Abstract
Context: Atractylenolide I (AT-I), an active compound isolated from Atractylodes macrocephala Koidz (Compositae), shows several pharmacological activities.
Objective: Our present study is designed to investigate the protective effect of AT-I on systemic inflammation in the mouse model of sepsis created by cecal ligation and puncture (CLP), and explore the possible mechanism.
Materials and methods: Sepsis mouse model was established by CLP, and the tested dosages of AT-I were 10, 20, and 40 mg/kg (ip). Pro-inflammatory cytokines in serum (TNF-α, IL-1β and IL-6) were determined by the ELISA method; serum lipopolysaccharide (LPS) level was measured by the Limulus Amebocyte Lysate (LAL) test; white blood cells (WBC) were counted by Blood cell analyzer; contents of alanine transaminase (ALT), aspartate transarninase (AST), creatinine (Cre), and blood urea nitrogen (BUN) in serum were determined by automatic biochemistry analyzer. For survival rate tests, CLP mice were observed within 7 days, and body temperature was measured at 0, 4, 8, 12, 24, 48 and 72 h after surgery.
Results: Our results indicated that AT-I significantly increased the survival rate of mice with sepsis (p < 0.05), whereas the WBCs and levels of LPS, pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), ALT, AST, Cre, and BUN decreased significantly after treatment with AT-I (p < 0.05).
Conclusion: In conclusion, the AT-I ameliorates sepsis syndrome by reduction of pro-inflammatory cytokines and LPS, and provides an improvement in liver and kidney functions.
Introduction
Sepsis, defined as systemic host response to microorganisms, is related to severe infections characterized by the Systemic Inflammatory Response Syndromes (SIRS), which is a whole body inflammatory state (Angus, Citation2008; Martin et al., Citation2003; Yang et al., Citation2013). Various microorganism infections including Gram-negative and Gram-positive organisms, viruses and fungi can result in sepsis (Huttunen & Aittoniemi, Citation2011). Furthermore, sepsis is one of the severe clinical syndromes with high mortality, leading to the associated multiple organ dysfunction syndromes (MODS) or septic shock (Venkataraman & Kellum, Citation2013; Vincent, Citation2008). Currently, effective drugs for treating sepsis are lacking, and the current drugs used for treating sepsis have severe side effects. Thus, finding novel and reliable therapeutic methods for treating sepsis or alleviating the syndromes of sepsis is very necessary.
Traditional Chinese medicines (TCM) have been widely used in folk medicine to treat infection and inflammatory diseases for thousands of years (Wang et al., Citation2013). The LPS and pro-inflammatory cytokines (including TNF-α and IL-6) have been reported to play an important role in the development of sepsis, and have also been considered as potential target points for the treatment of sepsis in the future (Li et al., Citation2007; Venkataraman & Kellum, Citation2013). To obtain the effective plant-derived agents for the treatment of sepsis, we have screened many TCMs or compounds used as anti-inflammatory or anti-infectious drugs in folk medicine in our preliminary experiment in vitro. Interestingly, we have found that atractylenolide I (AT-I) showed a good neutralization effect on LPS and inhibitory effects against TNF-α and IL-6. AT-I is one of the major active components of Atractylodes macrocephala Koidz (Compositae), which is a very important TCM used for treating digestive disorders and inflammatory diseases (Bose & Kim, Citation2013; Dong et al., Citation2007; Peng et al., Citation2011). Previous investigations have reported that AT-I had wide spectrum pharmacological activities in A. macrocephala, including anti-inflammation, antitumor, and antioxidant properties (Endo & Taguchi, Citation1979; Kang et al., Citation2011; Rittirsch et al., Citation2009). Based on our preliminary experiment, we aimed to investigate the protective effects of AT-I on systemic inflammation in a mice model of sepsis created by CLP and explore its possible mechanism.
Materials and methods
Materials
Atractylenolide I (AT-I) was purchased from Shanghai PueOne Biotechnology (Shanghai, China). LPS was purchased from Sigma Chemicals (St. Louis, MO). Mouse TNF-α, IL-1β, and IL-6 ELISA kits were purchased from eBioscinence (Sioux Falls, SD). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) kits were purchased from Jiancheng Institute of Biotechnology (Nanjing, China).
