Abstract
Context: Albizia myriophylla Benth (Leguminosae) is a medicinal plant widely used in Thailand and other Asian countries as a folk medicine remedy for many ailments.
Objective: The objective of this study is to investigate the chemical compositions, antibacterial activity, and cytotoxicity of A. myriophylla wood.
Materials and methods: The structure identification of the isolated compounds was established using spectroscopic methods. In vitro antibacterial activity against Streptococcus mutans, Staphylococcus aureus, and Bacillus cereus and the cytotoxicity against KB cells of extracts and compounds from A. myriophylla were performed using broth microdilution and resazurin microplate assays, respectively. The lupinifolin content in A. myriophylla extracts was quantified by high-performance liquid chromatography.
Results: A rare flavan-3,4-diol (1) together with eight known compounds (2–9) were isolated from the wood of A. myriophylla. Compounds 4–9 exhibited anti-S. mutans activity, of which lupinifolin (5) was the most potent with an MIC value of 0.98 µg/mL, followed by its dihydroxy derivative 4 with an MIC value of 62.5 µg/mL. Compounds 4 and 5 also displayed marked antibacterial activity against B. cereus and S. aureus (MIC value 15.63–125 µg/mL) and showed strong cytotoxic activity against KB cells (IC50 value 4.95–12.55 µg/mL). The lupinifolin contents in ethanol extracts from two different collections of this plant originating from central and southern Thailand were 93.85 and 0.04 mg/g, respectively.
Conclusion: This is the first report of compounds 1–4 from A. myriophylla. Compounds 4 and 5 showed potent antibacterial and cytotoxic activities compared with other isolates. The anti-S. mutans activity of A. myriophylla extracts seems to be related to the lupinifolin content.
Introduction
Plant-derived natural products, particularly those used in folk medicine, are shown to be valuable therapeutic sources for many ailments (Newman, Citation2003). Bioactive secondary metabolites produced by plants have proven to possess a wide range of therapeutic effects for human diseases (Ferrazzano et al., Citation2011). Recently, scientific interest in these metabolites has increased with the search for new therapeutic agents from plant sources, due to the fact that currently used drugs are relatively toxic or, to a certain extent, ineffective due to the spreading of resistance (Joycharat et al., Citation2013; Khaomek et al., Citation2008). In Thailand, medicinal plants are increasingly used by traditional healers and herbalists for the treatment of various ailments (Joycharat et al., Citation2012; Neamsuvan et al., Citation2012). Plants can be rationally selected for phytochemical and biological studies based on their uses in traditional medicine (Ekuadzi et al., Citation2014). Some products from herbal plants have been used successfully as medicinal agents against a variety of disorders and had advantages over synthetic drugs due to their minimal side effects (Venkatesh et al., Citation2010).
The genus Albizia of the Leguminosae family contains greatly valued multipurpose plants (Kokwaro, Citation1976). Many members of this genus are important in traditional medicines in some Asian and African countries (Kokwaro, Citation1976). Albizia myriophylla Benth (Thai local name “Cha Em Thai”) is widely distributed in southeastern Asian countries (Yoshikawa et al., Citation2002). It has been reported in the Thai and the Vietnamese systems of traditional medicines that the stems of this plant have been used to substitute for licorice due to their sweetness (Yoshikawa et al., Citation2002). Albizia myriophylla is among the most important medicinal plant and is used alone or in combination with other medicinal plants in various Thai herbal formulas, especially those for remedy of oral diseases including dental caries and aphthous ulcer (Neamsuvan et al., Citation2012). A broad range of pharmacological effects of this plant including antioxidant, anticandidal, and antibacterial activities has been previously established (Joycharat et al., Citation2013; Steinrut et al., Citation2011). Regarding the biological activity of the isolated secondary metabolites from A. myriophylla, only one report is currently available (Joycharat et al., Citation2013). In our previous work, the flavanone lupinifolin isolated from the wood extract of that originating from southern Thailand exhibited very strong antibacterial activity against Streptococcus mutans ATCC 25175 and its clinical isolates, with MIC values ranging from 0.5 to 4 µg/mL (Joycharat et al., Citation2013). To the best of our knowledge, there is no report on the antibacterial activity of this plant species against other bacterial strains such as Staphylococcus aureus ATCC 29213 and Bacillus cereus ATCC 11778. It has been reported that several plants of the genus Albizia possessed high cytotoxicity against many different cancer cell lines (Liu et al., Citation2010; Melek et al., Citation2007; Zou et al., Citation2005). Despite the notable cytotoxic potential of the plants belonging to the genus Albizia, knowledge in cytotoxicity of A. myriophylla is scarce. Preliminary antibacterial activity test of the ethanol extracts of A. myriophylla from two different areas in Thailand showed that the respective MIC values of those from central and southern parts of Thailand against S. mutans ATCC 25175 were 4 and 500 µg/mL (unpublished data from our laboratory). Due to our continued interest in bioactive substances from A. myriophylla wood, the aim of this study was to isolate and characterize the bioactive ingredients from the wood of two different collections of A. myriophylla originating from central and southern provinces of Thailand. Selected pharmacological assays of the isolated compounds including in vitro antibacterial activity against three pathogenic bacterial strains, S. mutans ATCC 25175, S. aureus ATCC 29213, and B. cereus ATCC 11778, as well as cytotoxicity against human oral epidermoid carcinoma (KB) cells were performed. The content of lupinifolin in ethanol extracts from two different collections of A. myriophylla originating from central and southern Thailand was quantified using high-performance liquid chromatography (HPLC) method and the anticariogenic activity against S. mutans of extracts from both collections of this plant was evaluated using broth microdilution assay.
