Abstract
Context: Genistein inhibits the proliferation and induces apoptosis of colorectal cancer cells; however, the underling molecular mechanisms remain to be determined.
Aim: The aim of this study was to investigate whether genistein reduces cell viability by suppressing the phosphorylation of AKT and activating the mitochondrial apoptosis pathway in colorectal cancer cells.
Materials and methods: The anti-proliferative effects of genistein (0, 25, 50, and 100 μM) on HCT-116 and LoVo cells were assessed using MTT assay. Genistein-induced apoptosis was measured by Hoechst 33258 staining and flow cytometry. The mRNA level of Bax was detected by real-time PCR. The protein levels of Bax, total Akt, and phosphorylated Akt were assessed by western blot.
Results: The IC50 values of genistein were 690, 135, and 61 μM in HCT-116 cells and 204, 135, and 93 μM in LoVo cells after treatment for 24, 48, and 72 h, respectively. After treatment with different concentrations of genistein (0, 25, 50, and 100 μM) for 48 h, the early apoptotic cells in HCT-116 increased from 1.99% ± 0.55% to 6.78% ± 2.12%, 23.16% ± 3.87%, and 36.99% ± 3.76%, respectively. The same concentrations of genistein increased the early apoptotic cells in LoVo from 2.56% ± 1.42% to 3.21% ± 1.52%, 18.22% ± 3.56%, and 23.56% ± 3.02%, respectively. Moreover, genistein increased the mRNA and protein levels of Bax, while it inhibited the phosphorylation of Akt in HCT-116 cells.
Conclusion: Genistein inhibited cell proliferation and induced apoptosis of colorectal cancer cells. Genistein induced the mitochondrial pathway of apoptosis in HCT-116 cells by inhibiting phosphorylation of Akt.
Introduction
Colorectal cancer is the second most frequently diagnosed cancer and the third in males globally. It is also the third leading cause of cancer death in females and the fourth in males worldwide. Intriguingly, the incidence and the mortality are high in developed countries, such as North America and Europe, but low in developing countries, including Eastern Asia and Africa (Jemal et al., Citation2011). The mechanism of the apparent different susceptibility to colorectal cancer of distinct populations is largely unknown.
Epidemiological studies showed that a high intake of soy foods is associated with a reduced risk for colorectal cancer in Asian countries (Ramadas & Kandiah, Citation2009; Yang et al., Citation2009). Isoflavones are naturally occurring dietary phytoestrogens distributed in the bark, leaf, bloom, and seed of some plants, specifically in soybeans. Genistin, the glycoside conjugate of genistein (4′,5,7-trihydroxyisoflavone), is one of the most abundant and biologically active soy isoflavones (Chang, Citation2014). In 1987, it was discovered that genistein is a specific inhibitor of tyrosine-specific protein kinases (Akiyama et al., Citation1987). Since then, studies have provided convincing evidence of the inhibitory effects of genistein on various cancers, including colorectal, breast, and prostate cancers, by inducing apoptosis or inhibiting cell proliferation both in vivo and in vitro (Chiyomaru et al., Citation2012; Li et al., Citation2013; Pampaloni et al., Citation2014). These data suggest that genistein is a promising small molecule for the prevention and treatment of cancer.
The pharmacology of genistein in colorectal cancer remains to be determined. It was reported that genistein inhibited cancer cell growth by attenuating the negative effect of epidermal growth factor (EGF) on FOXO3 (Qi et al., Citation2011); Moreover, genistein may prevent cell-cycle progression by decreasing Cyclin B1, checkpoint kinase 2 (Chk2) and proliferating cell nuclear antigen (PCNA), while up-regulating the expression of p21 (Fan et al., Citation2010; Han et al., Citation2013). It was suggested that genistein might reduce angiogenesis via suppressing the expression of vascular endothelial growth factor (VEGF) (Fan et al., Citation2010).