Experimental animals
This study was performed in accordance with the National Institute of Health’s approved guidelines, and the study protocols were approved by the Animal Research Ethics Committee of the Animal Care and Use Committee of the Central South University. The animals (mice) were housed between 20 and 22 °C and they were kept on a standard 12 h light/12 h dark cycle and pellet diet. KM mice (20 ± 2 g), purchased from the experimental animal centre of the Central South University (Changsha, China), were used in our study, and each animal was used only once in the experiment.
Preparation of cecal ligation and puncture model of mice
The cecal ligation and puncture (CLP) mice were prepared as described previously with minor modifications (Rittirsch et al., Citation2009; Yang et al., Citation2013). Briefly, mice were given general anesthesia with pentobarbital sodium (1.5%, 50 mg/kg), and an abdominal midline incision (approximately 1.5 cm) was made under sterile condition. After the cecum was isolated, it was exteriorized from the left side of the abdomen, ligate midpiece of the cecum. Then, the cecum was punctured with an 18-guage needle, and was returned to the peritoneal cavity. After relocating the cecum into the abdominal cavity, the abdominal incision was closed. Mice from the sham group underwent the same surgical procedures, but without ligation or puncture. Postoperatively, all the mice has free access to food and water.
Survival analysis after CLP surgery
For survival analysis, 125 CLP mice were divided randomly into five groups (n = 25) (sham: underwent the same operation without cecum puncture, and four AT-I-treating doses), and survival rate of the mice were observed during 7 days after CLP surgery; additionally, the body temperatures were measured in rectum at 0, 4, 8, 12, 24, 48, and 72 h after surgery.
Determination of serum WBC and LPS levels of CLP mice
Totally 75 mice were used in this investigation; 60 CLP mice were prepared as per the method described above, and were randomly divided into the following four groups (n = 15): AT-I treatment groups (0, 10, 20 and 40 mg/kg, ip); additionally, 15 sham mice were prepared as per the method mentioned above. After 12 h of CLP surgical operation, all the mice were sacrificed under anesthesia with sodium pentobarbital (40 mg/kg, ip), and the whole blood samples were collected from the heart. The white blood cells (WBC) were counted using the Blood cell analyzer (UniCel DxH 800, Beckman Coulter, Brea, CA). Additionally, the serum samples were separated by centrifugation (3500 × g for 20 min). The serum lipopolysaccharide (LPS) level was determined using the Limulus Amebocyte Lysate (LAL) test described previously (Fu et al., Citation2008) with an endotoxin detector (EDS-99, Zhanjiang A&C Biological Ltd, China).
Determination of serum cytokines of CLP mice
Serum samples were separated according to the method described above, and were stored at −70 °C until analysis. Serum levels of TNF-α (Cat. No. 85-BMS607/3), IL-1β (Cat. No. 85-BMS6002), and IL-6 (Cat. No. 85-BMS603/2) were determined using the ELISA method with the commercial kits.
Determination of liver and kidney function of CLP mice
An automatic biochemistry analyzer (AU5800, Beckman Coulter, USA) was used to measure the contents of alanine transaminase (ALT), aspartate transarninase (AST), creatinine (Cre), and blood urea nitrogen (BUN) in serum.
Statistical analysis
Data are presented as mean ± SD, and analyzed using the using SPSS software (SPSS for Windows 18.0, SPSS Inc., Chicago, IL). The chi-squared value of the exact test was used to analyze the significance of mice mortality differences among the groups. All other differences in means between two groups were analyzed with two-tailed Student’s t-test. Difference with a p value less than 0.05 was considered to be statistically significant.
Results
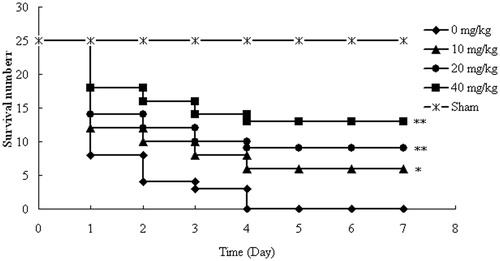
Effect of AT-I on survival of mice after CLP surgery
All mice in the control group (0 mg/kg) died with 4 days after CLP surgery. However, in the AT-I treatment group (10, 20, and 40 mg/kg), the survival rate of the mice significantly increased in a dose-dependent manner (p < 0.05, p < 0.01, p < 0.01, respectively) (). In addition, the median survival time values of the control group, AT-1 treatment groups (10, 20, 40 mg/kg), and sham group were 1, 1, 2, 7, and 7 days, respectively. Additionally, in the sham group, no death was observed within 7 days.
