Abstract
Context: The possibility of combining unripe plantain [Musa paradisiacae Linn (Plantaginaceae)] and cocoyam [Colocassia esculenta Linn (Araceae)] in the management of diabetes has not been investigated.
Objective: The objective of this study is to evaluate the antihyperglycemic and antihyperlipidemic actions of unripe plantain and cocoyam.
Materials and methods: Diabetes was induced in rats by intraperitoneal injection of streptozotocin (STZ) (65 mg/kg body weight). Twelve days after STZ induction, respective groups of diabetic rats were fed cocoyam (810 g/kg), unripe plantain (810 g/kg), and unripe plantain + cocoyam (405:405 g/kg) for 28 d. Body weights, feed intake, biochemical parameters, namely serum glucose, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), very low-density lipoprotein (VLDL), atherogenic index, coronary risk index, triacylglycerol, glycated hemoglobin (HbA1C), hepatic isocitrate dehydrogenase, malic enzyme, and glucose-6-phosphate dehydrogenase of the rats and phytochemical composition of the test and standard rat feeds were measured.
Results and discussion: Cocoyam or unripe plantain alone significantly (p < 0.05) ameliorated the body weights (18.89 and 19.95% decreases, respectively) and biochemical parameters as compared with those of STZ controls (31.21% decrease). While combination of cocoyam and unripe plantain significantly (p < 0.05) ameliorated the biochemical parameters of the rats (except HbA1C), it did not ameliorate their body weights (28.53% decrease). The feed intake of the experimental rats did not differ from each other (p > 0.05) at the end of experimentation and the feed samples contained considerable amounts of saponins, alkaloids, flavonoids, and tannins.
Conclusion: Cocoyam or unripe plantain alone showed better antihyperglycemic and anihyperlipidemic action than their combination.
Introduction
The abnormality produced in lipids is one of the major etiologic factors in the development of diabetic complications. It has been projected that by 2020–2025, the number of people in the developing world with diabetes will increase by more than 2–5-folds; from 84 million in 1995 to 228 million in 2025 and that 70% of deaths due to type 2 diabetes will occur in developing countries (WHO, Citation2003).
Since the use of synthetic drugs in the control of this disease and its complications poses enormous cost on the economy of developing nations with undesirable side effects, alternative strategies are urgently needed (WHO, Citation2003). Moreover, despite the use of new diagnostic devices, strict glycemic targets, better treatment guidelines, and increased awareness of the disease, baseline glycosylated hemoglobin has continued to remain relatively high in subjects diagnosed and treated with diabetes (Vaz & Patnaik, Citation2012). This has led to the recent trend of finding novel types of natural antidiabetic drugs from various medicinal plants.
Plantain [Musa paradisiacal Linn (Musaceae)] is cultivated in many tropical countries of the world. It ranks third after yam and cassava for sustainability in Nigeria (Akomolafe & Aborisade, Citation2007). Plantain is rich in fiber, iron, vitamins, minerals, and serotonin. Its hypoglycemic actions in diabetic animals have been reported by several authors (Chhanda et al., Citation2006; Ojewole & Adewunmi, Citation2003).
Cocoyam [Colocasia esculenta Linn (Araceae)] is an herbaceous perennial plant that grows from the fleshy corm (tuber) that can be boiled, baked, and mashed into a meal or used as snack. The corms supply easily digestible starch and are known to contain substantial amounts of protein, vitamin C, thiamine, riboflavin, niacin, and significant amounts of dietary fiber (Niba, Citation2003). The flour of Colocassia esculenta is commonly used in the southeastern part of Nigeria as a thickener in the preparation of soups.
In ethnomedicine, cocoyam is used in the management of diabetes mellitus, treatment of ringworm, etc. (Mwenye et al., Citation2010). The antidiabetic action of cocoyam in streptozotocin diabetic rat models has also been reported (Eleazu et al., Citation2013).
Although cocoyam and unripe plantain are used as single plants to manage diabetes mellitus in Nigeria, the possibility of combining them in a typical diabetic diet and the glycemic response elicited as a result of such combination has not been investigated. This has been necessitated by the fact that diabetic patients often complain of the monotony of staying on a particular diet (Ime et al., Citation2011).
