Abstract
Context: Pinellia ternata (Thunb.) Berit., a perennial herb belonging to Araceae, is one of the few medicinal plants to produce purine alkaloids. It is speculated that endophytic bacteria from P. ternata may produce guanosine or inosine. However, there is no report about endophytic bacteria in P. ternata.
Objective: In this study, endophytic bacteria were isolated from P. ternata and examined for the first time. This study finds a novel way to increase the yield of P. ternata herb, and to provide some new alkaloid producers.
Materials and methods: Plant material includes leaves, tubers, and roots of cultivated and wild P. ternata. The dilutions were smeared onto beef extract-peptone medium and cultured at 28 °C in darkness for 48–72 h. Co-culture treatments were prepared by inoculating 100 mL liquid 1/2 MS medium with bacterial culture broth at concentrations of 0 (control), 0.5%, and 1.5% (v/v).
Results: Of the 34 endophytic bacterial colonies isolated from P. ternata leaves, roots, and tubers, five strains were able to produce purine alkaloids. Results from 16s rDNA sequence analysis indicated that the bacteria belonged to Bacillus cereus, Aranicola proteolyticus, Serratia liquefaciens, Bacillus thuringiensis, and Bacillus licheniformis. Co-culture with living Serratia liquefaciens cells increased PLB growth by 58–71%. Co-culture with living Bacillus licheniformis cells increased PLB growth by 4–11%.
Conclusion: This study provides a novel way for improving the yield of P. ternata herb, and for the production of purine alkaloids by the fermentation industry.
Introduction
Pinellia ternata (Thunb.) Berit., a perennial herb belonging to Araceae, has been used in Chinese traditional medicine for over 2000 years. The herb is used for its anti-emetic, analgesic, and sedative effects (Han et al., Citation2006). Recent reports indicate that P. ternata has anti-anxiety, anticancer, and anti-inflammation effects as well as the ability to induce abortion in early pregnancy (He et al., Citation2007; Hu et al., Citation2008). Alkaloids are the main biologically active compounds in P. ternata. Two purine alkaloids, guanosine and inosine, have been isolated from P. ternata. Guanosine is a water-soluble compound that is commonly found in medicinal herbs (Zhang et al., Citation2005). In contrast, inosine is generally found in animals or microorganisms (Wu et al., Citation2003). Both alkaolids are known to have antitumor and antivirus activities (Kinahan et al., Citation1981).
Plant endophytes can produce the same or similar secondary metabolites as their host plants (Li et al., Citation1998). It is speculated that endophytic bacteria from P. ternata may produce guanosine or inosine. However, there is no report about endophytic bacteria in P. ternata.
Demand for P. ternata is increasing in recent years. The supply cannot keep pace with the increasing demand. It was reported plant endophytes could promote the growth of their host plant (Hinton & Bacon, Citation1995). We speculated endophytes of P. ternata may enhance the growth of the herb.
In this study, endophytic bacteria were isolated from P. ternata for the first time. Then the bacteria were screened to determine their capacity for producing guanosine or inosine. Strains which produced either of the two alkaloids were identified through 16s rDNA sequencing. Two bacteria strains, Serratia liquefaciens and Bacillus licheniformis, were co-cultured with PLBs of P. ternata to test their effect on the growth of PLBs. This study attempts to find a novel way to increase the yield of P. ternata herb, and to provide some new alkaloid producers.
Materials and methods
Plant material
Plant material includes both cultivated and wild P. ternata. Cultivated tubers, 0.5–1.5 cm in diameter, were collected in August and September 2010, from P. ternata grown in experimental fields at Northwest A&F University, Yangling, China. Wild tubers, 1.0–1.5 cm in diameter, were collected in October 2010, from P. ternata grown in Zhenping County, Ankang, China. Pinellia ternata is identified by Professor Yuejin Zhang. A voucher specimen of the plant is deposited at the Department of Life Science, Northwest A & F University.