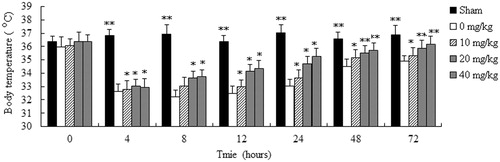
Effect of AT-I on body temperature of CLP-induced sepsis mice
The effects of AT-I on body temperature in mice with sepsis are showed in . In all groups, the body temperature of mice with CLP significantly decreased after surgery, and then the body temperature returned to normal progressively until the end of our observation period. However, the time to return to the normal temperature was significantly shorter in groups treated with AT-I compared with the CLP group (p < 0.05).
Effect of AT-I on LPS and WBC level of mice after CLP surgery
To determine whether AT-I could neutralize serum LPS of mice after CLP surgery in vivo, the LPS concentration in serum was measured. In our results, the concentrations of LPS in sham, control, and AT-I testing groups (10, 20, and 40 mg/kg) were 0.59 ± 0.26, 12.96 ± 1.82, 8.31 ± 1.43, 5.94 ± 1.08, and 4.29 ± 0.84 EU/mL, respectively (). LPS level increased sharply after CLP surgery. However, in the AT-I treatment groups (10, 20, 40 mg/kg), the LPS levels were significantly decreased in a dose-dependent manner (p < 0.05, p < 0.01, p < 0.01, respectively), compared with the control group (0 mg/kg).
Figure 3. Effects of AT-I on LPS and WBC level of mice after CLP surgery. Mice were divided into five groups (n = 15): sham, control (0 mg/kg), AT-I (10, 20, and 40 mg/kg, ip). Asterisks indicated significant difference from control group, *p < 0.05, **p < 0.01.
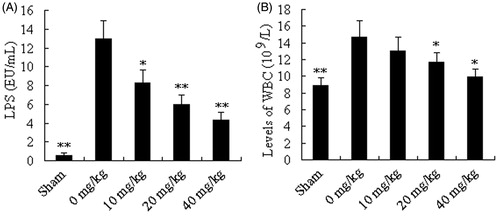
Similar to the LPS, the WBC level increased after CLP, but the WBC level could be significant decreased by treating with AT-I at the doses of 20 and 40 mg/kg (p < 0.05). And, WBC levels of sham, control, and AT-I testing groups (10, 20, and 40 mg/kg) were 8.86 ± 0.96, 14.67 ± 1.95, 13.05 ± 1.65, 11.65 ± 1.17, and 9.98 ± 0.88 109/L, respectively ().
Effects of AT-I on TNF-α, IL-1β and IL-6 levels after CLP surgery
Pro-inflammatory cytokines were all increased sharply at 12 h after CLP surgery in all groups. However, treatment of AT-I (10, 20, and 40 mg/kg) significantly decreased the levels of serum TNF-α and IL-6 (p < 0.05, p < 0.01, p < 0.01, respectively) in a dose-dependent manner. In addition, the serum level of IL-1β can also be decreased by treating with AT-I (20 and 40 mg/kg) (p < 0.05 and p < 0.01) ().
Table 1. Effect of AT-I on TNF-α, IL-1β and IL-6 of mice after CLP surgery.
Effect of AT-I on liver and kidney functions of mice after CLP surgery
shows that the AST and ALT significantly increased after CLP surgery, but this increase was suppressed by AT-I (10, 20 and 40 mg/kg) (p < 0.05, p < 0.01, p < 0.01, respectively) in a dose-dependent manner. Similar to AST and ALT, the Cre and BUN increased following CLP surgery. Treatment with AT-I (20 and 40 mg/kg) significantly decreased the contents of serum Cre and BUN (p < 0.05, p < 0.01).
Table 2. Effect of AT-I on liver and kidney functions of mice after CLP surgery.
Discussion
In previous research, the extracts of A. macrocephala possessed notable anti-inflammatory and protective effect on intestinal barrier function against insult of LPS (Bose & Kim, Citation2013). However, few related monomers were reported. To the best of our knowledge, our present investigation is the first evidence showing that AT-I has significant protective effect on systemic inflammation in a mice model of sepsis created by CLP. Our work also indicated that its possible mechanism is involved in decreasing of the pro-inflammatory cytokines and protecting the liver and kidney functions.
