Abstract
Context: Plants of the genus Heracleum L. (Apiaceae) have a long history of being used in traditional medicines for the treatment of alimentary tract disorders, and these biological effects have been ascribed to the presence of furanocoumarins (including bergapten).
Objectives: This study aimed to develop an efficient, preparative, counter-current chromatographic separation of bergapten in order to characterize its spasmolytic activity in isolated rat jejunum strips.
Materials and methods: Successful separation of the dichloromethane extract of the fruits of Heracleum leskovii Grossh. was achieved by high-performance countercurrent chromatography (HPCCC) using a two-phase solvent system composed of n-heptane/EtOAc/MeOH/H2O (6:5:6:5, v/v/v/v). The pharmacological assessment of bergapten (0.0001–50 μM) on jejunum smooth muscle strips isolated from rats was conducted under isotonic conditions, following up to three hours of incubation.
Results: The separation method was scaled up six-fold from analytical to semi-preparative conditions, affording bergapten of >99% purity in less than 30 min. This permitted bergapten to be available in quantity for spasmolytic tests on isolated jejunum strips from rats. Bergapten caused myorelaxation of the intestine preparations in the concentration range of 0.0001–1 μM. At higher doses, bergapten caused either relaxation or contraction of the smooth muscle.
Discussion and conclusion: Bergapten was successfully isolated by rapid HPCCC and its spasmolytic activity was confirmed, thereby providing a preliminary evidence base for the traditional medicine application. The data suggest that bergapten causes no irreversible changes to intestinal tissue.
Introduction
Functional gastro-intestinal diseases, including functional dyspepsia (FD) and irritable bowel syndrome (IBS), are well-documented ailments in many industrialized countries (Allescher, Citation2006; Drossman et al., Citation1993; Heaton et al., Citation1992). The symptomatic therapy of these diseases typically includes the application of antispasmodic agents. Such medications are typically taken chronically and their effectiveness on prolonged use is not always very satisfactory (Talley, Citation2003). Several attempts to include phytotherapeutical constituents into the treatment regimens of FD and IBS have been described (Bensoussan et al., Citation1998; Vejdani et al., Citation2006). Furanocoumarins, which have shown effectiveness in the treatment of alimentary tract disorders, are a significant group of active metabolites of plants in the Apiaceae (formerly Umbelliferae) family. Many of these plants are used in traditional medicine and are reputed to be effective for the treatment of alimentary tract disorders (Abbaskhan et al., Citation2012; Al-Essa et al., Citation2010). In pharmacological studies with isolated guinea pig ileum, STW5 (a plant-based product containing the roots of Angelica archangelica L. and the fruits of Carum carvi L.) was shown to exhibit spasmolytic activity (Ammon et al., Citation2006). Antispasmodic activity of plant extracts rich in furanocoumarins was also observed in the stomach fundus, corpus, antrum (Schemann et al., Citation2006), and jejunum (Abbaskhan et al., Citation2012). In addition, A. archangelica roots, which contain coumarins as the principal constituents, were found to relax the guinea-pig ileum (Izzo et al., Citation1996). Although the spasmolytic activity of the whole extract of A. archangelica is well recognized, the effects of the individual active constituents and their potential synergistic/antagonistic effects are not reported.
A principal constituent in A. archangelica, and participating in these activities, is the furanocoumarin bergapten (5-methoxypsoralen). According to the document “Reflection paper on the risks associated with furanocoumarins contained in preparations of A. archangelica” prepared by the Committee on Herbal Medicinal Products, European Medicines Agency (London, 31 October, 2007), there are significant knowledge deficiencies concerning furanocoumarins. Thus, while there are extensive data regarding their phototoxicity (Clark & Wilkinson, Citation1998; Müller et al., Citation1998; Raquet & Schrenk, Citation2014), little is known about the systemic effects of individual furanocoumarins. This preliminary study aims to enhance the functional pharmacological knowledge of one of these furanocoumarins, bergapten.
