Abstract
Context: The roots of Phytolacca americana L. (Phytolaccaceae) may be toxic. Despite heated controversy over the toxic compounds of P. americana, especially esculentosides, relevant studies remain scarce.
Objective: The objective of this study is to screen the toxic fractions and compounds of P. americana, to determine the controlling indices, and to provide evidence for unraveling the mechanism.
Materials and methods: Petroleum ether (PE), CH2Cl2, n-BuOH, and water fractions were isolated from 70% ethanol extract of P. americana. The n-BuOH fraction was dissolved in 50% ethanol and precipitated by adding ethyl ether. The resultant supernatants and precipitates were referred to as SUPs and SEDs fractions, respectively. SUPs fraction was separated by column chromatography into four main stimulating esculentosides that were identified by HR-ESI/MS and NMR as EsA, EsB, EsC, and EsF. The irritating effects of esculentosides on rabbit conjunctivae (500 μg/eye) was observed by pathological examination and those on macrophages (5, 25, 50 and 100 μg/mL) were evaluated by detecting changes of NO, TNF-α, and IL-1β levels.
Results and discussion: n-BuOH, SUP fractions, and EsC induced severe conjunctival edema. The four esculentosides induced dose-dependent releases of proinflammatory mediators NO, TNF-α, and IL-1β from macrophages, and releasing amounts peaked after 2 h of treatment. EsC and EsF induced macrophages to release mediators most significantly. EsC (50 μg/mL) functioned more effectively than EsF did, and similarly n-BuOH and SUPs fractions functioned more effectively than the esculentoside mixture. Thus, the four esculentosides exerted proinflammatory effects synergistically.
Conclusion: All extracted esculentosides, especially EsC, induced inflammatory stimulation. Phytolacca americana-induced irritation of the gastrointestinal tract may be associated with esculentosides such as EsC.
Introduction
Generally drugs have toxic and therapeutic effects simultaneously; therefore, traditional Chinese medicine (TCM), although relatively safe with few side effects, may also be toxic. The security of TCM has been spotlighted since the incidence of adverse events (Wang et al., Citation2012). Meanwhile, as complicatedly composed, the toxicity and side effects of TCM cannot be accurately explained.
Although the roots of Phytolacca americana L. (Phytolaccaceae) have commonly been used to treat systemic edema and ascites fullness (China Pharmacopoeia Committee, Citation2010; Qiao, Citation2010; Ye et al., Citation2011; Yuan et al., Citation1991), they were recorded as toxic since ancient times due to considerable side effects (China Pharmacopoeia Committee, Citation2010; Ye et al., Citation2011; Yuan et al., Citation1991). After being poisoned, patients suffer from sympathetic excitement (Chen, Citation1993; Zhang, Citation2011) and gastrointestinal irritation (Chen, Citation2010) within 20 min to 3 h (Wu & Li, Citation2008), mainly manifested as dizziness, palpitation, nausea, vomiting, diarrhea, and abdominal pain (Guo et al., Citation2005; Hu et al., Citation2011). Particularly, diarrhea is manifested as typical exudative type in the case of hemafecia (Field, Citation2003). Hence, P. americana should be processed cautiously (Ye et al., Citation2011) to be clinically applicable.
It has been previously reported that P. americana may lead to clinical nausea and vomiting by inducing the necrosis and shedding of local gastric epithelial cells (Chen et al., Citation2011). The extracts of P. americana also induced severe conjunctival edema of rabbits and proinflammatory mediator release of mice (Chen et al., Citation2011; Gong et al., Citation2013). As suggested by the exudative diarrhea upon poisoning, P. americana can result in intestinal mucosal inflammation (Bruckstein, Citation1988; Field, Citation2003), which is generally accompanied by the release of proinflammatory mediators.
Despite heated controversy over the toxic compounds of P. americana, especially esculentosides, relevant studies remain scarce. Therefore, we herein established a proinflammatory model to screen the toxic fractions and compounds of P. americana, to determine the controlling indices, and to provide evidence for unraveling the mechanism. Moreover, the toxic compounds were structurally analyzed and identified.