Materials and methods
General
The following instruments were used: UV, Genesys 10 Series (Madison, WI); IR, EQUINOX 55, Bruker FTIR (Bruker Corporation, New Orleans, LA); 1D- and 2D-NMR, FTNMR Bruker Advance 400 MHz (Bruker Corporation, New Orleans, LA); ESIMS, MAT 95 XL mass spectrometer (Thermofinnigan, Waltham, MA), and HRESIMS, Bruker Daltonics micrOTOF (Bruker Corporation, New Orleans, LA); HPLC, Agilent Technologies (Santa Clara, CA). Column chromatography (CC) was performed using silica gel (70–230 mesh, Merck, New York, NY), and Sephadex LH-20 (20–100 µm, Sigma, St. Louis, MO). Thin-layer chromatography (TLC) was performed on precoated sheets of silica gel 60 F254 (20 × 20 cm2, 0.25 mm, Merck, New York, NY). Fractions and compounds were monitored by TLC sprayed with an anisaldehyde-sulfuric acid solution. Bacterial culture media, brain heart infusion (BHI) agar and tryptic soy broth (TSB) were purchased from Difco (Detroit, MI). Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany). The microtiter plate was purchased from Corning Life Sciences (Union City, CA). All solvents for CC were of laboratory reagent grade and were purchased from commercial sources.
Plant materials
The wood of two different collections of Albizia myriophylla originating from southern and central regions of Thailand was collected during June 2011–October 2012. Botanical identification was performed by Asst. Prof. Dr. Oratai Neamsuvan of the Faculty of Traditional Thai Medicine, Prince of Songkla University where the voucher specimens (NJ0611 and NJ1012) were deposited.
Extraction and isolation
The details relating to the extraction method of A. myriophylla wood collected from southern region of Thailand were previously described (Joycharat et al., Citation2013). The wood of A. myriophylla originating from central region of Thailand (2.9 kg) was dried, ground, and exhaustively macerated with ethanol (EtOH) at room temperature three times, filtered, and concentrated to give a residue of EtOH extract (213 g, 7.34%). The EtOH extract was resuspended in a mixture of methanol (MeOH) and water and then partitioned with hexane, dichloromethane (CH2Cl2), and n-butanol (n-BuOH), successively. The yield of hexane extract (41.12 g, 1.42%), CH2Cl2 extract (32.17 g, 1.11%), and n-BuOH extract (34.53 g, 1.19%) was obtained.
For the isolation of compounds 1 and 3, the CH2Cl2 extract (12.36 g) of A. myriophylla from southern Thailand was applied to silica gel CC using gradient elution with acetone–CH2Cl2, and finally washed with MeOH. The fractions were then combined and numbered (ID-VID) according to their TLC patterns. Fraction IID (1.84 g) was further subjected to silica gel CC using gradient elution with acetone–hexane, and finally washed with MeOH. The fractions were then combined according to their TLC patterns to give five subfractions I–V. Subfraction III (750 mg) was further purified on silica gel CC using MeOH–CHCl3 (10:90) as the eluent, followed by three successive Sephadex LH-20 columns eluted with MeOH to yield 3,4,7,3′-tetrahydroxyflavan (1, 5.7 mg). Fraction IIID (1.46 g) was further applied to silica gel CC using gradient elution with acetone–CH2Cl2, and finally washed with MeOH. The fractions were then combined according to their TLC chromatograms to yield six subfractions I–VI. Subfraction IV (127 mg) was purified on silica gel CC eluted with acetone–CH2Cl2 (40:60), followed by repeated gel filtration chromatography, using three successive Sephadex LH-20 columns eluted with MeOH to yield butin (3, 11.7 mg).
For the isolation of compounds 2 and 4, the CH2Cl2 extract (20 g) of A. myriophylla from central Thailand was applied to silica gel CC using gradient elution with acetone–CH2Cl2, and finally washed with MeOH. The fractions were then combined and numbered (ID-XID) according to their TLC profiles. Fraction VID (0.35 g) was further separated on repeated silica gel CC ((i) CC acetone–CH2Cl2 gradient and (ii) CC acetone–CHCl3 gradient), followed by Sephadex LH-20 column eluted with MeOH to yield 2′″,3′″-dihydroxylupinifolin (4, 21.3 mg). Fraction VIID (0.29 g) was further purified over repeated silica gel CC ((i) CC acetone–CH2Cl2 gradient and (ii) CC CHCl3–MeOH gradient) to afford 1,2,3-benzenetriol (2, 5.5 mg).