Previous experiments in these laboratories showed that the fruits of Heracleum leskovii Grossh. (Apiaceae) provide an abundant source of bergapten (Skalicka-Woźniak & Głowniak, Citation2012). Plants from the genus Heracleum are noted for the treatment of alimentary tract disorders. Hogweed (Heracleum sphondylium L.) has been recommended in case of dyspepsia in Europe and Asia, and the aqueous extract has been used in Bulgaria as a spasmolytic in gastrointestinal disorders (Leporatti & Ivancheva, Citation2003). The leaf powder of Heracleum lanatum Michx., when taken with water, is used to treat gastric problems (Singh & Lal, Citation2008). These pharmacological effects have been observed in furanocoumarins, including bergapten (Van Wyk & Wink, Citation2010). In order to have sufficient quantity of purified bergapten available for pharmacological studies, the fruits of H. leskovii were processed efficiently through high-performance counter-current chromatography (HPCCC) and the process was scaled from analytical to preparative to effect rapid, preparative separation. The spasmolytic activity of bergapten was determined in isolated rat jejunum strips under isotonic conditions.
Materials and methods
Reagents and materials
All solvents used for separation were of analytical grade (Polish Reagents POCH, Gliwice, Poland). Methanol used for HPLC was of chromatographic grade (J.T. Baker Inc., Deventer, The Netherlands), water used was purified using a Millipore laboratory ultra pure water system (SimplicityTM system, Millipore, Molsheim, France). Acetylcholine chloride, dimethylsulfoxide (DMSO), isoproterenol hemisulfate, (Sigma Chemical Co, St. Louis, MO), CaCl2 (Merck, Darmstadt, Germany), NaH2PO4 (Fluka Chemie, AG, Buchs, Switzerland), inorganic salts: NaCl, KCl, MgSO4, NaHCO3; glucose (POCh, Gliwice, Poland) were used in the experiment. A standard sample of bergapten used for HPLC analysis was provided by Sigma-Aldrich (St. Louis, MO) (CAS number 484-20-8). Bergapten was dissolved in DMSO (0.5% w/v). Acetylcholine chloride and isoproterenol hemisulfate were dissolved in modified Krebs–Henseleit solution (M K-HS).
Isolation of bergapten
Apparatus
A Spectrum HPCCC (Dynamic Extractions, Slough, UK) consisting of analytical and semipreparative coils (22 mL and 137 mL capacity, respectively) was used. The instrument was connected to a Sapphire UV detector and an Alpha 10 pump (ECOM, Prague, Czech Republic). An Agilent 1100 instrument (Agilent Technologies, Santa Clara, CA) with a photodiode array detection (DAD) system was used for HPLC.
Sample preparation
The fruits of Heracleum leskovii Grossch. (Apiaceae) were collected in the Medicinal Plant Garden, Department of Pharmacognosy, Medical University, Lublin, Poland, in the summer of 2010. The plant material was identified by Mrs. Krystyna Dąbrowska, Botanical Garden of Maria Curie-Skłodowska University, Lublin, a botanical specialist. The fruits were dried at room temperature and ground to fine powder. A voucher specimen no. 26/27-28b of the plant material has been deposited in the Department of Pharmacognosy, Medical University, Lublin, Poland. A sample (100 g) of the dried and milled fruits was extracted three times under reflux with dichloromethane, filtered, and evaporated to afford the extract (12.6 g; yield 12.6%).
Selection of the two-phase solvent system
A two-phase solvent system providing a partition coefficient (K) of the target compound around 1, was developed based on the evaluation of different mixtures of heptane, ethyl acetate, methanol, and water (HEMWat) (), according to the method of Garrard (Citation2005). The K values (peak area of target compound in the stationary phase divided by the peak area in the mobile phase) were determined by the HPLC analysis. The optimal two-phase solvent system was prepared, thoroughly equilibrated at room temperature, and degassed by sonication prior to use.
Table 1. Partition coefficients (K) for bergapten in reversed-phase systems.