Akt, also known as protein kinase B (PKB), is a serine/threonine kinase and a major downstream effector of phosphatidylinositide-3-kinase (PI3K). Akt activation depends on its phosphorylation at T308 within its catalytic domain by phosphoinositol-dependent kinase 1 (PDK1) and at S473 within its C-terminal regulatory domain by mammalian target of rapamycin complex 2 (mTORC2) (Yang et al., Citation2010). Akt plays critical roles in regulating a wide range of cellular processes including cell survival, growth, proliferation, angiogenesis, metabolism, and migration (Manning & Cantley, Citation2007). Akt promotes cell-cycle progression by preventing the degradation of cyclin D1 while suppressing the cell-cycle inhibitors p21WAF1 and p27Kip1 (Bellacosa et al., Citation2004). At the same time, Akt prevents apoptosis by phosphorylating and inhibiting pro-apoptotic proteins like Bad and FOXO3a (Yang et al., Citation2010). Thereafter, aberrant activation of Akt is associated with the growth and survival of many human cancers. Previous study has demonstrated that Akt is significantly activated in colorectal cancer tissues, and phosphorylated Akt (p-Akt) was suggested as a biomarker for prognosis and a potential therapeutic target for colorectal cancer (Baba et al., Citation2011). Recently, it was shown that genistein induced apoptosis in human colon cancer HT-29 and Caco2 cells, accompanied with decrease of Akt phosphorylation (Gu et al., Citation2011; Qi et al., Citation2011), suggesting that genistein induces cell apoptosis and inhibit cell proliferation of colon cancer by suppressing the Akt signaling pathway. It is thus possible that inhibition of the activity of Akt may contribute to the suppression of cell growth and induction of apoptosis of colorectal cancer cells by genistein.
In the present study, we determined the cell viability, the expression of Bax, and phosphorylation Akt following the treatment of human colorectal cancer cells with genistein.
Materials and methods
Drug preparation
Genistein was purchased from Phytomarker (Tianjin, China) and dissolved in dimethyl sulfoxide (DMSO) and stored in small aliquots at −20 °C.
Cell culture
Human colorectal cancer cell lines, HCT-116 and LoVo, were obtained from American Type Culture Collection (Manassas, VA). The cells were maintained as a monolayer in DMEM (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT) at 37 °C in a humidified 5% CO2 incubator.
MTT assay
The effect of genistein on cell viability was assessed by MTT assay. HCT-116 and LoVo cells were seeded in 96-well plates at a density of 4 × 103 cells per well and incubated for 24 h. Then, media were replaced with fresh media containing different concentrations (0, 25, 50, and 100 μM) of genistein and cultured for 24, 48, and 72 h. After treatment, 20 μl MTT (5 mg/ml) was added to each well and further incubated for 4 h. The supernatant was aspirated and replaced with 150 μl/well of DMSO to dissolve the formazan salt formed. The optical density (OD) was measured at 570 nm using a microplate reader (Bio-Rad, Hercules, CA).
Flow cytometry
The apoptotic rates of the HCT-116 cells and LoVo cells were determined using an annexin V-FITC apoptosis detection kit (Boster, Hubei, China). In brief, cells were seeded in six-well plates overnight, then treated with various concentrations of genistein for 48 h, collected, and resuspended in binding buffer. Annexin V-FITC and propidium iodide (PI) were added according to the manufacturer’s instructions. The analysis was performed with an FACS Calibur Flow Cytometer (Becton Dickinson, San Jose, CA).
Hoechst 33258 staining
The morphological changes of the nuclei were observed by Hoechst 33258 staining (Apoptosis-Hoechst staining kit, Beyotime, Jiangsu, China). HCT-116 cells were seeded in six-well plates overnight. Following treatment with genistein for 48 h, cells were washed twice with PBS, and then stained with Hoechst 33258 for 5 min in the dark. The changes in nuclei were observed using a fluorescence microscope (Olympus, Tokyo, Japan) with a 480 nm excitation filter and captured.
Real-time PCR
Total cellular RNA was extracted from the treated cells using Trizol reagent (Gibco-BRL, Grand Island, NY). Single-strand cDNA was synthesized by Revert Aid TM First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD) using 5 μg of total RNA as template following the instructions. Quantity of Bax mRNA expression was determined by quantitative Real-time PCR using the ABI PRISM 7500 Sequence Detector System (Applied Biosystems, Carlsbad, CA) according to the manufacturer’s protocols. The primers were as follows: Bax forward: 5′-ACCAGCTCTGAGCAGATCATG-3′; Bax reverse: 5′-ATCATCCTCTGCAGCTCCATG-3′; GAPDH forward: 5′-TCACCCACACTGTGCCCATCTACGA-3′; GAPDH reverse: 5′-CAGCGGAACCGCTCATTGCCAATGG-3′. GAPDH mRNA was used as an internal control. The threshold cycle (Ct) value for each sample was normalized to GAPDH mRNA.
