an injection volume was 20 µL. Different flow rates were tried from 0.5 mL min−1 to 1.5 mL min−1 and a flow rate giving optimum results was fixed. Retention time for both norepinephrine and serotonin in the standard solutions was noted.
Optimization of sample preparation method
Different methods were tried for sample preparation with reference to previous literatures. Fixed concentration of standards, i.e., 200 ng mL−1 solution of norepinephrine and 750 ng mL−1 solution of serotonin, was spiked into homogenate of control samples and was extracted by four methods as given below and recovery values were estimated. From the four methods, one which gave maximum recovery was selected.
Mice were sacrificed by decapitation, brain tissues were removed immediately, washed in ice cold normal saline, dried, weighed precisely, and then prepared by the following methods.
Method 1: Brain samples were homogenized with 0.1 M perchloric acid and were centrifuged at 43 000 rpm for 15 min. The supernatant was separated and filtered through 0.25 µm nylon filters and injected into the chromatographic system (Dhir & Kulkarni, 2007).
Method 2: Brain samples were suspended in 10 mL g−1 of tissue in ice-cold 0.1 mol L−1 perchloric acid containing 1.34 mmol L−1 EDTA and 0.05%, w/v sodium bisulfite and were sonicated. Then, the homogenates were centrifuged at 35 000 rpm for 20 min at 4 °C. The supernatant was then filtered with 0.25 filters and injected into the chromatographic system (Parrot et al., Citation2011).
Method 3: Brain tissues were homogenized in ice cold methanol (4.0 mL g−1 of tissue). The 1.0 mL of homogenate was pipette out and centrifuged at 14 000 rpm for 20 min. Then the supernatant was evaporated to dryness by vacuum freeze. The dry residue was then reconstituted with 300 µL de-ionized water and vortex mixed for 10 s and added 300 µL solution of chloroform:isopropanol (100:30, v/v). Vortex mixed for 2 min and was centrifuged at 3000 rpm for 5 min. The upper aqueous layer was separated and injected into the HPLC system (Su et al., Citation2009).
Method 4: Brain tissues were kept in ice cold methanol (2 mL g−1 of tissue) and vortex mixed for 2–3 min to make a homogenate. Acetonitrile (1 mL g−1 of tissue) was added and vortex mixed again. Then, centrifuged at 14 000 rpm for 20 min. Supernatant (2 mL) was pipetted out and kept in drying tubes. Drying tubes were then kept in nitrogen chamber, where water was replaced with ice. Dried supernatant was reconstituted in 500 µL mobile phase and vortex for 30 s and was filtered through 0.22 µm nylon filters and was used for injecting into the HPLC system.
Method validation
Linearity of the method
Stock solutions of standard drugs, 1 mg mL−1 of norepinephrine, and serotonin were prepared. It was then diluted in the mobile phase so as to obtain different concentrations, 3.13–4000 ng mL−1 for norepinephrine and 3.91–4000 ng mL−1 for serotonin. These different dilutions were injected to the HPLC system and peak areas were recorded. Calibration curves of norepinephrine and serotonin were obtained by plotting peak areas versus applied concentrations at a range of 50–4000 ng mL−1 for norepinephrine and 31.25–4000 ng mL−1 for serotonin, respectively. Both limits of detection and quantification (LOD and LOQ) were determined according to the following equations as per ICH guidelines (ICH, Citation2005).
(1)
(2)
where σ is the standard deviation (SD) of y-intercept of calibration curves and S is the mean of slope of calibration curve.
Accuracy
Accuracy of the method was calculated by performing the recovery studies after spiking the samples with standard drugs at four different concentration levels (25, 50, 100, and 150%) of concentration in the homogenate of mice brain samples. The percentage recovery was calculated.
Repeatability, intra, and inter-day precision
Repeatability was determined by carrying out the estimation at different concentrations of standard solution of norepinephrine (100, 500, and 1000 ng mL−1) and serotonin (250, 500, and 1000 ng mL−1) for six times each in different chromatographic columns with same dimensions (Prodigy, Phenomenex, Torrance, CA and Phospher, Merck, Darmstadt, Germany) and with different analysts. Intra-day precision was determined at three different concentrations of standard solution for six times on the same day. Inter-day precision was determined by analyzing the above standard concentrations over a period of three different days.