Materials and methods
Animals
NZW rabbits and male ICR mice were obtained from animal facilities of Nanjing Medical University, housed in a temperature-controlled room and received water and food ad libitum. Animal welfare and experimental procedures were strictly followed according to the Guide for the Care and Use of the Laboratory Animals and the related ethics regulations of Nanjing University of Chinese Medicine.
Chemicals and reagents
Analytical grade ethanol, EtOAc, petroleum ether (60–90 °C), dichloromethane, butanol, ethyl ether, and methanol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were used for extraction and isolation. Silica gel (Qingdao Ocean Chemical Industry, Qingdao, China) was used for column chromatography. Dimethylsulfoxide (DMSO) was purchased from Sinopharm Chemical Reagent Co., Ltd. (AR grade, Shanghai, China). RPMI 1640 was purchased from GIBCO (Waltham, MA), and FCS was bought from SIJIQING (Hang Zhou, China). Trypan blue and No kit were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, PR China). Non-specific esterase stain was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing Jiancheng Bioengineering Institute). TNF-α and IL-1β ELISA kits were purchased from Ebio-Science (San Diego, CA), and 24 well Transwell sets and 48 well plates were purchased from Coring (Boom City, NY). All the reagents were of at least analytical grade. Saline was purchased from Nanjing Xiaoying Pharmaceutical Group Co., Ltd (Nanjing, PR China). D-Hanks buffer was laboratory made.
Instrumentation
NMR spectra were recorded on Bruker AVANCE AV 300 instrument operated at 300 MHz for 1H and 75 MHz for 13C using standard pulse sequences. Chemical shifts are reported on the δ scale in parts per million, with TMS as an internal standard. Electrospray ionization mass spectra (ESI-MS) were obtained on a Q-TOF micromass spectrometer (Waters Company, Milford, MA). Thin-layer chromatography (TLC) was performed on Qingdao Ocean TLC plates (0.25 mm thick, Qingdao Ocean Chemical Industry, Qingdao, China), with compounds visualized by spraying 10% (v/v) H2SO4 in ethanol solution and then heating on a hot plate. Laminar flow clean bench (Model: SW-CJ-1G) was purchased from Suzhou Antai Airtech Co., Ltd. (Suzhou, China). Microplate Reader (SPECTRA MAX 190) was obtained from Molecular Devices (Sunnyvale, CA).
Plant material
The roots of P. americana were collected from Shanxi Province, China with the help of Bozhou Yonggang Pieces Factory Co., Ltd. (Anhui, China) on 9 June 2011. The crude plant was identified by Professor Chungen Wang (Nanjing University of Chinese Medicine, Nanjing, China).
Extraction and isolation
The dried and crushed roots of P. americana (10.0 kg) were extracted with 75% EtOH in a 80 °C water bath. The supernatant was separated every day, and the solvent was removed under reduced pressure. Then the residue (3.0 kg) was partitioned with petroleum ether, dichloromethane, n-butanol saturated with water and water to provide PE fraction (122.4 g), CH2Cl2 fraction (130.4 g), n-BuOH fraction (1.412 kg), and water fraction (200.3 g).
To further enrich the toxic components, n-BuOH fraction was dissolved in 50% ethanol solution, into which was gradually added the mixture of ethyl ether and ethanol solution (v/v: 10/1), yielding precipitates (SEDs fraction, 1.25 kg) and supernatants (SUPs fraction, 124.5 g). The organic solvents in SUPs fraction were recycled.
SUPs fraction of P. americana (124.5 g) was chromatographed over a silica gel (1.25 kg) and eluted with EtOAc:MeOH (15:1) to give 40 fractions (FB1–FB40). FB17–FB34 (0.2 g) were combined and purified by sephadex LH-20 (20 g) chromatography with CH2Cl2:MeOH (1:1) to give compound 1 (140 mg). FC (19.6 g) was separated over silica gel chromatography (320 g) and eluted with EtOAc: MeOH (8:1) to yield 35 fractions (FC1–FC35). FC18–FC24 (0.6 g) were combined and purified by Sephadex LH-20 (20 g) chromatography with CH2Cl2:MeOH (1:1) to give compound 2 (580 mg). FD (12.1 g) was applied to a silica gel chromatography (180 g) with EtOAc–MeOH (5–1) to enrich gradually with EtOAc to obtain 30 fractions (FD1–FD30). FD3–FD6 (0.48 g) were combined and separated over ODS-C18 chromatography (20 g), eluted with MeOH–H2O (0:1–1:0) to obtain 10 fractions (Fr 1–10). Fr 5–8 (0.15 g) were combined and then purified by Sephadex LH-20 chromatography (20 g) with CH2Cl2:MeOH (1:1) to give compound 3 (10.6 mg). At last, FD8–FD18 (0.32 g) were combined and purified by Sephadex LH-20 chromatography (20 g) with CH2Cl2–MeOH (1:1) and continuous pTLC with CH2Cl2:Me2CO (2: 1) to give compound 4 (26.1 mg).