Western blot
Cells were lysed in ice-cold NP40 buffer (1% NP-40, 0.15 M NaCl, 50 mM Tris, pH 8.0) containing protease inhibitors (Sigma, St. Louis, MO). Cell lysates were centrifuged at 12 000 g for 10 min at 4 °C, and the supernatants were stored at −80 °C. The total protein concentrations were measured using a Bio-Rad assay kit (Bio-Rad, Hercules, CA). Equal amount of proteins from each sample (40 μg) were denatured in SDS sample buffer and separated on 10% SDS polyacrylamide gel. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) in buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol. After blocking with TBST containing 5% skim milk, the membranes were blotted with primary antibodies specific for T-Akt (1:1000), phospho-Akt, Bax, and β-actin (1:1000 dilution, Santa Cruz Biotechnology, Heidelberg, Germany). Anti-β-actin antibody was used as a loading control antibody. After washing, membranes were incubated with the appropriate horseradish peroxidase-linked secondary antibody. The membranes were then visualized using enhanced chemiluminescence (ECL) detection reagents (Millipore, Billerica, MA) and exposed to X-ray film. For the densitometry analysis of protein bands, Quantity One software (Bio-Rad, Hercules, CA) was used.
Statistical analyses
Results are shown as mean values ± standard deviation (SD). All statistical analyses were done using SPSS version 17.0 software (SPSS Inc., Chicago, IL). Data were determined by one-way ANOVA followed by Dunnet's multiple comparison test. For all comparisons, a p-value < 0.05 was considered a statistically significant difference (*p < 0.05).
Results
Genistein inhibits the growth of HCT-116 and LoVo cells
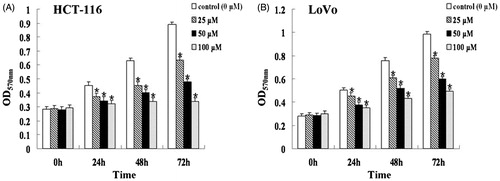
The MTT assay was used to evaluate viability of HCT-116 and LoVo cells exposed to different concentrations of genistein (0, 25, 50, and 100 μM) for 24, 48, and 72 h. At each time point, the OD570nm of treated HCT-116 cells decreased with the increasing concentration of genistein compared with that of each control group (0 µM) (, p < 0.05). Similar inhibitory effects of genistein on cell growth were observed for LoVo cells (, p < 0.05). The IC50 values of genistein were 690, 135, and 61 μM in HCT-116 cells and 204, 135, and 93 μM in LoVo cells after treatment for 24, 48, and 72 h, respectively. Thus, genistein inhibited the growth of colorectal cancer HCT-116 and LoVo cells in a time-dependent manner.
Figure 1. Genistein inhibited the proliferation of HCT-116 (A) and LoVo (B) cells. Cells were treated with 0, 25, 50, and 100 μM of genistein for 24, 48, and 72 h. The cell viability was determined by the MTT assay. The results were presented as mean ± SD for triplicate experiments. *p < 0.05 versus each control group (0 µM).
Genistein induces apoptosis in HCT-116 and LoVo cells
Genistein-induced apoptosis was assessed by flow cytometry. Annexin V(+)/PI(−) cells were scored as early apoptotic cells. After 48 h of treatment with genistein at different concentrations (0, 25, 50, and 100 μM), the early apoptotic HCT-116 cells increased from 1.99% ± 0.55% to 6.78% ± 2.12%, 23.16% ± 3.87%, and 36.99% ± 3.76%, respectively (p < 0.05). The same concentrations of genistein increased the early apoptotic LoVo cells from 2.56% ± 1.42% to 3.21% ± 1.52%, 18.22% ± 3.56%, and 23.56% ± 3.02% (p < 0.05). These results indicate that genistein induces apoptosis in HCT-116 and LoVo cells, and the rate of early apoptotic cells in HCT-116 cells was more than LoVo.
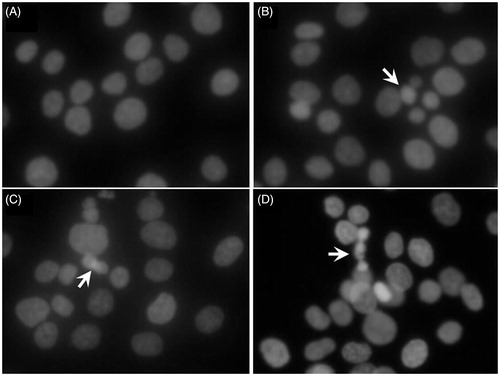
We further performed Hoechst 33258 staining to visualize nuclear changes and apoptotic body formation that were characteristic of apoptosis. As shown in , the morphological changes of cell apoptosis including condensation of chromatin and nuclear fragmentation were observed in the genistein-treated groups (25, 50, and 100 μM), while the cells in the control group (0 μM) were stained homogeneously blue.