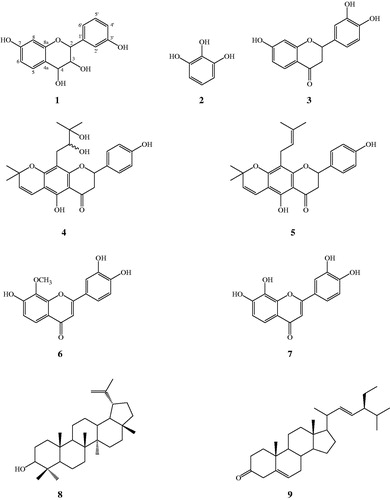
Additional quantities of lupinifolin (5, 98.6 mg), 8-methoxy-7,3′,4′-trihydroxyflavone (6, 4.3 mg), 7,8,3′,4′-tetrahydroxyflavone (7, 3.7 mg), lupeol (8, 14.8 mg), and stigmasta-5,22-dien-3-one (9, 13.4 mg) as well as a mixture of β-sitosterol and stigmasterol were obtained from the hexane or CH2Cl2 extracts of A. myriophylla from central Thailand according to the isolation method mentioned previously (Joycharat et al., Citation2013). The TLC Rf values of compounds 5–9 are 0.5 (20% acetone in hexane), 0.43 (40% EtOAc in CH2Cl2), 0.33 (40% EtOAc in CH2Cl2), 0.53 (20% acetone in hexane), and 0.75 (20% acetone in hexane), respectively. The structures of all the isolated compounds are shown in .
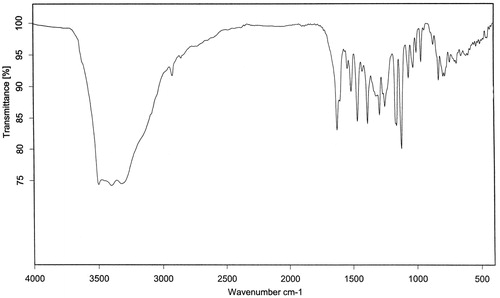
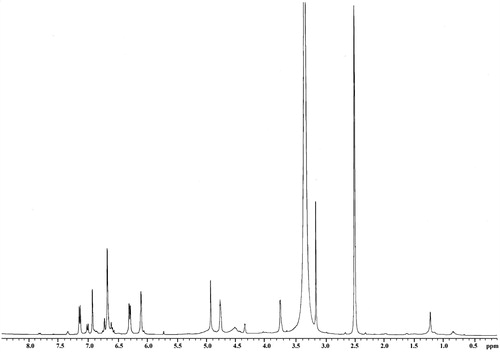
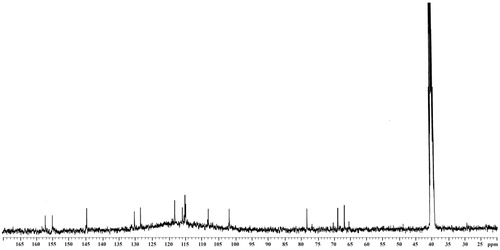
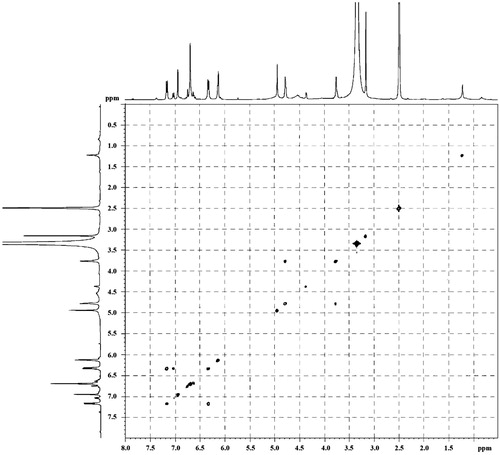
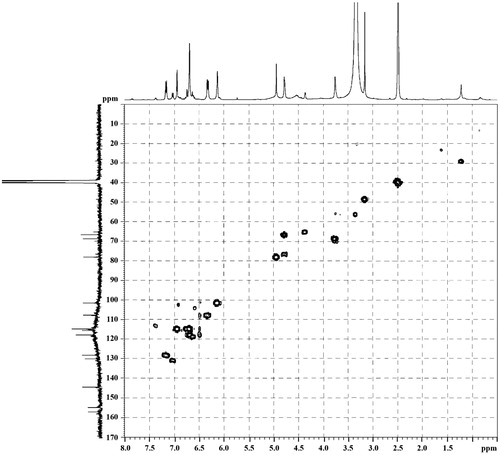
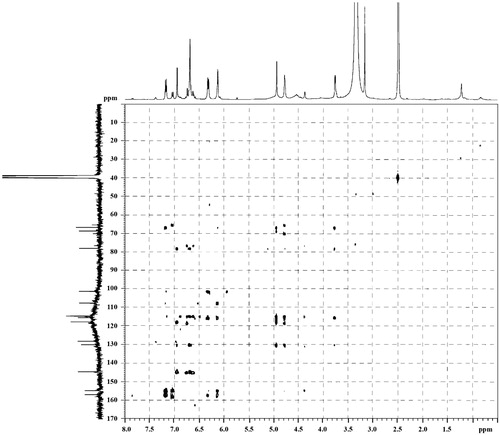
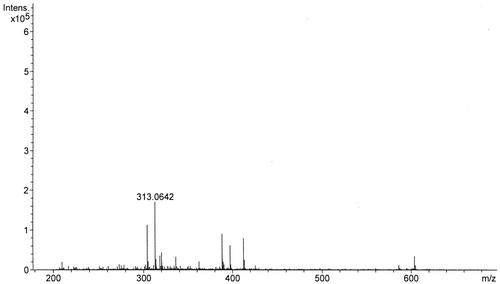
3,4,7,3′-Tetrahydroxyflavan (1): brownish amorphous solid; UV λmax (MeOH) nm (log ɛ): 313, 208. IR νmax (KBr) cm−1: 3497, 3398, 3319, 1621, 1461, 1382, 1155, 1119, 829, 781 (). 1H NMR, 13C NMR, and HMBC data (DMSO-d6): see and . HRESIMS: [M+K]+m/z 313.0642 ().