Since the alteration of lipid metabolism is one of the pathological bases for the development of diabetic complications and being that glycated hemoglobin is regarded as the best marker for glycemic control, this study was initiated to investigate the effect of cocoyam, unripe plantain or their combination on serum glucose, glycated hemoglobin, and lipid metabolism of streptozotocin-induced diabetes in rats.
Materials and methods
Plant materials
The unripe plantain, locally known in the Eastern part of Nigeria as seed plantain, was obtained from Umuahia main market in August 2013. It was identified by Mr. Ibe of the Forestry Department, Michael Okpara University of Agriculture, Umudike, and NRCRI. The root of the Colocassia esculenta variety, locally known in the eastern part of Nigeria as Edeofe, was obtained from National Root Crops Research Institute (NRCRI), Umudike, Nigeria, in August 2013. Identification of the plant was also carried out by Mr. Ibe. The plants were deposited in the herbarium of Michael Okpara University of Agriculture, Umudike, for authentication.
Chemicals
Streptozotocin (STZ), dl-isocitric acid, β-nicotinamide adenine dinucleotide phosphate-sodium salt, triethanolamine, hydrochloride Gly–Gly, free base, l(-)malic acid, free acid, and glucose-6-phosphate monosodium salt used were products of Sigma and Aldrich Chemical Co., Gillingham, Dorset, UK. The Randox kits used for lipid assays were products of Randox Laboratories Limited, Crumlin, Co., Antrim, UK.
Processing of the plant materials
The samples were properly peeled, soaked in water for about 10 min, rinsed, and oven dried at 70 °C until constant weight was obtained and processed to flour. The processed flours were pelletized, oven dried at 80 °C until constant weight before they were fed to the rats.
Animal experiments
Selection of animals
Forty-eight male albino rats of the Wistar strain (133.28–242.79 g) obtained from University of Nigeria, Nsukka, Enugu State, Nigeria, were used for the study. The animals were kept in metabolic cages in the animal house of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Nigeria. The animals were acclimatized for 2 weeks to their diets prior to the commencement of the experiment and were maintained at a room temperature of 27–30 °C. All animal protocols were approved by the ethical committee of Michael Okpara University of Agriculture, Umudike, which was in line with the guide for the care of use of laboratory animals as reported by the National Institute of Health’s Principles (NRC, Citation1985).
Induction of diabetes
After 2 weeks of acclimatization, freshly prepared solution of streptozotocin (0.1 g dissolved in 5 mL of freshly prepared sodium citrate buffer 0.1 M, pH 4.5) was injected intraperitoneally to 42 of the rats at a dosage of 65 mg/kg body weight at a fasting state while six of the remaining rats served as the non-diabetic group and received standard rat pellets. Blood was collected from the tail vein and blood glucose concentration was analyzed in the STZ-treated rats prior to the commencement of the dietary feeding using a blood glucose meter (Double G glucometer, Double G Industries, Ltd, Katy TX) and subsequently, twice in a week, throughout the duration of the experiment. The STZ-treated rats with fasting blood glucose levels >200 mg/dL after 12 d of induction of STZ and with evidence of glycosuria were considered to be diabetic and used for the study.
Experimental procedure
The STZ-treated rats with stable diabetic condition were then divided into subgroups (groups 2–5) comprising of six animals per group while the non-diabetic group formed the first group as follows:
Group 1. Non-diabetic rats fed standard rat feeds/pellets (non-diabetic control).
Group 2. Diabetic control rats fed standard pellets.
Group 3. Diabetic rats fed cocoyam pellets (810 g/kg).
Group 4. Diabetic rats fed with unripe plantain pellets (810 g/kg).
Group 5. Diabetic rats fed cocoyam + unripe plantain pellets (405:405 g/kg).
Their diets and water were both administered ad libitum for 28 d, after which the rats were stunned by blow, sacrificed, and blood was drawn from the heart using 10 mL syringes and poured into heparin tubes for HbA1C assays while the rest of the blood samples were collected in non-anti-coagulant tubes for serum assay of lipid profile.
Biochemical analysis
The liver was removed, washed with ice-cold physiological saline immediately, and stored in a deep freezer (−20 °C) until analyzed. Ten percent homogenate (w/v) of the liver was prepared in 150 mM KCl using a homogenizer at 4 °C (Singh et al., Citation2001) and centrifuged at 1000 × g for 5 min to remove cell debris. The supernatant was further re-centrifuged at 14 000 × g for 30 min at 4 °C and the supernatant was used as the enzyme source (Jin et al., Citation2003) for the assay of malic enzyme (ME), isocitrate dehydrogenase (IDH), and glucose-6-phosphate dehydrogenase (G6PD) activities.