Separation procedure
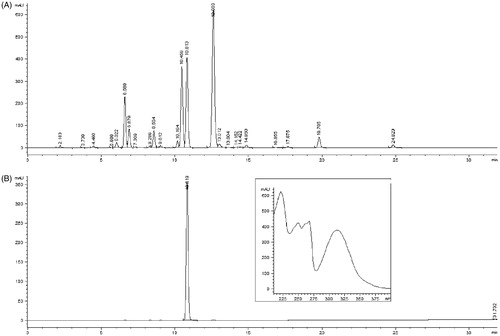
The upper phase (stationary) of the developed solvent system was pumped into the column. After the column was totally filled, the apparatus was rotated at 1600 rpm. At the same time, the lower phase (mobile) was pumped into the column at a flow rate of 1 mL/min (analytical conditions) or 6 mL/min (semi-preparative conditions) (head to tail). When the analytical column was used, the crude extract (84 mg) was dissolved in the two-phase solvent system (1 mL) and injected into the column through the 1 mL injection valve. When the separation was scaled to semi-preparative conditions, the extract (500 mg) was dissolved in the two-phase solvent system (6 mL) and the 6 mL injection valve was used. The effluent from the head end of the column was continuously monitored at 254 nm. Fractions were collected manually every minute, evaporated under reduced pressure, and dissolved in methanol. HPLC purity assessment was performed with a Zorbax Eclipse XDB C18 column (4.6 × 250 mm2, 5 µm) at 25°C and a flow rate of 1 mL/min. The mobile phase was A: methanol and B: water. The gradient was 0–5 min 50–60% A; 5–25 min 60–80% A; 25–30 min 80–100% A; 30–32 min 100% A. Detection was performed at 240 and 320 nm. Identification of bergapten was carried out by comparing the retention time and UV–DAD spectra with the data obtained from injecting the standard sample under the same conditions, and by spiking the standard into the sample.
Effect of bergapten on the spontaneous motor activity of isolated rat jejunum strips
Isolation and preparation of intestine strips
Jejunal strips were isolated from Wistar rats following euthanization in chambers filled with carbon dioxide (CO2). The protocol for the use of animals was approved by the local ethics committee (Approval number 8/2011). Immediately after euthanasia, the abdominal cavity of the rat was opened and a strip of the jejunum was excised, as described previously (Chlopecka et al., Citation2007). Each preparation of tubular, physiological shape was 15 mm in length, and all preparations were suspended in incubation chambers (5 mL) (Schuler Organ Bath, Hugo Sachs Elektronic, Harvard Apparatus, Germany). Modified Krebs–Henseleit solution (M K-HS) containing: NaCl (123.76 mM), KCl (5 mM), CaCl2 (2.5 mM), MgSO4 (1.156 mM), NaHCO3 (14.5 mM), KH2PO4 (2.75 mM), and glucose (12.5 mM), was employed as an incubation medium for the gastrointestinal strips, and was maintained at pH 7.35–7.45 throughout the long-term experiments, while being heated to 37°C and bubbled with carbogen (95% O2 + 5% CO2).
Registration of motoric activity of rat isolated intestine strips
Jejunum strips were distally attached to a hook and connected to an isotonic transducer (B 40, type 373, Hugo Sachs Elektronic, Germany). All experiments were conducted under a load of 0.5 g. Isotonic transducers were linked to an analog–digital registration set (PowerLab, ADInstruments, Sydney, Australia) by a bridge amplifier (DBA, type 666, Hugo Sachs Elektronic, March, Germany). The motor activities of the isolated intestine strips were recorded using the Chart v5.0 program (PowerLab, ADInstruments, Sydney, Australia). All calculations and analyses were completed using the LabChart Reader 7 program (PowerLab, ADInstruments, Sydney, Australia) and Excel (Microsoft Office XP Professional).
Experimental procedures
All experiments were initiated with a pre-incubation period (60 min) interrupted with several flushings with M K-HS. Subsequently, the preparations were treated with two reference substances: acetylcholine, a reference contractile agent (1 μM), and isoproterenol, a reference relaxant agent (0.1 μM). Next, the solvent for bergapten (DMSO, 0.5%) was added into the incubation vessels in order to exclude its effects on strip response to bergapten (neutral control). Once, the spontaneous motor activity was stabilized, the administration of bergapten in a concentration range of 0.0001–50 μM in a non-cumulative manner was conducted. At the termination of each experiment, acetylcholine was applied to verify the responsiveness of the strip throughout the long-term experiment. All experiments were repeated 7–8 times.