Table 1. The NMR spectral data of 3,4,7,3′-tetrahydroxyflavan (1) (in DMSO-d6, 400 MHz).
Antibacterial assay
Three bacterial strains including S. aureus ATCC 29213, B. cereus ATCC 11778, and S. mutans ATCC 25175 were obtained from the Department of Microbiology, Faculty of Science, Prince of Songkla University, Thailand. The bacterial cultures were stored in brain heart infusion (BHI) broth (Difco, Bordeaux, France) with 20% glycerol at −80 °C until use. All bacterial strains were cultured on BHI agar and incubated separately with 5% CO2 at 37 °C for 24 h for S. mutans ATCC 25175 or at 37 °C for 24 h for S. aureus ATCC 29213 and B. cereus ATCC 11778.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of extracts and compounds isolated from A. myriophylla were determined using a modified broth microdilution method (CLSI, Citation2009). Briefly, the extracts and compounds were dissolved in 10% DMSO (Merck, Darmstadt, Germany) and two-fold dilutions were prepared. Suspension of bacterial cultures in BHI broth was made from the overnight broth culture. The bacterial suspension (180 µL) was mixed with the diluted test agents (20 µL) in 96-well microtiter plate (Corning Life Sciences, Union City, CA). The final bacterial concentration was approximately 5 × 105 CFU/mL. The final concentration of the tested extracts ranged from 0.49 to 1000 µg/mL and that for the tested compounds was from 0.49 to 250 µg/mL. Chlorhexidine and penicillin G were included as the positive controls. DMSO was used as a negative control. The lowest concentration of the tested agents required to completely inhibit bacterial cell growth after incubation at 37 °C for 24 h was recorded as the MIC value. After MICs were recorded, an aliquot (100 µL) from the broth with no growth was pipetted and dropped onto agar plate and incubated at 37 °C for 48 h. The lowest concentration of the tested agents required to completely prevent the bacterial growth was reported as the MBC value. Each assay was performed in triplicate.
Cytotoxicity assay
Cytotoxic activity of the isolated compounds against KB cells was determined using the method described by Brien et al. (Citation2000). In short, cells at a logarithmic growth phase were harvested and diluted to 2.2 × 104 cells/mL in the growth medium. Successively, 5 µL of the tested compounds diluted in 5% DMSO and 45 µL of cell suspension were added into each well of 384-well plates. Plates were incubated at 37 °C in 5% CO2 incubator for 3 d. After incubation period, 12.5 µL of 62.5 µg/mL resazurin solution was added into each well and the plates were incubated for a further 4 h. The absorbance was measured on a SpectraMax M5 multi-detection microplate reader (Molecular Devices, Silicon Valley, CA) at the excitation and emission wavelengths of 530 nm and 590 nm. The reference compounds were ellipticine and doxorubicin. Water and 0.5% DMSO were used as negative controls. The activity is expressed as 50% inhibitory concentration (IC50). If % cell viability was < 50%, IC50 value is reported from a two-fold serial dilution. Compounds with an IC50 value more than 20 µg/mL are considered inactive.
Quantification of lupinifolin content by HPLC
Standard stock solution of lupinifolin (100 mg/L) was prepared in methanol (HPLC grade, Merck, Darmstadt, Germany). The extract solutions were prepared separately by dissolving 300 mg of individual extracts in 1 mL of methanol (HPLC grade, Merck, Darmstadt, Germany) and vigorously shaking. Lupinifolin content in the ethanol extracts of A. myriophylla was analyzed by HPLC-UV technique. The HPLC system (Agilent Technologies, Karlsruhe, Germany) consists of binary pump, auto injector, column thermostat, and variable wavelength detector. The stationary phase was a Zorbax Eclipse XDB-C8 column (4.6 × 250 mm2, 5 µm) connected to a cartridge guard column (Agilent Technologies, Karlsruhe, Germany). The mobile phase was an isocratic elution system consisting of methanol (HPLC grade, Merck, Darmstadt, Germany) and 15% glacial acetic acid (AR grade, Lab scan) in DI water (85:15, v/v%). The mobile phase was filtered through a 0.45 μm nylon membrane (Whatman, Sigma, St. Louis, MO) and degassed by sonicating prior to use. The flow rate of mobile phase was kept at 1.2 mL/min throughout the analysis and the detector wavelength was set at 254 nm. The injection volume of each sample was 5 μL. The column temperature was maintained at 25 °C. The chromatograms were processed using Chemstation software (Agilent Technologies, Karlsruhe, Germany). The peak of lupinifolin in the samples was characterized by direct comparison of its retention time with that of standard solution. The linearity of the analysis method was evaluated by regression analysis. Standard stock solution of lupinifolin was diluted with methanol (HPLC grade, Merck, Darmstadt, Germany) and six concentrations ranging from 1 to 50 mg/L were prepared. The calibration curve was obtained by plotting the value of peak area against each concentration of standard solution. The regression equation was given as y = ax + b, where y and x correspond to the peak area and concentration of standard, respectively.