Robustness
The robustness/ruggedness of an analytical procedure is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability during normal usage (Danaher et al., Citation2000). Robustness was determined by checking the changes in the result, while changing the ratio of the mobile phase from 85 to 95% of acetonitrile and 15 to 5% of 0.05% formic acid. Wavelength was varied in the range of 278–282 nm.
Results
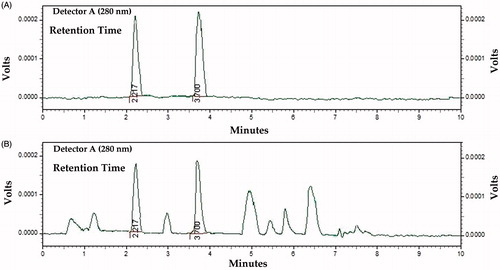
Optimum chromatographic conditions were determined after running different mobile phases. Optimum results were obtained with the mobile phase consisting of a mixture of 0.05% formic acid and acetonitrile in the ratio 90:10; v/v, run in an isocratic elution mode. The flow rate of the mobile phase was maintained at 1.0 mL min−1. A good separation was found to be achieved within 10 min using the conditions described. Symmetrical, sharp, and well-resolved peaks were observed for norepinephrine and serotonin. The elution order and the retention times for norepinephrine and serotonin were 2.17 ± 0.004 and 3.7 ± 0.005 min, respectively ().
Figure 2. (A) Chromatogram showing standard graphs of norepinephrine and serotonin. (B) Chromatogram showing the norepinephrine and serotonin in mice brain homogenate.
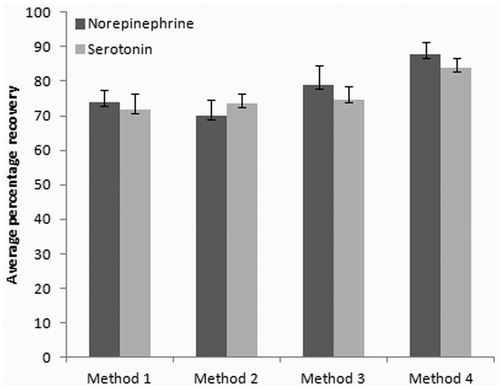
The comparison of recovery values obtained by four different methods after spiking a fixed concentration of standard norepinephrine (200 ng mL−1) and serotonin (750 ng mL−1) in the homogenate of control sample is shown in . Values of percentage recovery obtained for norepinephrine were 73.76 ± 3.28, 69.87 ± 4.65, 78.98 ± 5.54, and 87.25 ± 3.74 for methods 1, 2, 3, and 4, respectively. Recovery values in percentage obtained for serotonin were 71.62 ± 4.65, 73.65 ± 2.72, 74.67 ± 3.14, and 83.83 ± 2.98 for methods 1, 2, 3, and 4, respectively. Method 4 gave maximum percentage recovery for norepinephrine as well as serotonin and was selected for analysis.
Figure 3. Recovery values given by different sample preparation methods when a fixed amount of standards (norepinephrine, 200 ng mL−1 and serotonin, 750 ng mL−1) were spiked into normal brain samples.
Chromatogram of the sample obtained with optimized sample preparation method and chromatographic conditions are shown in . Standard norepinephrine and serotonin were run at different concentrations and calibration curves were constructed for both norepinephrine and serotonin. Both the curves were found to be linear. Regression coefficient (r2) values were calculated according to the equation, y = mx + c. Regression coefficient (r2) for norepinephrine was found to be 0.9943 and that for serotonin was 0.9961 (). The method was found be sensitive as it could detect (LOD = 25 ng mL−1) and quantify (LOQ = 50 ng mL−1) low amount of norepinephrine. Serotonin was found to be detectable at 15.63 ng mL−1 (LOD) and quantifiable at 31.25 ng mL−1 (LOQ).
Table 1. Analytical parameters for norepinephrine and serotonin.