Figure 2. Cell morphology under fluorescence microscopy by Hoechst 33258 staining in HCT-116 cells treated with genistein for 48 h. (A) Control group (0 μM). (B) Treated with genistein (25 μM). (C) Treated with genistein (50 μM). (D) Treated with genistein (100 μM). Arrows indicate the condensed and fragmented nuclei. The images were taken using an Olympus IX71FL fluorescence microscope (Olympus, Tokyo, Japan) (× 400).
Genistein induces the expression of Bax in HCT-116 cells
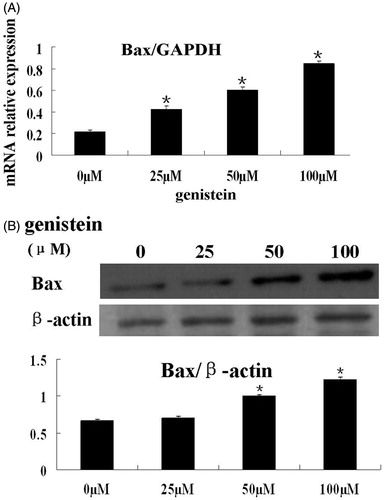
To further support the observation that genistein induces apoptosis in colorectal cancer cells, we determined the mRNA and protein levels of Bax by real-time PCR and immunoblotting, respectively. As shown in , Bax mRNA levels in HCT-116 cells were significantly upregulated by genistein (p < 0.05). Similarly, the protein expression of Bax in HCT-116 cells was also significantly increased by 50 and 100 μM genistein (, p < 0.05). Taken together, these results clearly demonstrated that induction of apoptosis is one of the mechanisms by which genistein inhibits cell viability of colorectal cancer cells.
Figure 3. Genistein decreased the expression of Bax in HCT-116 cells. (A) HCT-116 cells were treated with 0, 25, 50, and 100 μM of genistein for 48 h. The mRNA levels of Bax were determined by real-time PCR with GAPDH as internal control. (B) HCT-116 cells were treated with 0, 25, 50, and 100 μM of genistein for 48 h. The protein levels of Bax were determined by Western blot with β-actin as loading control. The results of real-time PCR and western blot assays are presented as mean ± SD for triplicate experiments. *p < 0.05 versus the control group (0 µM).
Genistein decreases the phosphorylation of Akt in HCT-116 cells
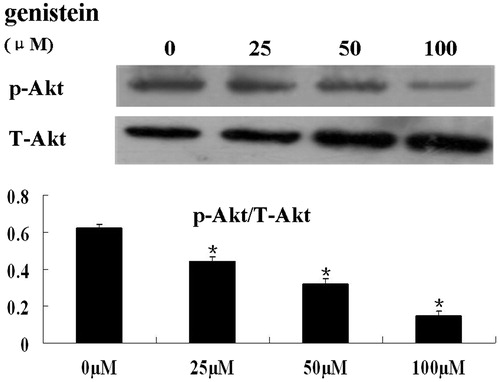
The PI3K-Akt pathway is a major cell growth-promoting signal and inhibits cell apoptosis via multiple mechanisms. To assess whether genistein inhibits cell proliferation and induces cell apoptosis by suppressing Akt signaling, we determined the phosphorylation of Akt status following treatment of HCT-116 cells with different concentrations of genistein by western blot analysis. Compared with the untreated control, genistein significantly decreased the phosphorylation of Akt (p < 0.05, ).
Figure 4. Treatment of HCT-116 cells with genistein resulted in reduced phosphorylation of Akt. HCT-116 cells were treated with 0, 25, 50, and 100 μM of genistein for 48 h. Total protein was extracted for immunoblotting of p-Akt and total Akt. The quantification of the western blot assays is presented as mean ± SD for triplicate experiments. *p < 0.05 versus the control group (0 µM).
Discussion
In this study, we demonstrated that genistein inhibited cell proliferation and induced apoptosis in colorectal cancer HCT-116 and LoVo cells, accompanied with upregulation of Bax and downregulation of the phosphorylation of Akt. Our findings suggest that genistein inhibits cancer cell growth at least, in part, by suppressing the PI3K-Akt signaling pathway.