Results
A rare flavan-3,4-diol, 3,4,7,3′-tetrahydroxyflavan (1), and a flavanone butin (3) were isolated from the CH2Cl2 extract of Albizia myriophylla collected from southern Thailand. A phenolic compound, 1,2,3-benzenetriol (2), and a prenylated flavonoid, 2′″,3′″-dihydroxylupinifolin (4), were isolated from this plant species originating from central Thailand. Furthermore, five other known compounds including lupinifolin (5), 8-methoxy-7,3′,4′-trihydroxyflavone (6), 7,8,3′,4′-tetrahydroxyflavone (7), lupeol (8), and stigmasta-5,22-dien-3-one (9) as well as a mixture of β-sitosterol and stigmasterol were obtained from both collections of A. myriophylla.
3,4,7,3′-Tetrahydroxyflavan (1) was obtained as a brownish amorphous solid. The HRESIMS spectrum of 1 showed [M + K]+ peak at m/z = 313.0642 matching a molecular formula of C15H14O5. Its IR spectrum showed absorption bands at 3423, 3398, and 3319 cm−1, suggesting the presence of hydroxy function. The 1H NMR spectrum of 1 showed one typical trisubstituted benzene system at δH 7.18 (1H, d, J = 8.2 Hz, H-5), 6.33 (1H, dd, J = 8.2, 1.7 Hz, H-6), and 6.14 (1H, br s, H-8), and one meta-disubstituted benzene system at δH 6.94 (1H, br s, H-2′), 6.76 (1H, m, H-4′), 6.60 (1H, m, H-5′), and 6.70 (1H, m, H-6′). The 13C NMR spectrum of 1 exhibited 12 aromatic carbons of the two aromatic rings and three oxymethine carbon signals at δC 78.11 (d, C-2), 66.69 (d, C-3), and 68.66 (d, C-4). The assignment of the four hydroxy groups to the flavan nucleus of 1 was accomplished by a detailed analysis of HMBC spectroscopic data (). These findings revealed that compound 1 was a flavan-3,4-diol derivative with the OH-substitution on positions C-7 in ring A and C-3′ in ring B of the flavonoid. The 2,3-cis configuration of 1 was determined according to a broad singlet at δH 4.94 for H-2 and its corresponding oxymethine carbon signal at δC 78.11 of C-2 (Wu et al., Citation2012). The low value of J3,4 4.0 Hz indicated the 3,4-cis configuration of 1 (Porter & Foo, Citation1982; Saayman & Roux, Citation1965). Thus, the structure of compound 1 was proposed to be 3,4,7,3′-tetrahydroxyflavan (1).
Antibacterial and anticancer activities of various extracts including those of EtOH, hexane, CH2Cl2, and n-BuOH as well as the isolated compounds 1–9 from A. myriophylla are presented in and . In our continuing study on the anti-Streptococcus mutans activity of A. myriophylla, the MIC values of all tested extracts from both collections of A. myriophylla against S. mutans ATCC 25175 ranged from 2 to 1000 µg/mL, of which the CH2Cl2 extract from central collection of this plant was found to be the most active with the same MIC and MBC of 2 µg/mL. Considering some phenolic compounds, those belonging to flavonoids have been described to have moderate or strong antibacterial activity (Cushnie et al., Citation2005). Thus, antibacterial activities against three pathogens including B. cereus ATCC 11778, S. aureus ATCC 29213, and S. mutans ATCC 25175 were determined with compounds 1–7 using the broth microdilution method. The results showed that flavanone 5 exhibited the best anti-S. mutans activity with an MIC value of 0.98 µg/mL, followed by its dihydroxy derivative 4 with an MIC value of 62.5 µg/mL. Both 4 and 5 also displayed pronounced antibacterial activity against B. cereus ATCC 11778 and S. aureus ATCC 29213 with MIC and MBC values ranging from 15.63 to 125 and 31.25 to 250 µg/mL, respectively. Compounds 6 and 7 showed moderate activity against the three tested bacterial strains with MIC values ranging from 62.5 to 250 µg/mL, whereas compounds 1 to 3 exhibited no antibacterial activity against the tested pathogens at the highest concentration tested of 250 µg/mL (). Besides antibacterial activity, the isolated compounds 1–9 were evaluated for their cytotoxicity against KB cells using resazurin microplate assay. Both prenylated flavonoids 4 and 5 demonstrated significant cytotoxic activity against KB cells, of which 5 was found to be more active with an IC50 value of 4.95 µg/mL. The other tested compounds were inactive against KB cells at the highest concentrations tested ().