Expression of the obtained results and statistical analyses
The results were expressed as the percent of the reaction induced by a reference substance applied in the optimal dose. All data were analyzed using Statistica PL for Windows (version 6.1, SPSS Inc, Chicago, IL). Results were expressed as mean values ± standard deviation (SD) of the average. Values of p ≤ 0.05 were considered to be significant. Three tests were used in the statistical analysis: a one-way analysis of variance (ANOVA), the Student t-test, and the LSD Fisher test. The change of the spontaneous motor activity was considered as significant if its strength of the reaction differed statistically from the force of the response to DMSO at a concentration of 0.5% (control).
Results
Separation of bergapten by high-performance counter-current chromatography
The selection of the two-phase solvent system for HPCCC is the most important, and also the most challenging aspect, because any change in the mobile phase composition is likely to change the stationary phase composition or volume (Li & Chen, Citation2005). In order to determine a suitable distribution ratio (K) of the target compound, different mixtures of heptane, ethyl acetate, methanol and water were evaluated, and the phases analyzed by HPLC. A solvent system composed of n-heptane, ethyl acetate, methanol, and water (6:5:6:5) was chosen as the most appropriate system among many tested solvent mixtures. The K values for bergapten in the tested solvent mixtures are presented in . A mixture of all four solvents in equal volume with the K = 1.17 would also be suitable; however, to decrease the separation time, the volume ratio of 6:5:6:5 was successfully used.
In order to determine suitable conditions for the HPCCC separation, experiments were performed on an analytical scale, and were then easily scaled to semi-preparative conditions. The method was scaled up six-fold to semi-preparative conditions, where the scale up factor was calculated as the ratio between the analytical and semi-preparative column volumes. After injection of the crude extract (500 mg), 40 fractions were collected and analyzed by HPLC. Bergapten (tR = 10.813), one of the main components of the crude extract, and a sample (95 mg) with purity >99% was isolated from fractions 22–28 (between 22 and 28 min). Chromatograms of the crude extract obtained from H. leskovii, together with the UV-DAD spectrum of the isolate, are shown in .
Effect of bergapten on the spontaneous motor activity of isolated rat jejunum strips
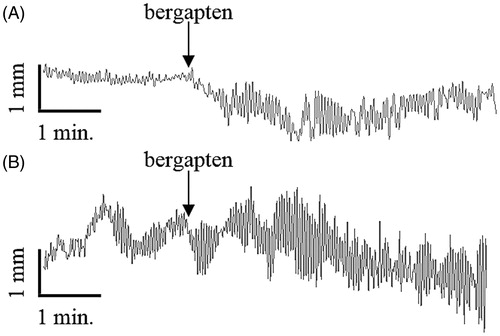
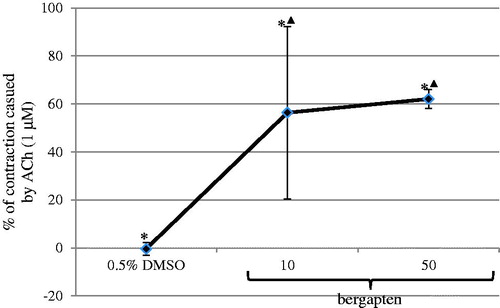
Bergapten affected significantly the spontaneous motor activity of rat jejunal smooth muscle in the wide concentration range 0.0001–50 μM ( and ). The observed reactions evoked by bergapten had a dual, dose-dependent character: either myorelaxation or contraction (). The administration of bergapten at the lowest dose (0.0001 μM) caused significant myorelaxation. Increasing the bergapten concentration (up to 50 μM) did not result in any statistically significant enhancement of the force of the relaxant response. The strongest myorelaxation was registered when the furanocoumarin was employed at 1 μM (). Interestingly, the administration of bergapten into the incubation vessels at doses of 10 and 50 μM did not always cause myorelaxation. Only half of the jejunum strips (four out of eight) exposed to bergapten at a concentration of 10 μM underwent myorelaxation, whereas the other four jejunal preparations experienced contraction ( and ). Similarly, the application of bergapten at a dose of 50 μM caused either myorelaxation (three out of seven jejunum strips) or contraction (four out of seven preparations) (). The force of the observed contractions amounted to 56.3 ± 35.9% (10 μM of bergapten) and 62.0 ± 3.9% (50 μM of bergapten) of the response to acetylcholine (1 μM). The disclosure of independent dual effects of bergapten on jejunal smooth muscle facilitates an understanding of the tendency of the myorelaxation force to drop when bergapten was applied in high doses (10 and 50 μM) in comparison with the strength of the reaction evoked by bergapten in the lower dose (1 μM).