The specific activities of nicotinamide adenine dinucleotide phosphate (NADP)-linked ME (EC 1.1.1.40), IDH (EC 1.1.1.42), and G6PD (EC 1.1.49) were determined using the methods of Geer et al. (Citation1980) and Noltmann et al. (Citation1961). A unit of enzyme activity was defined as 1 µmol nicotinamide adenine dinucleotide phosphate reduced (NADPH) formed per gram fresh weight of tissue per min at 37 °C. The HbA1C assay was carried out using the Biosystems (BioSystems S.A. Costa Brava 30, Barcelona, Spain) diagnostic kit method as described by Karl et al. (Citation1993) and the principle was based on the quantification of the HbA1C by a turbidimetric inhibition immunoassay after preparation of the hemolysate using tetradecyltrimethylammonium bromide as the detergent. The serum total triacylglycrols, serum total cholesterol, serum glucose, and serum HDL-cholesterol concentrations were analyzed using the Randox assay diagnostic kits as described by Tietz (Citation1995) and NCEP (Citation2001). The LDL-cholesterol and VLDL-cholesterol levels were calculated using the method of Friedewald et al. (Citation1972) as described below:
The atherogenic index and coronary risk index (CRI) were calculated using the formula shown below as described by Omonkhua et al. (Citation2013):
Atherogenic Index (AI) = LDL-cholesterol/HDL-cholesterol; Coronary Risk Index (CRI) = Total cholesterol/HDL-cholesterol.
Phytochemical analysis
The gravimetric method of Harbone (Citation1973) was used for the determination of alkaloids while the method of AOAC (Citation1990) was used for the determination of other phytochemical constituents of the test and standard rat feeds.
Statistical analysis
Data were subjected to analysis using the Statistical Package for Social Sciences (SPSS Inc., Chicago, IL), version 17.0. Results were presented as the means ± standard deviations of triplicate experiments. One-way analysis of variance (ANOVA) was used for comparison of the means. Differences between means were considered to be significant at p < 0.05 using the New Duncan Multiple Range Test.
Results
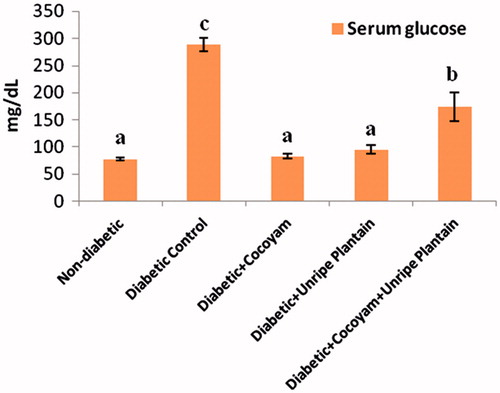
The serum glucose levels of the rats in the five groups decreased in the following order as shown in : diabetic control rats (288.60 ± 11.78 mg/dL) > unripe plantain + cocoyam group (174.25 ± 26.40 mg/dL) > unripe plantain group (95.10 ± 7.55 mg/dL) > cocoyam group (82.25 ±4.27 mg/dL) > non-diabetic group (78.24 ± 2.50 mg/dL). There were significant decreases (p < 0.05) in the serum glucose levels of the diabetic control rats compared with the non-diabetic rats () while the serum glucose levels of the diabetic rats fed cocoyam, unripe plantain, or their combination were significantly lower in comparison with the diabetic control rats (p < 0.05). Furthermore, the serum glucose levels of the diabetic rats given cocoyam or unripe plantain feeds did not differ significantly (p > 0.05) from the serum glucose levels of the non-diabetic rats.
Figure 1. Serum glucose levels of rats. Values are means ± SD of three determinations. a–cMeans with different superscripts are significantly different (p < 0.05).