Table 2. Antibacterial activity of extracts and the isolated compounds from two different collections of A. myriophylla.
Table 3. Anticancer activity of the isolated compounds from A. myriophylla.
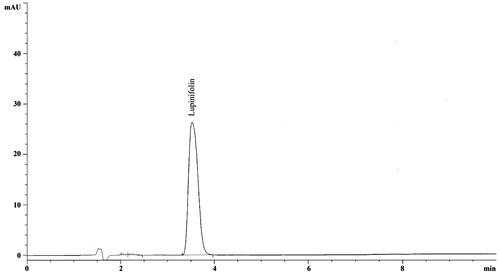
The quantity of lupinifolin (5) present in the ethanol extracts of two different collections of A. myriophylla was determined using the HPLC method. Both samples were assessed in triplicate to guarantee the reproducibility of the quantitative result. As can be seen from , the lupinifolin contents in ethanol extracts of A. myriophylla originating from central and southern Thailand were 93.85 and 0.04 mg/g (crude EtOH extract), respectively. The HPLC chromatogram of lupinifolin is shown in . It was interesting that the ethanolic wood extract of A. myriophylla from the central collection, that contained a high amount of lupinifolin, showed anti-cariogenic activity with the MIC value against S. mutans much better than that from the southern collection.
Table 4. Lupinifolin content and anti-cariogenic activity against S. mutans ATCC 25175 of two different collections of A. myriophylla.
Discussion
The isolation and characterization of a rare flavan-3,4-diol 1 from the wood of Albizia myriophylla in this study supported the previous literature of the general observation of the members of flavan-3,4-diols to be the wood components of plants (Stafford & Lester, Citation1985). It is reported that flavan-3,4-diols were involved as intermediate substances in flavan-3-ol biosynthesis catalyzed by reductase reaction (Drewes & Roux, Citation1965). The other isolated compounds were successively identified as 1,2,3-benzenetriol (2), butin (3), 2′″,3′″-dihydroxylupinifolin (4), lupinifolin (5), 8-methoxy-7,3′,4′-trihydroxyflavone (6), 7,8,3′,4′-tetrahydroxyflavone (7), lupeol (8), stigmasta-5,22-dien-3-one (9), and a mixture of β-sitosterol and stigmasterol by the analysis of their NMR spectra and by comparing with the data reported in the literature (Herath et al., Citation2009; Mahidol et al., Citation1997, Citation2002; Porter & Foo, Citation1982; Reynolds et al., Citation1986). Compounds 1–4 were first isolated herein from the genus Albizia.
The differences in the phytochemical composition of A. myriophylla collected from sampling sites differing in environmental conditions resulted in variation in the efficacy of its folk remedies for oral diseases. It is interesting to note that all the extracts of A. myriophylla from central Thailand demonstrated stronger activity against Streptococcus mutans ATCC 25175 compared with those from southern Thailand. In a previous study, the EtOH extract of this plant species purchased from a herb shop in southern province of Thailand exhibited pronounced anti-S. mutans activity with an MIC value of 3.9 µg/mL (Joycharat et al., Citation2012). Similarly, the EtOH extract of A. myriophylla from central Thailand, in this study, showed very good activity against S. mutans ATCC 25175 with an MIC value of 4 µg/mL. The significant differences in the antibacterial results of the extracts of this plant species collected from two geographically distinct regions could be explained by many factors which may affect the variation of their bioactive components including genetic and environment (Wahyuni et al., Citation2013). The variation of plant secondary metabolites under differing geographical conditions has been described previously (Zhao et al., Citation2012). Anti-S. mutans activity of several compounds isolated in this work, especially lupinifolin (5), has been reported previously (Joycharat et al., Citation2013). However, antibacterial activity of 5 against some other bacterial strains of medical importance including B. cereus ATCC 11778 and S. aureus ATCC 29213 has not been reported so far. A diverse range of antibacterial mechanisms of action of various flavonoids including inhibition of nucleic acid synthesis, inhibition of cytoplasmic membrane function, and inhibition of energy metabolism has been well documented (Cushnie & Lamb, Citation2005). Antibacterial activity against various kinds of pathogenic bacteria has been widely described for the members of prenylated flavonoids (Khaomek et al., Citation2008; Sutthivaiyakit et al., Citation2009). The presence of the 2,2-dimethyl-2H-pyran ring in compounds 4 and 5 may play an important role in their antibacterial activity (Sasaki et al., Citation2012). Previous research has revealed that combining the flavonoid nucleus with the lipophilicity of the prenyl group may result in a great potential for biological activity (Smejkal, Citation2014). In this study, the higher antibacterial activity of lupinifolin (5) compared with its closely related dihydroxy derivative 4 or other weak and inactive compounds tested could be partly associated with the more lipophilic property of its structure. The strongly cytotoxic activity of the members of flavonoids possesing an A-ring prenyl substitution against several cancer cell lines has been reported previously (Groweiss et al., Citation2000). Moreover, a decrease in the cytotoxic activity of prenylated flavonoids that have an oxidative change in the linear prenyl group has been observed and reported (Groweiss et al., Citation2000). The presence of an A-ring prenyl side chain at C-8 and the oxidative modification of the prenyl residue in compound 5 may play an important role in its cytotoxicity. Both 4 and 5 were previously reported to be active against P-388 leukemia cell (Mahidol et al., Citation1997, Citation2002). Compound 5 also possesses strong to moderate activity against KB, BC, and NCI-H187 cancer cell lines (Innok et al., Citation2009). The cytotoxic activity of 5 against KB cells in this study was in accordance with that previously described (Sutthivaiyakit et al., Citation2009). We reported, for the first time, the cytotoxicity of compound 4 against KB cells, with an IC50 value of 12.55 µg/mL.