Figure 2. Myorelaxant effect of bergapten dissolved in 0.5% DMSO on the spontaneous motor activity of isolated jejunum strips. The results are expressed as % of the relaxation caused by isoproterenol applied in the reference dose of 0.1 μM. The relaxation provoked by isoproterenol in the reference dose is expressed as 100%. The results are expressed as a mean from 7 to 8 independent experiments (±SD). *p ≤ 0.05 versus Isop, ▴p ≤ 0.05 versus DMSO, 0.5%.
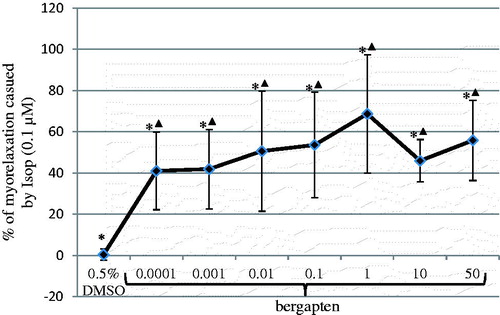
Figure 3. Contractile effect of bergapten dissolved in 0.5% DMSO on the spontaneous motor activity of isolated jejunum strips. The results are expressed as % of the contraction caused by acetylcholine applied at the reference dose of 1 μM. The contraction provoked by acetylcholine at the reference dose is expressed as 100%. The results are expressed as a mean from 7 to 8 independent experiments (±SD). *p ≤ 0.05 versus ACh, ▴p ≤ 0.05 versus DMSO, 0.5%.
Discussion
Plants containing coumarins are widely used in traditional medicine for the treatment of alimentary tract disorders, mostly as spasmolytics or carminative, however, little is known about pharmacological activities of the individual constituents. Before determination of the spasmolytic activity of bergapten, one of the main furanocoumarins in isolated rat jejunum strips, a rapid and efficient separation technique was developed for purification from a known plant source. HPCCC, applied in this study, is a new generation chromatograph where preparation times using HPCCC are minutes rather than hours, while maintaining resolution and elution times comparable to existing countercurrent chromatography techniques. This is the first time that bergapten could be isolated in such a short time and at such a high degree of purity. Previously, Li and Chen (Citation2005) isolated bergapten with four other coumarins from a crude ethanol extract of the dried fruits of Cnidium monnieri Cusson (Apiaceae), through a stepwise elution using a two-phase solvent systems composed of n-hexane/EtOAc/EtOH/H2O (5:5:4:6, v/v) and (5:5:6:4, v/v). The upper phase of the first solvent system was used as the stationary phase. The apparatus was rotated at 1000 rpm and the flow rate of the mobile phase was 1 mL/min. Bergapten, together with five other compounds, was isolated with satisfactory peak resolution; however, the separation took 13 h. Li and Chen (Citation2004) used a two-phase solvent system at a volume ratio of 5:5:5:5 for the separation of bergapten and imperatorin. The flow rate of the mobile phase was increased from 1 to 2 mL/min, but still the separation took several hours. A stepwise elution with a pair of two-phase solvent systems composed of light petroleum/EtOAc/MeOH/H2O at volume ratios of 5:5:5:5, 5:5:6:4 and 5:5:6.5:3.5 was also tested. Bergapten was isolated after 3 h, and the separation of all six compounds was completed after 8 h (Liu et al., Citation2004). The present isolation technique thus represents a significant improvement in the preparation of highly purified bergapten for biological study, and allows a decrease in the separation time to 30 min.