Rabbit conjunctivae test
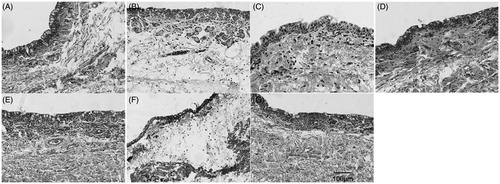
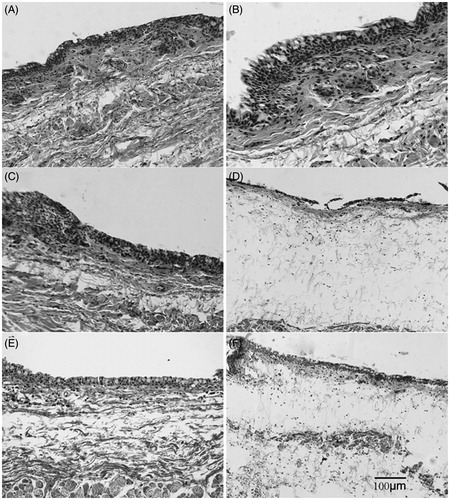
Rabbits were randomly divided into a normal saline group, six different isolating fraction groups (1.5 g/mL, suspended in normal saline or dissolved), four esculentoside groups (10 mg/mL, suspended in normal saline or dissolved), and a group of mixed esculentosides (three eyes each group) (18 rabbits in total). The rabbits were fixed in cages with their upper and lower eyelids raised, into which drug solutions were added dropwise (50 μL in each eye). After administration, the eyelids were passively closed for 10 s, 10 min after which the eyes were rinsed by 20 mL of normal saline. The eyes were observed 3 h later, prepared into conjunctival tissue sections, and were subjected to HE staining (Draize et al., Citation1944).
Separation and purification of macrophages
ICR mice were executed by cervical dislocation, immersed in 75% ethanol solution for 3 min, and intraperitoneally injected with 4 mL D-Hanks buffer. After 1 min of gentle abdominal massage, the abdominal fluid was collected and centrifuged at 1500 rpm for 10 min, yielding precipitates as the cell layer rich in macrophages. The cells were resuspended in RPMI 1640 culture medium containing 10% fetal bovine serum, adjusted to the density of 1 × 106/mL, inoculated in 48-well plates, and cultured at 37 °C in 5% CO2 for 2 h. After removing the non-adherent cells by D-Hanks buffer, the remaining ones were further cultured as the purified macrophages. As determined by trypan blue staining and non-specific esterase staining, the survival rate and the purity of the macrophages were 99% and 98%, respectively (Zhou et al., Citation2005).
Determination of NO released from macrophages
The prepared macrophages were divided into seven groups (six wells for each group). The six study groups were added PE fraction, CH2Cl2 fraction, n-BuOH fraction, water fraction, supernatant of treated n-BuOH fraction, and precipitate of treated n-BuOH fraction at identical concentrations (50 μL each). The control group was added the same volume of D-HANKS solution. Then 450 μL of cell culture medium was added in each group to adjust the final drug concentration to 50 μg/mL, and the resultant solution was placed at 37 °C for another 4 h. concentrations were determined using the Griess reaction according to Tracey et al. (Citation1995). The results were expressed as μmol/L.