Recently, researchers have recognized that there are many phytochemicals affecting the healing properties of herbal plants. The inconsistency of the active constituents affects the variation in the chemical quality of raw plant materials and may result in the reproducible therapeutic effect of their herbal products. The variation of bioactive phytochemicals in plants is common and often associated with geographical parameters. In this work, the variation of anti-S. mutans property of A. myriophylla extracts seems to be affected by sampling sites differing in geographical origin. Chemically both collections of A. myriophylla originating from central and southern parts of Thailand showed uniformity in the presence of compounds 5–9 in the wood. However, among the compounds studied, lupinifolin (5) which has the highest antibacterial and anticancer activities is most relevant to the bioactivities of this plant. As the batch-to-batch consistency of herbal products could be affected by many factors, for instance genetic and ecological factors, quality control has become an important requirement to support the clinical applications (Shi et al., Citation2014). Herbal products contain normally a complex mixture of phytochemicals, therefore selection of appropriate marker compounds that are relevant to the biological activities is currently an important approach to their quality control (Shi et al., Citation2014). Similarly, in order to facilitate an efficient standardization procedure for the future development of A. myriophylla extract as an herbal product, it is essential to define chemical markers responsible for its therapeutic activity. Our finding revealed that the quantity of lupinifolin in ethanolic wood extracts of A. myriophylla from two different geographical origins had a relationship with the anti-S.mutans activity in a dose-dependent manner. Thus, lupinifolin may be an effective marker compound representing the bioactivities of A. myriophylla.
Conclusion
The chemical investigation of the wood of Albizia myriophylla resulted in the isolation of a rare flavan-3,4-diol 1 together with eight known compounds (2–9). Compounds 1–4 were isolated herein from the genus Albizia for the first time. All the isolated compounds were evaluated for their anticancer activity against KB cells, whereas those belonging to the phenolic group were also tested for their antibacterial activity against some pathogens of medical importance. Compounds 4 and 5 showed significantly anticancer and antibacterial activities. The variation of anticariogenic activity against S. mutans of A. myriophylla extracts seems to be result from the sampling locations having different geographical origins. The HPLC analysis of lupinifolin (5) content in the ethanol extracts of two different collections of A. myriophylla revealed the relationship between the amount of 5 and the anti-S. mutans activity in a dose-dependent manner. Lupinifolin may be a marker compound responsible for the bioactivities of A. myriophylla. Further research on correlations between lupinifolin content and biological activities among geographically distinct plant populations would allow to be obtained information related to the quality control of A. myriophylla wood.
Acknowledgements
The authors would like to thank Dr. Alan Frederic-Geater from the Faculty of Medicine, Prince of Songkla University, for assistance with the English.
Declaration of interest
This work was supported by a research grant for General Researcher, The Annual Income Budget of Prince of Songkla University (TTM570374S), and by TRF-CHE Research Grant for New Scholar (MRG5580107) funded by the Thailand Research fund (TRF) and the Commission on Higher Education (CHE).