Bargapten, obtained through HPCCC separation, was subjected to spasmolytic tests. It caused myorelaxation of intestine when used in the concentration range of 0.0001–1 μM. At higher doses, bergapten caused either relaxation or contraction of smooth muscle (). Observation of a dual, sometimes opposing, character of a chemical is not very common; however, there are examples where compounds act quite differently depending on their concentration (e.g., phenylephrine, terbutaline, nicotine, coniine). It can be speculated that in the case of some jejunum preparations the relaxant and contractile responses are evoked simultaneously, and that the observed overall reaction is derived from both reaction responses. According to this hypothesis, the myorelaxation induced by bergapten at doses of 10 and 50 μM is diminished by the contractile component. Confirmation of this would be possible if one component (contractile or myorelaxant) of the response to bergapten could be inhibited, i.e., if the contraction or relaxation could have been abolished through the application of a membrane antagonist, or through the inhibition of intracellular pathways, focusing on calcium movements (Vuorela et al., Citation1997).
Generally, these results are in agreement with other studies, suggesting a relaxant effect of isolated furanocoumarins and plant extracts rich in these compounds on smooth muscle (He et al., Citation2007; Heinle et al., Citation2006; Zhang et al., Citation2010). However, previous studies typically focused on the myorelaxant effect of furanocoumarins on vascular smooth muscle (Chiu et al., Citation2001; He et al., Citation2007; Thastrup et al., Citation1983), and only very few analyzed their impact on gastrointestinal (GI) smooth muscle (Abbaskhan et al., Citation2012; Heinle et al., Citation2006; Schemann et al., Citation2006). According to these data, it can be postulated that bergapten contributes to the spasmolytic effect of A. archangelica extract and the phytomedicine STW 5 mentioned previously (Heinle et al., Citation2006; Schemann et al., Citation2006). However, it is surprising that in contrast to furanocoumarins of the psoralen type (e.g., imperatorin, xanthotoxin) bergapten caused a contractile response of the rat isolated jejunum strips when administered at high concentrations (10 and 50 μM). Possibly, bergapten, similarly to other furanocoumarins, acts as myorelaxant by exhibiting calcium channel blocking activity (Neuhaus-Carlisle et al., Citation1997). It is well recognized that imperatorin, a structurally similar compound, induces vasodilatation by inhibiting voltage-dependent calcium channel, calcium-activated potassium channel, receptor-operated Ca2+ influx, and Ca2+ release (He et al., Citation2007). Verifying the effect of bergapten on extracellular and intracellular Ca2+ influx would permit the determination of its myorelaxant mechanism of action and its abolition (Neuhaus-Carlisle et al., Citation1997). Elimination of the myorelaxation effect would also make it possible to test the contractile effect of furanocoumarins at lower concentrations (below 10 μM), which might be impossible to observe as long as the relaxant component dominates. In contrast, since it is expected that furanocoumarins interfere with voltage-dependent calcium channels, the application of bergapten could be preceded by the administration of a calcium blocker, like nifedipine or verapamil (Karaki et al., Citation1997; Vuorela et al., Citation1997). In this case, the possible elimination of the contractile component would permit observation of the relaxant response of the intestine strips to bergapten.
It is noteworthy that irrespective of the applied concentration of bergapten, the evoked reaction was always reversible. Flushing the intestine strips with fresh M K-HS resulted on every occasion in a rapid (up to 3 min) return to the spontaneous motility registered prior to furanocoumarin application. In addition, the final application of acetylcholine in the optimal concentration at the end of every experiment resulted in a contraction, the force of which did not change in comparison with the first administration of this reference agent. This finding suggests clearly that bergapten caused no damage to the intestinal smooth muscle cells, and did not change their functionality in an irreversible manner.
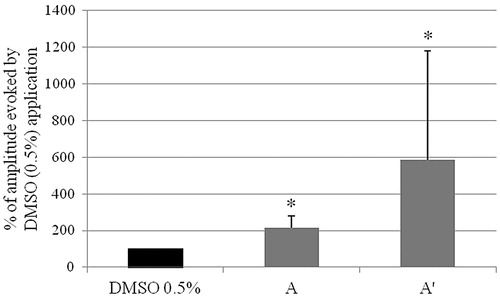
Furthermore, a significant increase in the amplitude of jejunum spontaneous motor activity was observed every time when bergapten was used at a concentration of 10 μM (). The amplitude of the spontaneous motility of strips that reacted with relaxation to bergapten (10 μM) administration increased to 215.13 ± 62.70% of the amplitude measured in the presence of the control agent (pure solvent, 0.5% DMSO). The increase was even stronger (ranging from 130.64 to 1742.76%, with an average of 586.77 ± 592.43% of the amplitude after exposure to DMSO, 0.5%) in the case of jejunum strips that contracted on the application of bergapten (10 μM) (′). These results indicate that bergapten also stimulates the force of the spontaneous contractions. The effect of bergapten on the amplitude of spontaneous motor activity is, however, not very surprising. A similar impact was observed in case of the extract of celandine, Iberis amara L. (Brassicaceae) (Heinle et al., Citation2006; Schemann et al., Citation2006). Increasing the force of the spontaneous contraction is a desirable effect for a phytoconstituent regarding the paralytic behavior of the intestine found in irritable bowel syndrome and for antral hypomotility in functional dyspepsia (Tack et al., Citation1998).