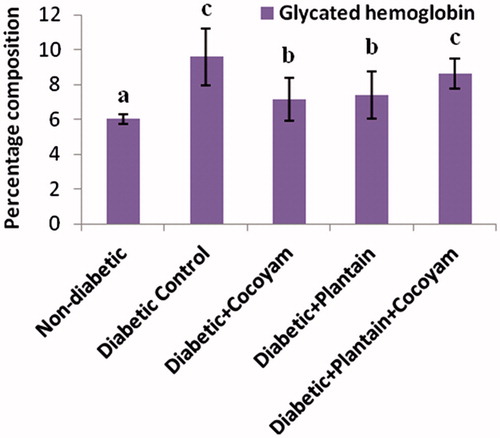
The percentage glycated hemoglobin (HbA1C) levels of the rats in the five groups decreased in the following order as shown in : diabetic control rats (9.57 ± 1.63) > unripe plantain + cocoyam group (8.64 ± 0.85) > unripe plantain group (7.39 ± 1.33) > cocoyam group (7.15 ± 1.21) > non-diabetic group (6.03 ± 0.28). There were significant decreases (p < 0.05) in the HbA1C levels of the diabetic control rats compared with the non-diabetic rats () while the HbA1C levels of the diabetic rats fed cocoyam or unripe plantain feeds did not differ significantly from each other (p > 0.05), but were significantly lower (p < 0.05) than the HbA1C levels of the diabetic control rats and diabetic rats fed cocoyam + unripe plantain feeds but significantly higher (p < 0.05) than the HbA1C levels of the non-diabetic rats.
Figure 2. Glycated hemoglobin levels of rats. Values are means ± SD of three determinations. a–cMeans with different superscripts for each parameter are significantly different (p < 0.05).
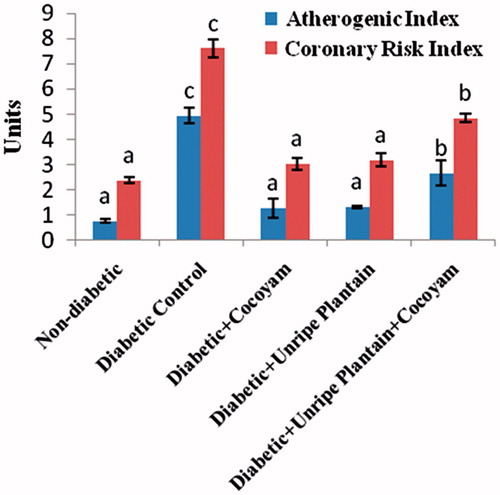
The coronary risk and atherogenic risk indices of the diabetic control rats were significantly higher (p < 0.05) than those of the non-diabetic rats (). Feeding of cocoyam, unripe plantain, or their combination to the diabetic rats of groups 3–5, resulted in significant reduction (p < 0.05) of their atherogenic and coronary risk indices compared with the diabetic control rats ().
Figure 3. Atherogenic and Coronary Risk Indices of rats (units); values are means ± SD of three determinations. a–cMeans with different superscripts are significantly different (p < 0.05).
There were significant increases (p < 0.05) in the serum total cholesterol, serum triglycerides, VLDL-cholesterol, and LDL-cholesterol but significant decreases in the serum HDL-cholesterol levels of the diabetic control rats compared with the non-diabetic rats ().
Table 1. Lipid profile (mg/dL) of rats.
The diabetic rats fed cocoyam, unripe plantain, or their combination recorded significant reduction (p < 0.05) of their serum total cholesterol, triacylglycerol, and LDL-cholesterol, but significant increase (p < 0.05) of their HDL-cholesterol levels compared with the diabetic control rats ().
There were significant decreases (p < 0.05) in the hepatic levels of malic enzyme, isocitrate dehydrogenase, and glucose-6-phosphate dehydrogenase in the diabetic control rats compared with the non-diabetic rats (). Feeding of cocoyam, unripe plantain, or their combination to the diabetic rats of groups 3–5, resulted in significant (p < 0.05) elevation of the hepatic levels of malic enzyme, isocitrate dehydrogenase, and G6PD in the rats compared with the diabetic control rats (). In addition, the malic enzyme, isocitrate dehydrogenase, and G6PD activities of the diabetic rats fed cocoyam or unripe plantain were not significantly different (p > 0.05) from each other ().
Table 2. Lipogenic enzyme activities in the liver of rats (units/g).