Spiking the brain homogenate of control samples with standard solutions at low, medium, and high levels (25, 50, 100, and 150%) gave recoveries in the range of 86.04–89.01% for norepinephrine and 86.43–89.61% for serotonin (). The values of % relative standard deviation (RSD) at three levels of concentrations for repeatability as well as both intra and inter-day precision studies for standard norepinephrine and serotonin are shown in . % RSD for repeatability and intra- and inter-day precision studies for samples were less than 10.11% for norepinephrine and 9.44% for serotonin ().
Table 2. Accuracy test results (n = 6).
Table 3. Repeatability and intra- and inter-day precision (n = 6) for the standard solution of norepinephrine and serotonin.
Table 4. Repeatability and intra- and inter-day precisions of sample.
The robustness of the developed method was checked by making small but deliberate changes in the method parameters. The % RSD obtained for method variables such as detection wavelength and ratio of the mobile phase were lower than the standard limits and are shown in .
Table 5. Robustness data of the HPLC method using control samples.
Discussion
The present work contributes a new and a simple method for the estimation of norepinephrine and serotonin in mice brain samples using HPLC-UV. There are so many methods reported for the extraction of neurotransmitters from brain tissues. So we used four different sample preparation methods and the best one was ascertained on the basis of maximum recovery.
Noradrenaline and serotonin are brain neurotransmitters, which actively influence mental behavior patterns and have become a centre of neuropsychopharmacological studies for many years (Blows, Citation2000). A change in the concentrations of brain of norepinephrine and serotonin in brain disorders like depression, schizophrenia, panic disorder, and Parkinson’s disease are already established (Fujino et al., Citation2003). It indicates the need for developing simple, sensitive, and reliable methods for the estimation of these bioamines in brain samples. There are advanced methods which can detect these compounds at lower levels (Vaarmann et al., Citation2002), but it requires expensive equipments and solvents. Here, we could detect up to a lower level of 25 ng mL−1 of norepinephrine and 15.63 ng mL−1 of serotonin using inexpensive chemicals with HPLC-UV. It is comparable with that of the sensitivity of method given by Su et al. (Citation2009) which used expensive HPLC-MS-MS.
For comparing the recovery values in samples extracted by different sample preparation methods, the concentrations of standard drugs were selected as 200 ng mL−1 of norepinephrine and 750 ng mL−1 of serotonin (Welch & Welch, Citation1969) because it is almost equivalent to the amount of these drugs present in the homogenate of control brain samples and lies in between linearity range. Clearly resolved, sharp, symmetrical peaks showed that good separation of norepinephrine and serotonin from other components present in the brain extract was achieved. Calibration curves with a regression coefficient greater than 0.99 shows the linearity of the method. Low LOD and LOQ values show the high sensitivity of the method.
Good recovery values within the limit of ICH guidelines were obtained and are comparable with that obtained by HPLC-MS methods (Carrera et al., Citation2007; Su et al., Citation2009). The values of % RSD at all three levels of concentrations for repeatability and intra and inter-day precision studies indicate the method is precise and reproducible. Low %RSD values obtained for the deliberate variations in the wavelength and ratio of the mobile phase show the robustness of the method.
Conclusion
A sensitive, precise, and reliable isocratic HPLC-UV method for simultaneous separation and quantification of norepinephrine and serotonin in whole mice brain samples has been developed. The method was performed using inexpensive chemicals and reagents and the procedure is also simple. It can be further extrapolated to other monoamines and its metabolites also.
Declaration of interest
The authors report that they have no conflicts of interest. The authors thank CSIR, New Delhi, India, for sponsoring the work.