Epidemiological studies have provided evidence that postmenopausal women who have ever used hormone replacement therapy are at lower risk of colon and rectal cancer (Fernandez et al., Citation1998). However, the use of exogenous hormones is associated with a decreased risk for estrogen receptor-β-positive (ERβ-positive), but not estrogen receptor-β-negative (ERβ- negative) colorectal cancer (Rudolph et al., Citation2013). Although ERα and ERβ are the two known subtypes through which estrogens exert their effects on various tissues, ERβ is the predominant estrogen receptor subtype in the human colon and decreased levels of ERβ are associated with colonic tumorigenesis (Campbell-Thompson et al., Citation2001; Pampaloni et al., Citation2014). Experimental studies indicate that 17β-estradiol (E2), as the main circulating estrogen hormone, exerts a protective function against cancer growth and induces the translation of ERβ mRNA in colon cancer cells by binding to Erβ (Caiazza et al., Citation2007). Genistein has a similar structure to estrogens and has been shown to bind to estrogen receptors (Tadaishi et al., Citation2014), indicating that genistein may prevent tumorigenesis of colorectal cancer. Indeed, in this study, we found that genistein inhibited cell proliferation and induced apoptosis in the both HCT-116 and LoVo cancer cell lines. Considering that both HCT-116 and LoVo cells express ERβ but lack ERα (Arai et al., Citation2000), the inhibitory effects of genistein on the two cancer cell lines may be through activating ERβ signaling.
We previously showed that calycosin, another member of isoflavones family, suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R (insulin-like growth factor 1 receptor) and PI3K/Akt pathways. As the expression of ERβ gradually increased with calycosin, the levels of IGF-1R decreased, accompanied with attenuated phosphorylation of Akt (Chen et al., Citation2014). Akt suppresses cell apoptosis by inhibiting Bax (Gu et al., Citation2013). Bax, as a member of the Bcl-2 family, interacts with the permeability transition pore to lower the mitochondrial membrane potential and release cytochrome c, inducing mitochondrial pathway of apoptosis (Huang et al., Citation2014; Park et al., Citation2014). Our results showed that genistein upregulated Bax expression but inhibited phosphorylation of Akt. These data suggest that the mitochondrial apoptotic pathway (regulating the Bax) and the phosphorylation of Akt are involved in genistein-induced apoptosis in colorectal cancer HCT-116 cells.
However, our result from flow cytometry showed that the apoptosis induced by genistein was more remarkable on HCT-116 than on LoVo cells. Recent studies reported that the expression of Dickkopf-1 (DKK1) was significantly higher in HCT116 cells compared with that in LoVo cells (Yu et al., Citation2013). DKK1 is one of the key regulators of the Wingless-int (Wnt)/β-catenin signaling pathway, which plays an important role in development and in regulating adult stem cell systems (Huang et al., Citation2013). Moreover, aberrant regulation of this pathway promotes tumorigenesis of human colorectal cancer (Klaus & Birchmeier, Citation2008). DKK1, as a repressor of cancer cell growth, prevents β-catenin-mediated signal transduction, thereby promoting cell differentiation and apoptosis (Wang et al., Citation2012). Furthermore, β-catenin is a downstream signaling molecule of PI3K/AKT pathway (Wang et al., Citation2011). In addition, it was recently reported that genistein inhibited the WNT/β-catenin pathway by inducing DKK1 expression in human colorectal cancer (Wang et al., Citation2012). Therefore, we speculate that overexpression of DKK1 might contribute to the enhanced inhibition of cell proliferation and induction of apoptosis by genistein in HCT-116 cells.
In summary, we found that genistein inhibited cell proliferation and induced apoptosis in the human colorectal cancer HCT-116 and LoVo cells. Moreover, the current study expanded the knowledge that genistein induced the mitochondrial pathway of apoptosis in HCT-116 cells by inhibiting phosphorylation of Akt. Our finding will accelerate the development of genistein as an agent for the prevention and treatment of colorectal cancer.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of this article. This study is supported by the National Natural Science Foundation of China (No. 81160107), Guangxi Science Research and Technique Development Plan Foundation (No. 1104003-69), and Guangxi Natural Science Foundation (No. 2010GXNSFB013083).