Natural compounds isolated from plants are very important resources for discovering novel effective and safe drugs (Li & Vederas, Citation2009; Qi et al., Citation2010; Qiu, Citation2007). Moreover, previous investigations have reported many plant-derived natural constituents that showed significant protective effects on sepsis (Alkharfy et al., Citation2011; Fu et al., Citation2008; Liu et al., Citation2009). Previous animal experiments have demonstrated that the LPS can induce inflammatory response syndrome, leading to severe pro-inflammatory reaction with subsequent acute hypotension, multi-organ failure (MOF), and even death (Davies & Cohen, Citation2011; Huttunen & Aittoniemi, Citation2011). Additionally, it is reported that neutralization or removal LPS would be a successful approach for the treatment of sepsis (Cruz et al., Citation2009; Davies & Cohen, Citation2011; Setoguchi et al., Citation2011). In our present study, our results demonstrated that the serum LPS can be significantly neutralized by treating with AT-I in a dose-dependent manner, which indicated that AT-I might have therapeutic effect on sepsis. In addition, LPS can induce over-expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, leading to sepsis (Davies & Cohen, Citation2011; Liu et al., Citation2011). TNF-α is one of the key mediators for initiating systemic inflammatory response, and IL-1β and IL-6 are the later cytokines. In addition, the three cytokines are involved in the activation of cytokine cascade reaction in sepsis (Ma et al., Citation2006), and important in the pathogenesis of septic shock and MOF. Furthermore, in previous investigations, it is reported that AT-I possessed significant anti-inflammatory effects both on acute and chronic inflammations, and the AT-I can significantly inhibit the pro-inflammatory cytokines (NO, TNF-α, IL-1β, and IL-6) in the Freunds complete adjuvant (FCA) in the induced mouse of the air-pouch model (Li et al., Citation2007; Wang et al., Citation2009). In addition, AT-I can also selectively antagonize the toll-like receptor 4 (TLR-4) (Li et al., Citation2006). From our present investigation, we examined the effect of AT-I on serum TNF-α, IL-1β and IL-6 of mice after CLP surgery, and the results revealed that AT-I can significantly decrease the levels of TNF-α, IL-1β and IL-6 after CLP in a dose-dependent manner. Additionally, the mice serum WBC level after CLP surgery can also be decreased by treating with AT-I, which is another evidence for demonstrating the anti-inflammatory effect of AT-I in the CLP model.
Liver and kidney are the two target organs susceptible to be attacked by sepsis, and liver and kidney functions could affect the development and prognosis of sepsis (Kang & Zhang, Citation2012; Wang et al., Citation2011). AST and ALT are two key markers of liver function, and Cre and BUN are two important indices of the kidney function. In our present study, our result indicated that the AT-I had significantly protective effects on the liver and kidney functions. Furthermore, our present study also demonstrated that the AT-I can obviously increase the survival rate in mice with sepsis induced by CLP; and also decrease the time returned to normal body temperature after CLP.
In conclusion, the AT-I has significant protective effect on sepsis mice induced by CLP surgery, and the mechanism might be related to down-regulation of pro-inflammatory cytokines, decrease of LPS, and protection of liver and kidney functions. Our present study suggested that AT-I can be developed as an effective and safe agent for treating sepsis in the future.
Declaration of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
This work was supported in part by the National Natural Science Foundation of China (No.81201487).