Figure 5. The effect of the application of bergapten (10 μM) dissolved in DMSO (0.5%) on the amplitude of the spontaneous motor activity of rat isolated jejunum strips. (A) The amplitude of the spontaneous motor activity of jejunum strips which reacted through relaxation to a bergapten (10 μM) application. (A′) The amplitude of the spontaneous motor activity of jejunum strips which reacted through contraction to a bergapten (10 μM) application. The results are expressed as % of the amplitude of the spontaneous motor activity of isolated jejunum strips registered after DMSO (0.5%) application. The amplitude registered after DMSO (0.5%) administration is expressed as 100%. The results are expressed as a mean from 7 to 8 independent experiments (±SD). *p ≤ 0.05 versus DMSO, 0.5%.
Conclusions
An HPCCC method was developed for the rapid, preparative isolation of bergapten (5-methoxypsoralen) of high purity (>99%) from the fruits of H. leskovii. Bergapten exhibited relatively strong antispasmodic activity on isolated rat gastrointestinal smooth muscle if used at low concentrations (0.0001–1 μM). However, the effects of bergapten when applied at higher doses (10 and 50 μM) were not clear, since the preparations responded either with a contraction or a relaxation effect. Due to the dual characters of the response of the isolated smooth muscle to high doses of bergapten, further investigation is necessary, including the study of the underlying mechanism of action. Nevertheless, the obtained data are sufficient to indicate that bergapten do not cause irreversible changes to jejunal smooth muscle tissue. These results demonstrated that Heracleum leskovii, known as a harmful and invasive plant, can be a potential source of the bioactive compound, bergapten.
Declaration of interest
The authors report that they have no conflicts of interest. This work was partially supported (isolation part) by Grant no. N N405 617538 from the National Science Centre in Krakow, Poland.
References
- Abbaskhan A, Choudhary MI, Ghayur MN, et al. (2012). Biological activities of Indian celery, Seseli diffusum (Roxb. ex Sm.) Sant. & Wagh. Phytother Res 26:783–6
- Al-Essa MK, Shafagoj YA, Mohammed FI, Afifi FU. (2010). Relaxant effect of ethanol extract of Carum carvi on dispersed intestinal smooth muscle cells of the guinea pig. Pharm Biol 48:76–80
- Allescher H. (2006). Functional dyspepsia – A multi-causal disease and its therapy. Phytomedicine 13:2–11
- Ammon HPT, Kelber O, Okpanyi SN. (2006). Spasmolytic and tonic effect of Iberogasts (STW 5) in intestinal smooth muscle. Phytomedicine 13:67–74
- Bensoussan A, Talley NJ, Hing M, et al. (1998). Treatment of irritable bowel syndrome with Chinese herbal medicine. J Am Med Assoc 280:1585–9
- Chiu WF, Huang YL, Chen CF, Chen CC. (2001). Vasorelaxing effect of coumarins from Cnidium monnieri on rabbit corpus cavernosum. Planta Med 67:282–4
- Chlopecka M, Dziekan N, Mendel M, et al. (2007). Evaluation of the time-stability of an alternative research model based on isolated rat gastrointestinal strips. J Physiol Pharm 58:73–86
- Clark SM, Wilkinson SM. (1998). Phototoxic contact dermatitis from 5-methoxypsoralen in aromatherapy oil. Contact Dermatitis 38:289–90
- Drossman DA, Li Z, Andruzzi E, et al. (1993). US householder survey of functional gastrointestinal disorders: Prevalence, sociodemography and health impact. Dig Dis Sci 38:1569–80
- Garrard IJ. (2005). Simple approach to the development of a CCC solvent selection protocol suitable for automation. J Liq Chromatogr Rel Technol 28:1923–35