References
- Akiyama T, Ishida J, Nakagawa S, et al. (1987). Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262:5592–5
- Arai N, Ström A, Rafter JJ, et al. (2000). Estrogen receptor beta mRNA in colon cancer cells: Growth effects of estrogen and genistein. Biochem Biophys Res Commun 270:425–31
- Baba Y, Nosho K, Shima K, et al. (2011). Phosphorylated AKT expression is associated with PIK3CA mutation, low stage, and favorable outcome in 717 colorectal cancers. Cancer 117:1399–408
- Bellacosa A, Testa JR, Moore R, et al. (2004). A portrait of AKT kinases: Human cancer and animal models depict a family with strong individualities. Cancer Biol Ther 3:268–75
- Caiazza F, Galluzzo P, Lorenzetti S, et al. (2007). 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochem Biophys Res Commun 359:102–7
- Campbell-Thompson M, Lynch IJ, Bhardwaj B. (2001). Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res 61:632–40
- Chang TS. (2014). Isolation, bioactivity, and production of ortho-hydroxydaidzein and ortho-hydroxygenistein. Int J Mol Sci 15:5699–716
- Chen J, Hou R, Zhang X, et al. (2014). Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS One 9:e91245
- Chiyomaru T, Yamamura S, Zaman MS, et al. (2012). Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One 7:e43812
- Fan YZ, Li GH, Wang YH, et al. (2010). Effects of genistein on colon cancer cells in vitro and in vivo and its mechanism of action. Zhonghua Zhong Liu Za Zhi 32:4–9
- Fernandez E, La Vecchia C, Braga C, et al. (1998). Hormone replacement therapy and risk of colon and rectal cancer. Cancer Epidemiol Biomarkers Prev 7:329–33
- Gu S, Papadopoulou N, Nasir O, et al. (2011). Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/bad in vitro and in vivo and blocks migration via vinculin/actin signaling. Mol Med 17:48–58
- Gu Y, Liu SL, Ju WZ, et al. (2013). Analgesic-antitumor peptide induces apoptosis and inhibits the proliferation of SW480 human colon cancer cells. Oncol Lett 5:483–8
- Han J, Kurita Y, Isoda H. (2013). Genistein-induced G2/M cell cycle arrest of human intestinal colon cancer Caco-2 cells is associated with Cyclin B1 and Chk2 down-regulation. Cytotechnology 65:973–8
- Huang WJ, Bi LY, Li ZZ, et al. (2014). Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm Biol 52:466–70
- Huang Z, Li S, Song W, et al. (2013). Lysine-specific demethylase 1 (LSD1/KDM1A) contributes to colorectal tumorigenesis via activation of the Wnt/β-catenin pathway by down-regulating Dickkopf-1 (DKK1). PLoS One 8:e70077
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90
- Klaus A, Birchmeier W. (2008). Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–98
- Li Y, Chen H, Hardy TM, et al. (2013). Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS One 8:e54369
- Manning BD, Cantley LC. (2007). AKT/PKB signaling: Navigating downstream. Cell 129:1261–74
- Pampaloni B, Palmini G, Mavilia C, et al. (2014). In vitro effects of polyphenols on colorectal cancer cells. World J Gastrointest Oncol 6:289–300
- Park JW, Kim SC, Kim WK, et al. (2014). Expression of phosphoenolpyruvate carboxykinase linked to chemoradiation susceptibility of human colon cancer cells. BMC Cancer 14:160
- Qi W, Weber CR, Wasland K, et al. (2011). Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 11:219
- Ramadas A, Kandiah M. (2009). Food intake and colorectal adenomas: A case–control study in Malaysia. Asian Pac J Cancer Prev 10:925–32
- Rudolph A, Toth C, Hoffmeister M, et al. (2013). Colorectal cancer risk associated with hormone use varies by expression of estrogen receptor-β. Cancer Res 73:3306–15
- Tadaishi M, Nishide Y, Tousen Y, et al. (2014). Cooperative effects of soy isoflavones and carotenoids on osteoclast formation. J Clin Biochem Nutr 54:109–15
- Wang H, Li Q, Chen H. (2012). Genistein affects histone modifications on Dickkopf-related protein 1 (DKK1) gene in SW480 human colon cancer cell line. PLoS One 7:e40955
- Wang L, Li CL, Wang L, et al. (2011). Influence of CXCR4/SDF-1 axis on E-cadherin/β-catenin complex expression in HT29 colon cancer cells. World J Gastroenterol 17:625–32
- Yang G, Shu XO, Li H, et al. (2009). Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr 89:577–83
- Yang WL, Wu CY, Wu J, et al. (2010). Regulation of Akt signaling activation by ubiquitination. Cell Cycle 9:487–97
- Yu Q, Yang L, Ding Y, et al. (2013). Expression of Dickkopf-1 in human colon carcinoma cell lines. Nan Fang Yi Ke Da Xue Xue Bao 33:923–5, 933