References
- Bergquist J, Silberring J. (1998). Identification of the catecholamines in the immune system by electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 12:683–8
- Blows WT. (2000). Neurotransmitters of the brain: Serotonin, noradrenaline (norepinephrine), and dopamine. J Neurosci Nutr 32:234–8
- Carrera Victoria, Sabater Esther, Vilanova Eugenio, Sogorb Miguel A. (2007). A simple and rapid HPLC-MS method for the simultaneous determination of epinephrine, norepinephrine, dopamine and 5-hydroxytryptamine: Application to the secretion of bovine chromaffin cell cultures. J Chromatogr B 847:88–94
- Cooper BR, Wightman MR, Jorgenson JW. (1994). Quantification of epinephrine and norepinephrine secretion from individual adrenal medullary cells by microcolumn high-performance liquid chromatography. J Chromatogr 653:25–34
- Danaher M, O'Keeffe M, Glennon JD. (2000). Validation and robustness testing of a HPLC method for the determination of avermectins and moxidectin in animal liver samples using an alumina column clean-up. Analyst 125:1741–4
- Dhir A, Kulkarni SK. (2007). Involvement of nitric oxide NO signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol 568:177–85
- Hows MEP, Lacroix L, Heidbreder C, et al. (2004). High-performance liquid chromatography/tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples. J Neurosci Methods 138:123–32
- Fujino K, Yoshitake T, Kehr J, et al. (2003). Simultaneous determination of 5-hydroxyindoles and catechols by high-performance liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J Chromatogr A 1012:169–77
- International Conference on Harmonisation. (2005). Validation of analytical procedure: Text and methodology Q2 (R1). Geneva: IFPMA
- Koenig AM, Thase ME. (2009). First-line pharmacotherapies for depression – What is the best choice? Pol Arch Med Wewn 119:478–86
- Komura J, Sakamoto M. (1990). Determination of biogenic amines and their metabolites in regional tissue of mouse brain by high performance liquid chromatography using an ultraviolet detector. J Liq Chromatogr 13:1291–9
- Kulkarni SK, Dhir A. (2008). On the mechanism of antidepressant action of berberine chloride. Eur J Pharmacol 589:163–72
- Lunte SM, O’Shea TJ. (1994). Pharmaceutical and biomedical applications of capillary electrophoresis/electrochemistry. Electrophoresis 15:79–86
- Manini P, Andreoli R, Cavazzini S, et al. (2000). A new method for the analysis of styrene mercapturic acids by liquid chromatography/electrospray tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 744:423–31
- Parrot S, Neuzeret P-C, Denoroy L. (2011). A rapid and sensitive method for analysis of brain monoamine neurotransmitters using ultra-fast liquid chromatography coupled to electrochemical detection. J Chromatogr B 879:3871–8
- Pitchot W, Hansenne M, Pinto E, et al. (2005). 5-Hydroxytryptamine 1A receptor, major depression, and suicidal behavior. Biol Psychiatry 58:854–8
- Saha RN, Sajeev C, Jadhav PR, et al. (2002). Determination of celecoxib in pharmaceutical formulations using UV spectrophotometry and liquid chromatography. J Pharm Biomed Anal 28:741–51
- Samanidou V, Pantazidou K, Kovatsi L, et al. (2012). A simple HPLC method for the simultaneous determination of two selective serotonin reuptake inhibitors and two serotonin–norepinephrine reuptake inhibitors in hair, nail clippings, and cerebrospinal fluid. J Sep Sci 35:839–45
- Semerdjian-Rouquier L, Bossi L, Scatton B. (1981). Determination of 5-hydroxy tryptophan, serotonin and 5-hydroxyindoleacetic acid in rat and human brain and biological fluids by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr 218:663–70
- Su F, Wang F, Zhu R, Li H. (2009). Determination of 5-hydroxytryptamine, norepinephrine, dopamine and their metabolites in rat brain tissue by LC-ESI-MS-MS. Chromatographia 69:207–13
- Svenningson P, Chergui K, Rachleff I, et al. (2006). Alterations in 5-HT1B receptor function by p11 in depression-like states. Science 311:77–80
- Vaarmann A, Kask A, Maeorg U. (2002). Novel and sensitive high-performance liquid chromatographic method based on electrochemical coulometric array detection for simultaneous determination of catecholamines, kynurenine and indole derivatives of tryptophan. J Chromatogr B Analyt Technol Biomed Life Sci 769:145–53
- Welch AS, Welch BL. (1969). Solvent extraction method for simultaneous determination of norepinephrine, dopamine, serotonin and 5-hydroxyindoleacetic acid in a single mouse brain. Analyt Biochem 30:161–79
- Yoshitake T, Yoshitake S, Fujino K, et al. (2004). High-sensitive liquid chromatographic method for determination of neuronal release of serotonin, noradrenaline and dopamine monitored by microdialysis in the rat prefrontal cortex. J Neurosci Methods 140:163–8