References
- Brien JO, Wilson I, Orton T, Pognan F. (2000). Investigation of the alamarblue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–6
- Clinical and Laboratory Standards Institute. (2009). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard 8th Edition. Clinical and Laboratory Standards Institute document M07-A8. Wayne (PA): Clinical and Laboratory Standards Institute
- Cushnie TPT, Lamb AJ. (2005). Antimicrobial activity of flavonoids. Inter J Antimicrob Agents 26:343–56
- Drewes SE, Roux DG. (1965). Natural and synthetic diastereoisomeric (−)-3′,4′,7-trihydroxyflavan-3,4-diols. Biochem J 98:681–7
- Ekuadzi E, Dickson R, Fleischer T, et al. (2014). Flavonoid glycosides from the stem bark of Margaritaria discoidea demonstrate antibacterial and free radical scavenging activities. Phytother Res 28:774–80
- Ferrazzano GF, Amato I, Ingenito A, et al. (2011). Plant polyphenols and their anti-cariogenic properties: A review. Molecules 16:1486–507
- Groweiss A, Cardellina JH, Boyd MR. (2000). HIV-inhibitory prenylated xanthones and flavones from Maclura tinctoria. J Nat Prod 63:1537–9
- Herath W, Mikell JR, Khan IA. (2009). Microbial metabolism. Part 10: Metabolites of 7,8-dimethoxyflavone and 5-methoxyflavone. Nat Prod Res 23:1231–9
- Innok P, Rukachaisirikul T, Suksamrarn A. (2009). Flavanoids and pterocarpans from the bark of Erythrina fusca. Chem Pharm Bull (Tokyo) 57:993–6
- Joycharat N, Limsuwan S, Subhadhirasakul S, et al. (2012). Anti-Streptococcus mutans efficacy of Thai herbal formula used as a remedy for dental caries. Pharm Biol 50:941–7
- Joycharat N, Thammavong S, Limsuwan S, et al. (2013). Antibacterial substances from Albizia myriophylla wood against cariogenic Streptococcus mutans. Arch Pharm Res 36:723–30
- Khaomek P, Ichino C, Ishiyama A, et al. (2008). In vitro antimalarial activity of prenylated flavonoids from Erythrina fusca. J Nat Med 62:217–20
- Kokwaro JO. (1976). Medicinal plants of East Africa, East African. Nairobi: Literature Bureau
- Liu R, Ma SG, Liu Y, et al. (2010). Albizosides D and E, two new cytotoxic triterpene saponins from Albizia chinensis. Carbo Res 345:1877–81
- Mahidol C, Prawat H, Kaweetripob W, Ruchirawat S. (2002). Two new pyranoflavanones from the stems of Derris reticulata. Heterocycles 57:1287–92
- Mahidol C, Prawat H, Ruchirawat S, et al. (1997). Prenylated flavanones from Derris reticulate. Phytochemistry 45:825–9
- Melek FR, Miyase T, Ghaly NS, Nabil M. (2007). Triterpenoid saponins with N-acetyl sugar from the bark of Albizia procera. Phytochemistry 68:1261–6
- Neamsuvan O, Tuwaemaengae T, Bensulong F, et al. (2012). A survey of folk remedies for gastrointestinal tract diseases from Thailand’s three southern border provinces. J Ethnopharmacol 144:11–21
- Newman DJ, Cragg GM, Snader KM. (2003). Natural products as sources of new drugs over the period. J Nat Prod 66:1022–37
- Porter LJ, Foo YP. (1982). Leucocyanidin: Synthesis and properties of (2R,3S,4R)-(+)-3,4,5,7,3′,4′-hexahydroxyflavan. Phytochemistry 21:2947–52
- Reynolds WF, McLene S, Poplawski J, et al. (1986). Total assignment of 13C and 1H spectra of three isomeric triterpenol derivatives by 2D NMR: An investigation of the potential utility of 1H chemical shifts in structural assignments of complex natural products. Tetrahedron 42:3419–28
- Saayman HM, Roux DG. (1965). Configuration of guibourtacacidin, and synthesis of isomeric racemates. Biochem J 96:36–42
- Sasaki H, Kashiwada Y, Shibata H, Takaishi Y. (2012). Prenylated flavonoids from Desmodium caudatum and evaluation of their anti-MRSA activity. Phytochemistry 82:136–42
- Shi Z, Song D, Li R, et al. (2014). Identification of effective combinatorial markers for quality standardization of herbal medicines. J Chromatogr A 1345:78–85
- Smejkal K. (2014). Cytotoxic potential of C-prenylated flavonoids. Phytochem Rev 13:245–75
- Stafford HA, Lester HH. (1985). The conversion of (+)-dihydromyricetin to its flavan-3,4-diol (leucodelphinidin) and to (+)-gallocatechin by reductases extracted from tissue cultures of Ginkgo biloba and Pseudotsuga menziesii. Plant Physiol 78:791–4
- Steinrut L, Itharat A, Ruangnoo S. (2011). Free radical scavenging and lipid peroxidation of Thai medicinal plants used for diabetic treatment. J Med Assoc Thai 94:178–82
- Sutthivaiyakit S, Thongnak O, Lhinhatrakool T, et al. (2009). Cytotoxic and antimycobacterial prenylated flavonoids from the roots of Friosema chinense. J Nat Prod 72:1092–6
- Venkatesh P, Mukherjee PK, Kumar NS, et al. (2010). Anti-allergic activity of standardized extract of Albizia lebbeck with reference to catechin as a phytomarker. Immunopharmacol Immunotoxicol 32:272–6
- Wahyuni Y, Ballester AR, Tikunov Y, et al. (2013). Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics 9:130–44
- Wu XD, Cheng JT, He J, et al. (2012). Benzophenone glycosides and epicatechin derivatives from Malania olefera. Fitoterapia 83:1068–71
- Yoshikawa M, Morikawa T, Nakano K, et al. (2002). Characterization of new sweet triterpene saponins from Albizia myriophylla. J Nat Prod 65:1638–42
- Zhao BT, Jeong SY, Moon DC, et al. (2012). High performance liquid chromatography used for quality control of Achyranthis Radix. Arch Pharm Res 35:1449–55
- Zou K, Thong WY, Liang H, et al. (2005). Diastereoisomeric saponins from Albizia julibrissin. Carbohydr Res 340:1329–34