- He JY, Zhang W, He LC, Cao YX. (2007). Imperatorin induces vasodilatation possibly via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Eur J Pharmacol 573:170–5
- Heaton KW, O’Donnell LJD, Braddon FEM, et al. (1992). Symptoms of irritable bowel syndrome in a British urban community: Consulters and nonconsulters. Gastroenterology 102:1962–7
- Heinle H, Hagelauer D, Pascht U, et al. (2006). Intestinal spasmolytic effect of STW 5 (Iberogast®) and its components. Phytomedicine 13:75–9
- Izzo AA, Capasso R, Senatore F, et al. (1996). Spasmolytic activity of medicinal plants used for the treatment of disorders involving smooth muscles. Phytother Res 10:S107–8
- Karaki H, Ozaki H, Hori M, et al. (1997). Calcium movements, distribution and functions in smooth muscle. Pharmacol Rev 49:157–230
- Leporatti ML, Ivancheva S. (2003). Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. J Ethnopharmacol 87:123–42
- Li HB, Chen F. (2004). Preparative isolation and purification of bergapten and imperatorin from the medicinal plant Cnidium monnieri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A 1061:51–4
- Li HB, Chen F. (2005). Simultaneous separation and purification of five bioactive coumarins from the Chinese medicinal plant Cnidium monnieri by high-speed countercurrent chromatography. J Sep Sci 28:268–72
- Liu R, Feng L, Sun A, Kong L. (2004). Preparative isolation and purification of coumarins from Cnidium monnieri (L.) Cusson by high-speed counter-current chromatography. J Chromatogr A 1055:71–6
- Müller L, Kasper P, Kersten B, Zhang J. (1998). Photochemical genotoxicity and photochemical carcinogenesis – Two sides of a coin? Toxicol Lett 102–3:383–7
- Neuhaus-Carlisle K, Vierling W, Wagner H. (1997). Screening of plant extracts and plant constituents for calcium-channel blocking activity. Phytomedicine 4:67–9
- Raquet N, Schrenk D. (2014). Application of the equivalency factor concept to the phototoxicity and genotoxicity of furocoumarin mixtures. Food Chem Toxicol 68:257–66
- Schemann M, Michel K, Zeller F, et al. (2006). Regio-specific effects of STW 5 (Iberogast®) and its components in gastric fundus, corpus and antrum. Phytomedicine 13:90–9
- Singh K, Lal B. (2008). Ethnomedicines used against four common ailments by the tribal communities of Lahaul-Spiti in western Himalaya. J Ethnopharmacol 115:147–59
- Skalicka-Woźniak K, Głowniak K. (2012). Pressurized liquid extraction of coumarins from fruits of Heracleum leskowii with application of solvents with different polarity under increasing temperature. Molecules 17:4133–41
- Tack J, Piessevaux H, Coulie B, et al. (1998). Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenerology 115:1346–52
- Talley N. (2003). Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol 98:750–8
- Thastrup O, Fjalland B, Lemmich J. (1983). Coronary vasodilatatory, spasmolytic and cAMP-phosphodiesterase inhibitory properties of dihydropyranocoumarins and dihydrofuranocoumarins. Acta Pharmacol Toxicol 52:246–53
- Van Wyk BE, Wink M. (2010). Medicinal Plants of the World. An Illustrated Scientific Guide to Important Medicinal Plants and Their Uses, 4th ed. London: Timber Press
- Vejdani R, Shalmani HRM, Mir-Fattahi M, et al. (2006). The efficiency of an herbal medicine, carmit, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: A pilot study. Dig Dis Sci 51:1501–7
- Vuorela H, Vuorela P, Törnquist K, Alaranta S. (1997). Calcium channel blocking activity: Screening methods for plant derived compounds. Phytomedicine 4:167–81
- Zhang Y, Wang QL, Zhan YZ, et al. (2010). Role of store-oerated calcium entry in imperatorin-induced vasodilatation of rat small mesenteric artery. Eur J Pharmacol 647:126–31