The feed composition that was given to the group 3 diabetic rats comprising 81% cocoyam flour, 9% soya bean flour, 4% vitamin mixture, 2% salt, and 4% groundnut oil, the feed composition that was given to the group 4 diabetic rats comprising 81% unripe plantain flour, 9% soya bean flour, 4% vitamin mixture, 2% salt, and 4% groundnut oil while the feed composition that was given to the group 5 diabetic rats comprising 40.5% unripe plantain flour, 40.5% cocoyam flour, 9% soya bean flour, 4% vitamin mixture, 2% salt, and 4% groundnut oil.
The diabetic rats fed cocoyam recorded 18.89% loss of weight, the diabetic rats fed unripe plantain recorded 19.95% loss of weight, the diabetic rats fed unripe plantain + cocoyam recorded 28.53% loss of weight compared with the diabetic control rats that recorded 31.21% loss of weight, unlike the non-diabetic rats that recorded 15.50% gain in weight ().
Table 3. Body weight of rats (g).
The average feed intake of the diabetic rats of groups 2–5 was not significantly different (p > 0.05) from each other by the last week of experimentation but were significantly lower (p < 0.05) than the average feed intake of the non-diabetic rats by the last week of experimentation ().
Table 4. Feed intake per rat (g/week).
Phytochemical analysis of the test and standard rat feeds revealed that the cocoyam feed contained higher quantities of saponin, alkaloid and tannin than the unripe plantain feed, or the combination of unripe plantain and the standard rat feeds although its percentage tannin content was not significantly different (p > 0.05) from that of unripe plantain feed ().
Table 5. Phytochemical composition of test and standard rat feeds (%).
The unripe plantain feed contained higher quantities of alkaloids, flavonoids, saponins, and tannins than the unripe plantain + cocoyam and standard rat feeds ().
Discussion
The normalization of the blood glucose concentrations of the diabetic rats fed cocoyam or unripe plantain to the extent that was observed in this study suggests the hypoglycemic actions of cocoyam or unripe plantain.
However, it was observed that the combination of cocoyam and unripe plantain at the dosage used was not very effective in attenuating the elevated blood glucose of the diabetic rats compared with when the plants were fed individually which is a significant finding in this study.
HbA1C is a product of the irreversible condensation of glucose with the N-terminal residue of the β-chain of hemoglobin A. The level of HbA1C is a useful and reliable tool for the assessment of glycemic control in diabetics as it reflects the average blood glucose concentration over the preceding 6–8 weeks (Cosson et al., Citation2013; Haseena et al., Citation2011; Zhang & Feng, Citation2007), equivalent to the life time of the erythrocytes.
The higher levels of HbA1C in the diabetic rats compared with the non-diabetic rats indicate their poor glycemic control. Several authors have reported the ameliorating actions of different anti-diabetic plants on the HbA1C status of streptozotocin diabetic rat models in short-term studies (Kumar et al., Citation2013; Sharma et al., Citation2011).
Although the duration of this study was short, the significant reduction of the HbA1C levels of the diabetic rats fed cocoyam or unripe plantain establishes the potentials of cocoyam or unripe plantain in the management of diabetes and its complication since the risk of retinopathy and renal complications are directly increased with elevated glycated hemoglobin values. However, the values obtained for the combination of cocoyam and unripe plantain suggest poor management of diabetes mellitus with a combination of unripe plantain and cocoyam at the dosage used in this study.
Hypercholesterolemia and hyper-lipidaemia are recognized complications of diabetes mellitus (Sharma et al., Citation1996; Umesh et al., Citation2004) resulting from alterations in lipid metabolism characterized by elevated levels of cholesterol, triacylglycerol, LDL-cholesterol, and this explains the elevated levels of cholesterol, triacylglycerol, and LDL-cholesterol in the diabetic control rats. The abnormally high concentration of serum lipids in diabetes is mainly due to an increase in the mobilization of free fatty acids from the peripheral depots (Merzouk et al., Citation2000).
Increased VLDL-cholesterol level denotes the increased production of LDL-cholesterol since LDL-cholesterol is formed from VLDL-cholesterol. The effective control of glycemic imbalance will reduce the VLDL-cholesterol and triacylglycerol levels (Lemhadri et al., Citation2006).
Coronary risk is well established by the elevated levels of total cholesterol, especially LDL-cholesterol (Bagri et al., Citation2009). Studies have shown that atherogenic index is a good predictor of cardiovascular disease risk as well as efficient monitor of the effectiveness of lipid-lowering therapies since the LDL-cholesterol/HDL-cholesterol ratio has been considered more prognostic than LDL-cholesterol or HDL-cholesterol alone (Nadeem et al., Citation2013).