References
- Alkharfy KM, Al-Daghri NM, Al-Attas OS, Alokail MS. (2011). The protective effect of thymoquinone against sepsis syndrome morbidity and mortality in mice. Int Immunopharmacol 11:250–4
- Angus DC. (2008). Discussant management of sepsis. JAMA 5:1469–77
- Bose S, Kim H. (2013). Evaluation of in vitro anti-Inflammatory activities and protective effect of fermented preparations of rhizoma Atractylodis Macrocephalae on intestinal barrier function against lipopolysaccharide insult. Evid-Based Complement Alternat Med 2013:363076
- Cruz DN, Antonelli M, Fumagalli R, et al. (2009). Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 301:2445–52
- Davies B, Cohen J. (2011). Endotoxin removal devices for the treatment of sepsis and septic shock. Lancet Infect Dis 11:65–71
- Dong HY, Dong YL, He LC, Pei WJ. (2007). Study on constituents and anti-inflammatory activity of rhizome Atractylodes macrocephala. Chin Pharm J 42:1055–9
- Endo K, Taguchi T. (1979). Antiinflammatory principle of Atractylodes rhizomes. Chem Pharm Bull 27:2954–8
- Fu JF, Cao HW, Wang N, et al. (2008). An anti-sepsis monomer, 2′,5,6′,7-tetrahydroxyflavanonol (THF), identified from Scutellaria baicalensis Georgi neutralizes lipopolysaccharide in vitro and in vivo. Int Immunopharmacol 8:1652–7
- Huttunen R, Aittoniemi J. (2011). New concepts in the pathogenesis, diagnosis and treatment of bacteremia and sepsis. J Infection 63:407–19
- Kang J, Zhang BL. (2012). Effect of curcumin on protection of organ function and serum levels of IL-18 in LPS-induced sepsis in rats. J Clin Pediatr 30: 869–73
- Kang TH, Bang JY, Kim MH. (2011). Atractylenolide III, a sesquiterpenoid, induces apoptosis in human lung carcinoma A549 cells via mitochondria-mediated death pathway. Food Chem Toxicol 49:514–19
- Li CQ, He LC, Dong HY, Jin JQ. (2007). Screening for the anti-inflammatory activity of fractions and compounds from Atractylodes macrocephala Koidz. J Ethnopharmacol 114:212–17
- Li CQ, He LC. (2006). Establishment of the model of white blood cell membrane chromatography and screening of antagonizing TLR4 receptor component from Atractylodes macrocephala Koidz. Sci Chin Ser C Life Sci 49:182–9
- Li JWH, Vederas JC. (2009). Drug discovery and natural products: End of an era or an endless frontier? Science 325:161–5
- Liu X, Cheng J, Zheng XC, et al. (2009). Targeting CpG DNA to screen and isolate anti-sepsis fraction and monomers from traditional Chinese herbs using affinity biosensor technology. Int Immunopharmacol 9:1021–31
- Liu X, Zheng XC, Wang N, et al. (2011). Kukoamine B, a novel dual inhibitor of LPS and CpG DNA, is a potential candidate for sepsis treatment. Brit J Pharmacol 162:1274–90
- Ma HY, Kou JP, Zhu DN, et al. (2006). Liu–Shen–Wan, a traditional Chinese medicine, improves survival in sepsis induced by cecal ligation and puncture via reducing TNF-α levels, MDA content and enhancing macrophage phagocytosis. Int Immunopharmacol 6:1355–62
- Martin GS, Mannino DM, Eaton S. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–54
- Peng W, Han T, Xin WB, et al. (2011). Comparative research of chemical constituents and bioactivities between petroleum ether extracts of the aerial part and the rhizome of Atractylodes macrocephala. Med Chem Res 20:146–51
- Qi FH, Li A, Inagaki Y, et al. (2010). Chinese herbal medicines as adjuvant treatment during chemoor radio-therapy for cancer. BioSci Trend 4:297–307
- Qiu J. (2007). Traditional medicine: A culture in the balance. Nature 448:126–8
- Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4:31–6
- Setoguchi D, Nakamura M, Yatsuki H. (2011). Experimental examination of anti-inflammatory effects of a 5-HT3 receptor antagonist, tropisetron, and concomitant effects on autonomic nervous function in a rat sepsis model. Int Immunopharmacol 11:2073–8
- Venkataraman R, Kellum JA. (2013). Sepsis: Update in the management. Adv Chronic Kidney Dis 20:6–13
- Vincent JL. (2008). Clinical sepsis and septic shock-definition, diagnosis and management principles. Langenbecks Arch Surg 393:817–24
- Wang CH, Duan HJ, He LC. (2009). Inhibitory effect of atractylenolide I on angiogenesis in chronic inflammation in vivo and in vitro. Eur J Pharmacol 612:143–52
- Wang QH, Kuang HX, Su Y. (2013). Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol 146:9–39
- Wang R, Li YH, Zhang JL, Fu LB. (2011). Effects of sodium ferulate on Bcl-2 and Bax expression in septic rats kidney. Chin J Clin Pharmacol 27:46–9
- Yang C, Wu K, Li SH, You Q. (2013). Protective effect of curcumin against cardiac dysfunction in sepsis rats. Pharm Biol 51:482–7