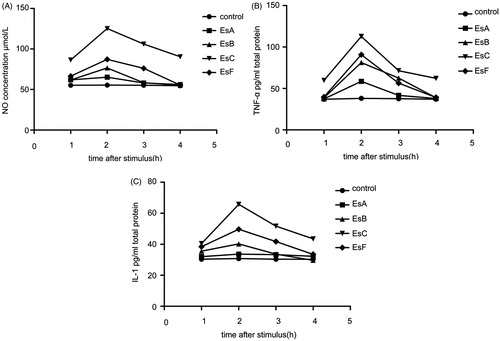
Dose–response and time–response relationships between esculentosides and release of TNF-α, IL-1β, and NO
EsA, EsB, EsC, and EsF were isolated from SUPs fraction, dissolved with 0.25% DMSO, and filtered. In dose–response relationship experiments, five groups were set for each esculentoside (six wells for each group). Different concentrations of EsA, EsB, EsC, and EsF were added and diluted to 5, 25, 50, and 100 μg/mL with D-HANKS solution. The control group was added D-HANKS solution containing 0.25% DMSO. The contents of TNF-α, IL-1β, and NO were measured after 4 h. In time–response relationship experiments, eight groups were set for each esculentoside (six wells for each group). For each group, 50 μg/mL EsA, EsB, EsC, and EsF were added. The control group was added D-HANKS solution containing 0.25% DMSO. The contents of TNF-α, IL-1β, and NO were measured at 1, 2, 3, and 4 h, respectively. The contents of TNF-α and IL-1β were determined by Elisa. The results were expressed as pg/mL.
Comparisons between the effects of esculentosides on TNF-α, IL-1β, and NO
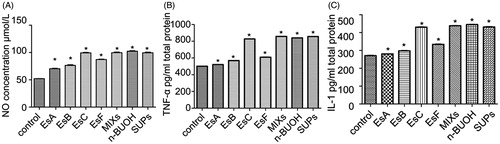
Macrophages were divided into eight groups (six wells for each group), and the study groups were stimulated by EsA, EsB, EsC, EsF, their mixture in equal proportions, n-BuOH fraction, and SUPs fraction, respectively (final concentrations: 50 μg/mL). The contents of TNF-α, IL-1β, and NO were determined after 2 h.
Statistical method
All data were analyzed by statistical software SPSS 16.0 (SPSS Inc., Chicago, IL) and expressed as (mean ± SEM). Inter-group comparisons were performed by using independent two-sample t-test, and the comparisons among multiple groups employed analysis of variance and test for homogeneity of variance.
Results
Effects of isolated fractions on rabbit conjunctival edema
Petroleum ether (PE), CH2Cl2, n-BuOH, and water fractions, which were isolated from the 70% ethanol extract of P. americana, were used to test the irritating effects on rabbit conjunctivae. shows that n-BuOH fraction induced conjunctival edema most obviously, while the other three barely exerted effects. Therefore, n-BuOH fraction was dissolved in 50% ethanol and precipitated by adding ethyl ether, with the resultant supernatant and precipitates referred to as SUPs and SEDs fractions, respectively. SUPs fraction induced intense edema, whereas SEDs fraction failed to so, indicating that the toxicity of n-BuOH fraction was concentrated in SUPs fraction.
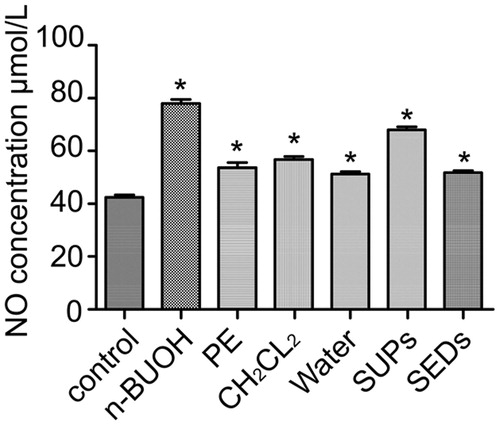
Effects of isolated fractions on nitric oxide (NO) release from macrophages
Compared with the control group, all fractions stimulated macrophages extremely significantly (p < 0.01; ). Besides, n-BuOH and SUPs fractions were more stimulating than other fractions. The findings are consistent with those of rabbit conjunctivae.