The significant reduction of the serum total cholesterol, VLDL-cholesterol, triacylglycerol, LDL-cholesterol, atherogenic, and coronary risk indices together with the concomitant significant increase in the HDL-cholesterol levels of the diabetic rats fed cocoyam, unripe plantain, or their combination suggests anti-hyperlipidemic actions of cocoyam, unripe plantain, or their combination. However, cocoyam or unripe plantain showed better anti-hyperlipidemic action than when combined at the dosage used in this study.
The triacylglycerol lowering property of cocoyam or unripe plantain or their combination as observed in this study could indirectly have contributed to their anti-hyperglycemic activity through the glucose-fatty acid cycle (Randle et al., Citation1994) which reports that increased supply of triacylglycerol could constitute a source of increased free fatty acid availability and oxidation that can impair insulin action, glucose metabolism and utilization, leading to the development of hyperglycemia as earlier mentioned. Therefore, reduction of triacylglycerol after feeding of cocoyam or unripe plantain would also facilitate glucose oxidation, utilization, and subsequently reduction of hyperglycemia.
Malic enzyme catalyzes the oxidative decarboxylation of cytoplasmic malate (Vasudevan et al., Citation2011; Voet et al., Citation2006). The reaction helps to transfer cytoplasmic oxaloacetate to the mitochondrion which is important in the tricarboxylic acid (TCA) cycle for the formation of citrate with acetyl-CoA for generating malate which can feed the cytosolic gluconeogenic pathway (Bukato et al., Citation1995; Singh et al., Citation2001).
Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate and requires either nicotinamide adenine dinucleotide oxidized (NAD+) or nicotinamide adenine dinucleotide phosphate oxidized (NADP+), producing nicotinamide NADH and NADPH, respectively (Jin et al., Citation2003).
Glucose-6-phosphate dehydrogenase catalyzes the first and rate limiting step in the pentose phosphate pathway (conversion of glucose-6-phosphate to 6-phosphoglucnolactone). It is another crucial enzyme in lipogenesis that supplies NADPH that is required to keep glutathione in its reduced state.
During diabetes, lipogenesis is decreased while lipolysis is increased in the hepatic tissue which results from the underutilization of glucose resulting in increased lipolysis and stimulation in the activities of gluconeogenic enzymes (Gomathi & Andmalarvili, Citation2009) and this explains the reduced hepatic levels of ME, IDH, and G6PD in the diabetic control rats. The increased hepatic ME and IDH activities of the diabetic rats fed cocoyam or unripe plantain indicates better utilization of energy yielding intermediates by the TCA cycle.
The decrease in the hepatic levels of G6PD of the diabetic rats could lead to decreased generation of NADPH needed for the regeneration of glutathione and other reducing agents needed for the maintenance of tissue integrity and oxidative balance while the increased hepatic levels of this enzyme in the diabetic rats fed cocoyam, unripe plantain, or their combination are an indication of improvement in glucose utilization by the pentose phosphate pathway.
To make a better comparison of change in weight, the percentage change in weight was calculated. The higher percentage loss of weight of the diabetic control rats compared with the non-diabetic rats despite their increased feed intake can be explained on the basis of increased muscle wasting and loss of structural proteins as these structural proteins are the known to be major contributors to body weight.
The increased body weights of the diabetic rats fed cocoyam or unripe plantain compared with the diabetic control rats indicate the protective action of cocoyam or unripe plantain against muscle wasting and also suggest that control over muscle wasting may have resulted from glycemic control (Ramesh et al., Citation2011).
Alkaloids and tannins are polyphenolic compounds with antioxidant properties. There are indications that apart from scavenging of free radicals, polyphenols could also act by direct interactions with important cellular receptors or key signaling pathways leading to the modification of the redox status of cells which results in the triggering of a series of redox-dependent reactions (Umesh et al., Citation2004). In addition, tannins exert their action on carbohydrate metabolism through the inhibition of α-glucosidase and α-amylase, the key enzymes responsible for the digestion of dietary carbohydrates to glucose (Umesh et al., Citation2004). The higher amounts of these polyphenolic compounds in the unripe plantain or cocoyam feeds compared with their combination and the standard rat feeds could be one explanation for the higher antidiabetic actions shown by unripe plantain or cocoyam in this study compared with their combination or the standard rat feeds.