Isolation of endophytic bacteria from P. ternata
Fresh leaves, tubers, and roots were rinsed with tap water and air-dried. Roots, leaves, and tubers were cut into 1 cm, 1 × 1 cm2, and 0.5 × 1 × 1 cm3 segments, respectively. The tissue segments were surface-sterilized in 75% (v/v) ethanol for 1 min, 0.1% (m/v) mercuric chloride solution for 4–6 min (roots and leaves 4 min, tubers 6 min), and rinsed three times with sterile distilled water, then drained. The tissues (3 g for leaves or roots, 7 g for tubers) were ground in 10 mL distilled water and then 10-fold serial dilutions (10−1–10−4) of the homogenates were prepared using distilled water. The dilutions were smeared onto beef extract-peptone medium and cultured at 28 °C in darkness for 48–72 h. Morphologically, distinct colonies on the culture medium were selected, purified, and then conserved at 4 °C. The control in the experiment was the water which had been used to rinse the sterilized plant material for the last time. An aliquot of this water was smeared onto beef extract-peptone medium and cultured at 28 °C. No microbes were observed growing on the culture medium, indicating that the plant materials had been completely surface-sterilized. Colonies that grew from the plant homogenates must have been endophytic bacteria (Chen et al., Citation2006; He et al., Citation2002).
Selection bacteria production alkaloids of P. ternata
After selection and purification, the bacteria were grown in shaker flasks containing 100 mL liquid Luria–Bertani (LB) medium. The flasks were shaken on an orbital shaker (250 rpm) in darkness at 28 °C for 3 d. At the end of the incubation, Fermentation broths were concentrated, bacterial cells were broken by ultrasonication, and then the fermentation broths were homogenized and centrifuged at 10 000 rpm for 10 min. Chitosan (0.03% v/v) was added to the supernatant to precipitate carbohydrates and then the liquids were put in a refrigerator at 4 °C for 12 h. The supernatants were filtered through a 0.45 μm microfiltration membrane and the subsequent filtrates were analyzed by HPLC. The control in the experiment was the flasks containing 100 mL liquid Luria–Bertani (LB) medium without bacteria.
Guanosine and inosine contents of the fermentation broths were determined using a standard HPLC system equipped with a 1525 binary pump and a 2996 photodiode array detector. The column was a Sunfire C-18 reverse-phase column (Waters Corporation, Milford, MA), 250 mm × 4.6 mm i.d., 5 µm particle diameter, 100 Å average pore size with a mobile phase composed of methanol/water (2:98, v/v). The flow rate was 1.0 mL min−1 and the column temperature was 30 °C. Alkaloid content was quantified by UV absorption at 248 nm for inosine and 254 nm for guanosine. Alkaloid contents were calculated according to the standard curves of reference standards.
Identification of endophytic bacteria
Endophytic bacteria were identified by 16s rDNA sequence analysis. The work was done by Shanghai Shengong Company, which is one of the most authoritative bacteria identification institute in Shanghai, China. After identification, the bacterial strains were deposited at the Microbe Research Center, Northwest A & F University, Yangling, China.
Effect of endophytic bacteria on PLB growth
For the co-culture treatment, culture broth containing living cells was prepared by growing Serratia liquefaciens and Bacillus licheniformis cells in shaker flasks containing liquid LB medium (pH 7.0). The flasks were shaken on an orbital shaker (250 rpm) in darkness at 30 °C for 24 h. The optical density (OD) in the medium reached a maximum value of 0.8 (pH 6.5).
Co-culture treatments were prepared by inoculating 100 mL liquid 1/2 MS medium with bacterial culture broth at concentrations of 0 (control), 0.5, and 1.5% (v/v). After the culture medium was prepared, we transferred 3.0 g of 5-week-old undifferentiated PLBs to each flask. The flasks were placed on an orbital shaker (110 rpm) for 35 d at 25 °C and 12 h white fluorescent light (1200 l×) per day.
The growth index (GI) of the PLBs was calculated over a 35-d culture period by dividing the final PLB fresh weight by the initial PLB fresh weight. The differentiation rate (DR) of the PLBs was calculated by dividing the number of differentiated PLBs by the total number of PLBs (differentiated plus undifferentiated PLBs).
Statistical analysis
All data presented are the average of three replicates. Statistical analysis was confirmed by Duncan's multiple range test (DMRT) using SAS 8.0 software for windows (SAS Inc., Cary, NC).