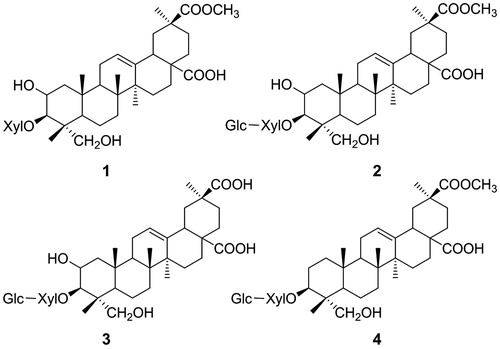
Identification of compounds 1–4
Compounds 1–4 were all of previously known structures (). They were identified as esculentoside B (1), esculentoside A (2), esculentoside C (3), and esculentoside F (4), respectively, by comparison of their spectral (UV, IR, NMR, and MS, Supplementary Material Tables 1–5) and/or mp data with those of corresponding authentic samples or data from the literature (Liu, Citation2006; Takahashi et al., Citation2001; Wang & Yi, Citation1984).
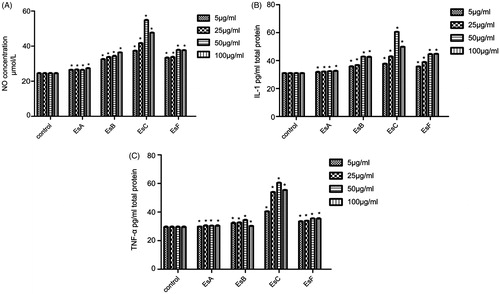
Effects of esculentosides on the release of NO, TNF-α, and IL-1β from macrophages
With increasing doses of the four main esculentosides isolated from SUPs fraction, more NO, TNF-α, and IL-1β were released from macrophages (), suggesting that they were all proinflammatory. Maximum NO and TNF-α were released with 50 and 100 μg/mL esculentosides, while maximum IL-1β was released at 50 μg/mL. In addition, the releasing amounts of NO, TNF-α, and IL-1β became evident after 1 h of treatment and peaked after 2 h ().
Comparisons among the effects of esculentosides, n-BuOH fraction, and SUPs fraction on the release of NO, TNF-α, and IL-1β from macrophages
Of the four esculentosides, EsC and EsF induced macrophages to release NO, TNF-α, and IL-1β most significantly (p < 0.01; ). Meanwhile, EsC functioned more effectively than EsF did and similarly to the esculentoside mixture. The proinflammatory effect of EsC was comparable with those of n-BuOH and SUPs fractions.
Figure 6. Effects of esculentosides, n-BuOH fraction, and SUPs fraction on the release of NO, TNF-α, and IL-1β from macrophages. EsC functioned more effectively than EsF did and similar to the esculentoside mixture did. The proinflammatory effect of EsC was equivalent to those of n-BuOH and SUPs fractions. Results are reported as mean ± SEM (n = 6). *p < 0.01, compared with the control.
Comparisons between the irritating effects of esculentosides on rabbit conjunctival edema
Of the four esculentosides, only EsC gave rise to severe rabbit conjunctival edema (), revealing that EsC was significantly proinflammatory. The results are consistent with those of macrophages in vitro. Nevertheless, when mixed in equal proportions, the four esculentosides exhibited similar irritating effects to EsC did, inferring that they functioned synergistically.
Discussion
Drugs are bound to induce adverse reactions in human gastrointestinal tract such as diarrhea (Chassany et al., Citation2000; Zhao et al., Citation2011). Phytolacca americana is a commonly used traditional Chinese medicine, but it may lead to diarrhea by significantly irritating the gastrointestinal tract. Therefore, the roots of P. americana should be processed to alleviate diarrhea (Ye et al., Citation2011) that occurs for complicated reasons. In this study, proinflammatory fractions and esculentosides were isolated from 75% ethanol extract.
By establishing a proinflammatory model, we found that SED fractions isolated from n-BuOH fractions exerted less significant stimulating effects on rabbit conjunctivae and macrophages than SUP fractions did, suggesting that precipitation with ethyl ether enriched the toxic components in SUP fractions. Moreover, the content of EsC in SUP fractions was significantly higher than that of n-BuOH fractions.