Conclusion
This study demonstrated the anti-diabetic and anti-hyperlipidemic actions of cocoyam, unripe plantain, or their combination. Furthermore, the study revealed that a combination of cocoyam and unripe plantain at the dosage used in the dietary management of diabetes and related complication may not be very effective compared with the plants used individually. This may be as a result of its lower amounts of polyphenolic compounds compared with unripe plantain or cocoyam. This study therefore underscores the need to try different doses of combination of cocoyam and unripe plantain to establish a case of antagonism between these plants.
Declaration of interest
The authors report that they have no conflicts of interest. The authors wish to thank the management of National Root Crops Research Institute, Umudike, Nigeria, for sponsoring this study.
References
- Akomolafe OM, Aborisade AT. (2007). Effects of stimulated storage conditions on the quality of plantain (Musa paradisiaca) fruits. Int J Agric Res 2:1037–42
- Association of Official Analytical Chemists. (1990). Official methods of analysis of the Association of Official Analytical Chemists. In: W Horwitz, ed. Official Methods of Analysis, 13th edn. Washington, DC: Association of Official Analytical Chemists, 233–4
- Bagri P, Ali M, Aderi V, et al. (2009). Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidermia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol 47:50–4
- Bukato G, Kochan Z, Swierczynski J. (1995). Purification and properties of cytosolic and mitochondrial malic enzyme isolated from human brain. Int J Biochem Cell Biol 27:47–54
- Chhanda M, Rajkumar M, Debidas G. (2006). Comparative study on the anti-hyperglycemic and anti-hyperlipidemic effects of separate and composite extracts of seed of Euglena jambolana and root of Musa paradisciaca in streptozotocin induced diabetic male albino rats. J Pharmacol Ther 85:27–33
- Cosson E, Chiheb S, Cussac-Pillegand C, et al. (2013). Haemoglobin glycation may partly explain the discordance between HbA1c measurement and oral glucose tolerance test to diagnose dysglycaemia in overweight/obese subjects. Diabetes Metab 39:118–25
- Eleazu CO, Iroaganachi M, Eleazu KC. (2013). Ameliorative potentials of cocoyam (Colocasia esculenta L.) and unripe plantain (Musa paradisiaca L.) on the relative tissue weights of streptozotocin-induced diabetic rats. J Diabetes Res 2013:1–8
- Friedewald WT, Levy RI, Fredrickson DS. (1972). Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–505
- Geer BW, Krochko D, Oliver MJ, et al. (1980). A comparative study of the NADP-malic enzymes from Drosophila and chick liver. Comp Biochem Physiol 65B:25–34
- Gomathi N, Andmalarvili T. (2009). Effect of Hibiscus rosasinensis on carbohydrate metabolizing enzymes in monosodium glutamate induced obesity in female rats. J Cell Tissue Res 9:1969–74
- Harbone JB. (1973). Phytochemical methods, a guide to modern techniques of plants analysis. In: Harbone JB, ed. Comparative Biochemistry of the Flavonoids. New York: Academic Press, 221–2
- Haseena B, Kaladevi S, Varun V, et al. (2011). Ameliorating effect of Semecarpus anacardium on TCA cycle enzymes in high fat diet STZ induced Type 2 Diabetic rat model. J Pharm Res 4:4577–80
- Ime FA, Atangwho IJ, Regina I, et al. (2011). Hypoglycaemic effect and proximate composition of some selected Nigerian traditional diets used in management of diabetes mellitus. Eur J Food Res Rev 1:94–101
- Jin HL, Eun SY, Jeen-Woo P. (2003). Enzyme catalysis and regulation. Alcohol-induced liver injury implications for cytotoxicity and isocitrate dehydrogenase by peroxynitrite: Inactivation of NADP+-dependent. J Biol Chem 278:16168–76
- Karl J, Burns G, Engel WD, et al. (1993). Development and standardization of a new immunoturbidimetric assay HbA1c Assay. Klin Lab 39:991–6