Results and discussion
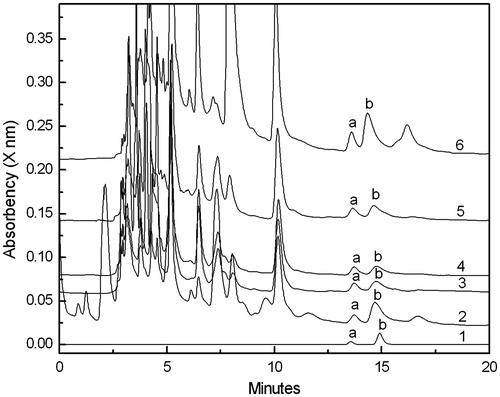
Homogenates from surface sterilized cultivated and wild P. ternata leaves, tubers, and roots were cultured on the beef extract-peptone medium. We identified 34 morphologically distinct colonies growing on the medium. Among these, 14 colonies were isolated from tubers, nine colonies were isolated from roots, and 11 colonies were isolated from leaves. The colonies were cultured separately in the liquid LB medium for 3 d and then water extracts from the fermentation broth were analyzed by HPLC. Results showed that bacteria from five colonies produced guanosine and inosine (). The 16s rDNA sequence analysis indicated that the bacteria belonged to Bacillus cereus, Aranicola proteolyticus, Serratia liquefaciens, Bacillus thuringiensis, and Bacillus licheniformis.
Figure 1. Guanosine and inosine content of fermentation broths containing endophytic bacteria isolated from P. ternata. Alkaloid content was quantified by UV absorption at 248 nm for inosine and 254 nm for guanosine, but only absorption at 254 nm is shown. (1) Standard solution; (2) Bacillus thuringiensis; (3) Aranicola proteolyticus; (4) Bacillus cereus; (5) Serratia liquefaciens; and (6) Bacillus licheniformis. Peak a: inosine; Peak b: guanosine.
It is very important to isolate endophytes correctly. Several sterilants were tested for P. ternata. It showed surface sterilization was thoroughly by 75% (v/v) ethanol and 0.1% (m/v) mercuric chloride solution. Sterilizing periods were tested. Some endophytes may be killed with a time longer than 7 min, while surface microbes cannot be killed thoroughly with a time shorter than 4 min. It took a longer sterilizing time for tubers than for leaves and stems. It showed a sterilizing period within 4–6 min was suitable.
A comparison of guanosine and inosine production by the endophytic bacteria is shown in . All the five bacterial strains produced larger amounts of guanosine when compared with inosine production. There was a relatively wide range in the amount of guanosine produced by the bacteria. Guanosine production was greatest for strains belonging to Bacillus thuringiensis and Bacillus licheniformis. In contrast, inosine production was nearly the same among the five strains.
Table 1. Alkaloid production by endophytic bacteria isolated from P. ternataa.
It should be noted that alkaloid production by these five endophytic strains was low, compared with that of 1 g/L inosine by mutant of E. coli (Matsui et al., Citation2001), 6 g/L inosine by B. subtilis (Asahara et al., Citation2010), and 20 g/L guanosine by mutants of B. subtilis (Kuninaka, Citation2008). The production of guanosine and inosine by Bacillus subtilis has been increased by optimizing fermentation conditions, breeding new bacterial strains, and regulating the metabolic pathway of the bacteria (Bai et al., Citation2003; Liu et al., Citation2004; Qian et al., Citation2003; Zhu et al., Citation2004). Similar methods might be employed to increase guanosine or inosine production from the bacterial strains isolated in our study.
The effects of co-culture with living cells on PLB growth and differentiation are shown in . Co-culture with living Serratia liquefaciens cells increased PLB growth by 58–71% and PLB differentiation by 81–96%. Co-culture with living Bacillus licheniformis cells increased PLB growth by 4–11% and decreased PLB differentiation by 42–74%.
Table 2. Effect of Serratia liquefaciens and Bacillus licheniformis on PLB proliferation and differentiation.
PLBs of P. ternata are a kind of highly differentiated tissue. PLBs are similar to field-grown tubers morphologically and in microstructure (Liu et al., Citation2010). Both the two bacterial strains, Serratia liquefaciens and Bacillus licheniformis, increased the growth rate of PLBs. It indicates that endophytic bacteria may promote the growth of P. ternata field-grown tubers. Further studies may pay attention to bacterial manure to improve the yield of P. ternata herb.