The four isolated esculentosides all stimulated macrophages to release NO, TNF-α, and IL-1β. Particularly, only EsC induced the releases of the three inflammatory mediators dose dependently. Of the four esculentosides, EsC was most proinflammatory while dramatically stimulating macrophages to release NO, TNF-α, and IL-1β, which was followed by EsF and EsA descendingly. Meanwhile, only EsC induced severe conjunctival edema. Hence, EsC exerted the most potent proinflammatory effects.
The toxicity of TCM depends significantly on the structures of certain ingredients (Cai et al., Citation1990, Citation1998). EsA, which can inhibit cell growth and ICAM-1 induction, accumulate calcein in cells (Wang et al., Citation2008) and treat nephritis (Ju et al., Citation1999), did not show obvious proinflammatory or irritating effects herein. Since the content of EsA remained constant (Chen et al., Citation2011) while that of EsC reduced appreciably after vinegar processing, the toxicity of P. americana may be associated more with EsC than with EsA. The results may be ascribed to the missing hydroxyl group at the of C2 position of EsC. Regardless, further studies on the structure–activity relationship are still in need.
In this study, the four esculentosides mixed in equal proportion had similar effects on macrophages to EsC at the same dose did. In addition, significantly less inflammatory factors were released in the presence EsA, EsB, and EsF than those released in the presence of the mixture of EsA, EsB, EsC, and EsF in equal proportion. They showed the same effects on the rabbit conjunctivae as those on macrophage-released inflammatory factors. The results indicated that the four esculentosides worked synergistically in inducing inflammation, which has never been reported before.
We herein established a proinflammatory model to screen the toxic fractions and compounds of P. americana. Macrophages participate in natural innate immunity by phagocytizing and degrading antibody-coated and complement-opsonized foreign particles such as microbes and antigens, as well as by killing microbes through secretion of enzymes, reactive oxygen radicals, and lipid-derived mediators (Pathmakanthan & Hawkey, Citation2000). Macrophages are also the principal effector cells of cell-mediated immunity secreting a host of cytokines. In this study, all extracted esculentosides, especially EsC, stimulated macrophages to evidently release NO, TNF-α, and IL-1β. It has previously been reported that TNF-α secreted from macrophages-like cells caused cell damage to human intestinal epithelial-like Caco-2 monolayers. The disrupted Caco-2 monolayers showed both apoptotic and necrotic characteristics, as evidenced by morphological and biochemical analyses (Satsu et al., Citation2006). This phenomenon has also been observed in the intestinal mucosa of inflammatory bowel disease patients (Souza et al., Citation2005). Activated macrophages can inhibit enterocyte gap junctions by releasing NO, which suggests macrophage-secreted TNF-α and NO can lead to intestinal epithelial cell death or inhibit enterocyte gap junctions, thus inducing mucosal barrier dysfunction and diarrhea. Therefore, the findings in this study indicate that P. americana-induced irritation of the gastrointestinal tract may be associated with esculentosides such as EsC.
Supplementary material available online
Supplementary Material Tables 1–5
Declaration of interest
This work was supported by Special Scientific Research for Traditional Chinese Medicine of State Administration of Traditional Chinese Medicine of China (Nos. 201207004-1 and 200807039) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- Bruckstein AH. (1988). Acute diarrhea. Am Fam Physician 38:217–28
- Cai BC, Hattori M, Namba T. (1990). Processing of nux vomica. II. Changes in alkaloid composition of the seeds of Strychnos nux-vomica on traditional drug-processing. Chem Pharm Bull (Tokyo) 38:1295–8
- Cai BC, Wang TS, Kurokawa M, et al. (1998). Cytotoxicities of alkaloids from processed and unprocessed seeds of Strychnos nux-vomica. Zhongguo Yao Li Xue Bao 19:425–8