- Kumar V, Danish A, Firoz A, et al. (2013). Enhanced glycemic control, pancreas protective, antioxidant and hepatoprotective effects by umbelliferon-α-d-glucopyranosyl-(2I→1II)-α-d-glucopyranoside in streptozotocin induced diabetic rats. Springer Plus 2:1–20
- Lemhadri A, Hajji L, Michel J, Eddouks M. (2006). Cholesterol and triglyceride lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol 106:321–6
- Merzouk H, Madani S, Chabane S, et al. (2000). Time course of changes in serum glucose, insulin, lipids and tissue lipase activities in macrosomic offspring of rats with Streptozotocin induced diabetes. Clin Sci (Lond) 98:21–30
- Mwenye OL, Herselman MT, Benesi L, Chipungu FP. (2010). Ethno-Botanical and Morphological Characterisation of Cocoyams (Colocasia esculenta L. Schott and Xanthosoma sagittifolum L. Schott) germplasm in Malawi. Second RUFORUM Biennial Meeting Entebbe, Uganda, 193–99
- Nadeem I, Muhammad S, Yousaf K, et al. (2013). Anti-hyperlipidemic and renoprotective activities of methanolic extract of Canscora decussata extract in alloxan-induced diabetic rabbits. Bangladesh J Pharmacol 8:323–7
- National Research Council (NRC). (1985). Guide for the Care and Use of Laboratory Animals. Bethesda (MD): National Institute of Health, 8523
- NCEP. (2001). Third report of the national cholesterol education programme. Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult treatment panel 111). JAMA 285:2486–97
- Niba LL. (2003). Processing effects on susceptibility of starch to digestion in some dietary starch sources. Int J Food Sci Nutr 54:97–109
- Noltmann EA, Gubler CJ, Kuby SA. (1961). Glucose-6-phosphate dehydrogenase (Zwischenferment) isolation of the crystalline enzyme from yeast. J Biol Chem 236:1225–30
- Ojewole JA, Adewunmi CO. (2003). Hypoglycemic effect of methanolic extract of Musa paradisiaceae (Musaceae) green fruits in normal and diabetic mice. Methods Find Exp Clin Pharm 25:453–6
- Omonkhua A, Onoagbe I, Ajileye A, et al. (2013). Long term antidiabetic, anti-hyperlipidaemic and anti-atherogenic effects of Carica papaya leaves in streptozotocin diabetic rats. Eur J Med Plants 3:508–19
- Ramesh B, Karuna R, Sreenivasa R, et al. (2011). Anti-hyperglycemic and antioxidant activities of alcoholic extract of Commiphora mukul gum resin in streptozotocin induced diabetic rats. Pathophysiology 18:255–61
- Randle PJ, Priestman DA, Mistry SC. (1994). Glucose-fatty acid interactions and the regulation of glucose disposal. J Cellular Biochem 55:1–11
- Sharma SR, Dwivedi SK, Swarup D. (1996). Hypoglycemic and hypolipidaemic effects of Cinnamomum tamala Nees leaves. Indian J Exp Biol 34:372–4
- Sharma M, Siddique M, Akhter M, et al. (2011). Evaluation of antidiabetic and antioxidant effects of seabuckthorn (Hippophae rhamnoides L.) in streptozotocin-nicotinamide induced diabetic rats. Open Conf Proc J 2:53–8
- Singh SN, Praveen V, Shoba S, et al. (2001). Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol 76:269–77
- Tietz NW. (1995). Clinical Guide to Laboratory Tests. 3rd edn. Philadelphia, PA: WB Saunders Company
- Umesh CS, Yadav KM, Najma ZB. (2004). Effects of sodium-orthovanadate and Trigonella foenum Graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J Biosci 29:81–91
- Vasudevan DM, Sreekumari S, Vaidyanathan K. (2011). Textbook of Biochemistry for Medical Students, 6th edn, 2nd edn. New Delhi, India: Jaypee Brothers Medical Publishers Pvt. Ltd
- Vaz JA, Patnaik A. (2012). Diabetes mellitus: Exploring the challenges in the drug development process. Perspect Clin Res 3:109–12
- Voet D, Voet J, Pratt C. (2006). Fundamentals of Biochemistry. Life At The Molecular Level, 2nd ed. New York: John Wiley and Sons, Inc
- World Health Organization. (2003). Screening for Type 2 Diabetes. Report of a World Health Organization and International Diabetes Federation Meeting, Geneva, 120
- Zhang Z, Feng CF. (2007). Non-enzymatic glycosylation reaction contributes to a rise of blood glucose in alloxan-induced diabetic rats. Int J Diabetes Metab 15:52–9