Conclusions
In summary, we isolated endophytic bacteria of P. ternata for the first time. Among the 34 bacterial colonies isolated, five endophytic bacteria strains had the ability to produce the same alkaolids as their host plant. These bacteria were identified by 16s rDNA sequence analysis. Further studies may focus on improving guanosine and inosine yields in fermentation broth. Two bacterial strains, Serratia liquefaciens and Bacillus licheniformis, may be studied as manure to improve field-grown P. ternata yield.
Acknowledgements
The authors sincerely thank Dr. Jeff Gale for critical reading of the manuscript.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by a Scientific Research Program Funded by Shaanxi Provincial Education Department (Fund number appended later).
References
- Asahara T, Mori Y, Zakataeva NP, et al. (2010). Accumulation of gene-targeted Bacillus subtilis mutations that enhance fermentative inosine production. Appl Microbiol Biotechnol 87:2195–207
- Bai JX, Zhu XH, Zhang YP, Du GJ. (2003). Study on the microorganism fermentation of guanosine with breeding from production of inosine Bacillus. Food Fermn Ind 15:19–24
- Chen HH, Yang Y, Jiang Y, et al. (2006). Identification of plant endophytic actinomycetes. Microbiology 33:182–5
- Han MH, Yang XW, Zhang M, Zhong GY. (2006). Phytochemical study of the rhizome of Pinellia ternata and quantification of phenylpropanoids in commercial Pinellia tuber by RP-LC. Chromatographia 64:647–53
- He H, Cai XQ, Hong YC, et al. (2002). Selection of endophytic antifungal Bacteria from Capsicum. Chinese J Bio Control 18:171–5
- He YB, Hu XF, Chen HM, Chen JS. (2007). Isolation and identification of bacterial pathogen causing soft-rot on Pinellia ternata. Acta Phytopath Sin 37:337–42
- Hinton DM, Bacon CW. (1995). Enterobacter cloacae is an endophytic symbiont of corn. Mycopathologia 129:117–25
- Hu XF, Ying FX, He YB, Gao YY. (2008). Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. Eur J Plant Pathol 120:305–10
- Kinahan JJ, Kowal EP, Grindey GB. (1981). Biochemical and antitumor effects of the combination of thymidine and 1-β-D-arabinofuranosylcytosine against eukemla L1210. Cancer Res 41:445–51
- Kuninaka A. (2008). Nucleotides and related compounds. In: Rehm H-J, Reed G, eds. Biotechnology: Products of Primary Metabolism, vol. 6, 2nd ed., Chapter 15. Weinheim, Germany: Wiley-VCH Verlag GmbH, 561–612
- Li JY, Sidhu R, Ford EJ. (1998). The induction of production in the endophytic fungus-periconia sp. from Torreya grandifolia. J Ind Microbio Biotechnol 20:259–64
- Liu XX, Chen SX, Chu J, et al. (2004). Effect of sodium citrate on the growth metabolism and inosine accumulation by Bacillus subtilis. Acta Microbiol Sin 5:628–30
- Liu YH, Zong SL, Jian LL. (2010). Use of protocorm-like bodies (PLBs) for studying alkaloid metabolism in Pinellia ternata. Plant Cell Tiss Org 100:83–9
- Matsui H, Kawasaki H, Shimaoka M, Kuirahashi O. (2001). Investigation of various geno-type characteristics for inosine accumulation in Escherichia coli W3110. Biosci Biotecnhnol Biochem 65:570–8
- Qian JC, Cai XP, Chu J, Zhang YP. (2003). Analysis of three nucleotide sequences involved in the purine nucleotides biosynthesis in inosine and guanosine-producing Bacilus subtilis. Acta Microbiol Sin 2:200–5
- Wu H, Li W, Zhang KW. (2003). Studies on a distinguish principle of Pinellia ternata. China J Chinese Mater Med 28:836–9
- Zhang KW, Wu H, Li W. (2005). Determination of inosine and guanosine in Rhizoma Pinellia Ternata. Chin J Pharm Anal 25:487–9
- Zhu XH, Du GJ, Wang HD, et al. (2004). Feed of adenine by given way effects on fermentative production of guanosine. Food Fermn Ind 4:55–7