- Chassany O, Michaux A, Bergmann JF. (2000). Drug-induced diarrhoea. Drug Saf 22:53–72
- Chen JX. (2010). Clinical analysis on 26 cases of integrated Traditional Chinese and Western Medicine in treating acute Phytolacca acinosa poisoning. J Zhejiang Univ Trad Chin Med 34:221
- Chen L, Wu H, Wang M, Shi RJ. (2011). Comparative study of mucosa irritation of crude and processed Radix Phytolaccae. China J Chin Materia Medica 36:859–63
- Chen L, Wu H, Wang M, et al. (2011). Processing technique of Phytolaccae Radix stir-baked with vinegar. Chin Tradit Herbal Drugs 42:1101–4
- Chen XC. (1993). A case of Phytolacca americana L. poisoning. Hunan Tradit Chin Med 9:39–40
- China Pharmacopoeia Committee. (2010). Chinese Pharmacopoeia, Beijing, 304
- Draize JH, Woodward G, Calvery HO. (1944). Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther 82:377–90
- Field M. (2003). Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111:931–43
- Gong L, Wu H, Yu HL, et al. (2013). Comparative study on toxicity of extracts from Phytolaccae radix before and after being processed with vinegar. China J Chin Materia Medica 38:6–9
- Guo BK, Zhang FQ, Zhang L, Liu Y. (2005). Clinical analysis on 10 cases of acute pokeberry root poisoning. Chin J Ind Med 18:32–3
- Hu Y, Zeng CY, Mei QX. (2011). 82 Cases of acute poisoning of Phytolacca americana L. Lishizhen Med Materia Medica Res 22:3041
- Ju DW, Zheng QY, Cao XT, et al. (1999). Therapeutic effects of esculentoside A on passive Heymann nephritis in rats and its inhibition on cytokine production. Acta Pharm Sin 34:9–12
- Liu JQ. (2006). The isolation and vitro antineoplasmic activity of compounds from Radix Phytolacca acinosa [master's thesis]. Jilin, China: Jilin University, 21–2
- Pathmakanthan S, Hawkey CJ. (2000). A lay doctor’s guide to the inflammatory process in the gastrointestinal tract. Postgrad Med J 76:611–17
- Qiao FK. (2010). Clinical application experience of ancient modern famous doctor treatment of edema using Shanglou. J Chin Med 25:933–4
- Satsu H, Ishimoto Y, Nakano T, et al. (2006). Induction by activated macrophage-like THP-1 cells of apoptotic and necrotic cell death in intestinal epithelial Caco-2 monolayers via tumor necrosis factor-alpha. Exp Cell Res 312:3909–19
- Souza HS, Tortori CJ, Castelo-Branco MT, et al. (2005). Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: Evidence of altered expression of FasL and perforin cytotoxic pathways. Int J Colorectal Dis 20:277–86
- Takahashi H, Namikawa Y, Tanaka M, Fukuyama Y. (2001). Triterpene glycosides from the cultures of Phytolacca americana. Chem Pharm Bull (Tokyo) 49:246–8
- Tracey WR, Tse J, Carter G. (1995). Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: Pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther 272:1011–15
- Wang L, Bai L, Nagasawa T, et al. (2008). Bioactive triterpene saponins from the roots of Phytolacca americana. J Nat Prod 71:35–40
- Wang YG, Ma ZC, Liang QD, et al. (2012). Thoughts and methods for toxicity study on Chinese materia medica. Chin Tradit Herbal Drugs 43:1875–9
- Wang ZL, Yi YH. (1984). Studies on the active principles of the Chinese drug “shanglu” (Phytolacca esculenta Van Houtte). II. The isolation and structure of esculentoside E and F. Yao Xue Xue Bao 19:825–9
- Wu ZM, Li Y. (2008). Phytolacca americana L. poisoning and identification. China Pharm 17:60–1
- Ye DJ, Zhang SC, Wu H. (2011). Science of Chinese Medicine Processing, 2nd ed. Beijing: People’s Medical Publishing House Co., Ltd, 402–4
- Yuan ST, Wang ZJ, Cheng M. (1991). Development of traditional Chinese medicine Phytolacca americana L. (I). J Chin Med Mater 14:42–9
- Zhang JH. (2011). 12 cases of acute poisoning of Phytolacca americana L. Jilin Med J 32:734–5
- Zhao Y, Zuo ZY, Liu X, Sun YZ. (2011). Documentary study on correlation between adverse reactions of Chinese materia medica and their natures. Chin Tradit Herbal Drugs 42:392–7
- Zhou MH, Wang CL, Zhao XR, Cao XH. (2005). Purification and macrophage immunomodulatory activity of lectin from Musca domestica pupae. J Tianjin Univ Sci Technol 25:5–8