Abstract
Context: The traditional uses of Alpinia zerumbet (Pers.) B.L.Burtt & R.m.SM (Zingiberaceae), popularly known as colonia or pacová, suggest that the species has antihypertensive, diuretic, and sedative properties. We previously reported that an ethanol extract of Alpinia zerumbet (HEA) significantly reduced the immobility time in the tail suspension test (TST), similar to the tricyclic antidepressant imipramine. Moreover, HEA presented antioxidant and anxiolytic-like effects in mice.
Objective: The objective of this study is to investigate the involvement of monoaminergic and glutamatergic systems in the antidepressant-like effects of this species.
Materials and methods: A hydroethanolic extract prepared with the leaves of A. zerumbet was assayed in the TST in male Swiss mice (800 mg/kg, p.o.). Synthesis inhibitors (AMPT, inhibitor of tyrosine hydroxylase, 100 mg/kg, i.p.; and PCPA, irreversible tryptophan hydroxylase inhibitor, 100 mg/kg, i.p.) and a specific glutamate antagonist (AMPA receptor antagonist NBQX, 10 mg/kg, i.p.) were used prior testing.
Results: Pre-treatment with the noradrenergic/dopaminergic inhibitor AMPT fully abolished the anti-immobility effects of HEA, with the two-way ANOVA yielding a significant interaction between pre-treatment and treatment (F1,32 = 10.0, p < 0.01); no interaction was observed with the serotonergic inhibitor PCPA (F1,32 = 0.33, p > 0.05) or NBQX (F1,32 = 0.21, p > 0.05).
Conclusion: These results indicated that HEA most likely acts through the dopaminergic and/or noradrenergic system but not through the serotoninergic or glutamatergic systems. This study reinforces the idea that the available biodiversity in Brazil can serve as a basis for innovation in the development of new drugs.
Introduction
Epidemiological studies demonstrate that millions of people worldwide suffer from psychiatric disorders and that this prevalence is steadily increasing, especially in developing countries (Krishnan & Nestler, Citation2010), with associated global costs being estimated to reach US $6 trillion over the next 15 years (Insel et al., Citation2013). Depression is a major public health problem and is associated with functional disability and high mortality. The projections of the World Health Organization (WHO) show that depression will be the second leading cause of disability in 2020 (Sobocki et al., Citation2006). Despite the heavy investment of the pharmaceutical industry in the production of new drugs, drug therapy for depression is still far from ideal and is associated with serious adverse effects. Therefore, the search for new compounds for the treatment of depression with improved efficacy and reduced incidence of adverse and toxic effects is an active field.
The use of plants for medicinal purposes to treat, cure, or prevent diseases is one of the oldest forms of medical practice. Medicinal plants are an important source of compounds with therapeutic potential, and Brazil has the highest biodiversity in the world, comprising 50 000 species of plants. This fact, coupled with the traditional use of medicinal plants in Brazilian folk medicine, has aroused the interest of researchers and the pharmaceutical industry in developing new drugs (Calixto, Citation2005). Alpinia zerumbet (Pers.) B.L.Burtt & R.m.SM (Zingiberaceae), locally known as colonia or pacová, is traditionally used as an antihypertensive, a diuretic, and a sedative agent (de Araújo et al., Citation2005; de Moura et al., Citation2005). This species is native to East Asia; however, it is wide cultivated and traditionally used as a medicinal plant in Brazil (Almeida, Citation1993). Pre-clinical studies have shown that the essential oil of this species has potential antipsychotic and anxiolytic effects (de Araújo et al., Citation2005; Satou et al., Citation2010). Recently, our group showed that a hydroethanolic extract of A. zerumbet (HEA) presents antioxidant-, antidepressant-, and anxiolytic-like activities in mice (Roman Junior et al., Citation2013). Thus, to characterize the psychopharmacological properties of A. zerumbet further, this study investigates the involvement of the monoaminergic and glutamatergic systems in the antidepressant-like effect of this species in the tail suspension test (TST) in mice.
Materials and methods
Animals
Experiments were performed with 108 two-month-old male Swiss mice from our own breeding facility. The mice were maintained in cages with 12 h light/dark cycle (lights on at 08:00 h and off at 20:00 h) under controlled environmental conditions (22±) 1 °C, with free access to food [Nuvilab CR1] and water) for at least 2 weeks before the experiments. All the procedures were carried out in accordance with institutional policies regarding the handling of the experimental animals (ethics committee approval #010/2013, September 2013) and were conducted according to the National Research Council’s guidelines.
Extract
The leaves of Alpinia zerumbet were collected in Chapecó (SC-Brazil) (26°58′36.06″ S, 52°44′27.18″ W) in October 2013 by Walter Antonio Roman Jr and identified by Osmar dos Santos Ribas (the voucher MBM #306196 is deposited at the Municipal Botanical Museum of Curitiba). The A. zerumbet hydroethanolic extract (HEA) was prepared as described previously (Roman Junior et al., Citation2013). The dose that was used in this study (800 mg/kg) did not modify locomotion in the open field test (Roman Junior et al., Citation2013).
Characterization of HEA
A chromatographic analysis of the HEA was performed using a Varian® Pro-Star HPLC system (Spectralab Scientific Inc, Markham, ON, Canada) consisting of an automatic injector, ternary gradient detectors, pumps, and a UV/Vis Kromasil® C18 reversed-phase ODS column (Spectralab Scientific Inc, Markham, ON, Canada) (5 µm; 25 × 4.5 mm2). The mobile phase consisted of two solvent solutions, H2O:H3PO4 (0.1% v/v) (solvent A) and MeOH (solvent B). Both the solvents were filtered through 0.45 μm Millipore PTFE membranes (Waters Corp., Milford, MA). The separations were performed using a linear gradient as follows: 70% solvent A for 15 min, 60% solvent A for 10 min, and 100% solvent B for 20 min. The UV absorbance at 254 nm was measured, and the results were compared with the retention times of an authentic external standard followed by UV spectrum analysis. The flow rate of the mobile phase was 1.0 mL min−1, and the injection volume was 20 μL. The chromatographic runs were performed at 25 °C. Different concentrations (12.5, 25, 50, 100, and 200 µg/mL) of rutin (Sigma-Aldrich® ≥94%; Sigma-Aldrich, St. Louis, MO) were analyzed in triplicate to generate a calibration curve. The HEA was dissolved in H2O (50 g/500 mL) and partitioned into equal volumes of solvents of increasing polarity. After freeze drying, the EtOAc fraction was dissolved in MeOH (10 mg/mL) and filtered through a Micropore® filter (Waters Corp., Milford, MA) (0.45 µm) before the chromatographic profile was generated. The results are expressed as the concentration of rutin per gram of HEA (Victório et al., Citation2010).
Drugs
d,l-α-Methyl-ρ-tyrosine (AMPT), 4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA), and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt hydrate (NBQX) were acquired from Sigma (St. Louis, MO). All the drugs were dissolved in saline (0.9% NaCl) and injected intraperitoneally (i.p.) or orally (p.o.) in a constant volume of 0.1 mL/10 g body weight. The administration schedule and the doses of AMPT, PCPA, and NBQX were selected from the literature (Linck et al., Citation2012; Rodrigues et al., Citation2002; Rojas-Corrales et al., Citation2005) and tested in pilot experiments in our laboratory; all the selected doses did not modify locomotion in the open field or the immobility time in the TSTs (data not shown).
Investigation of the monoaminergic system in the antidepressant-like effects of HEA
AMPT and PCPA were used to inhibit the synthesis of dopamine/noradrenaline and serotonin, respectively. Groups of mice (N = 8–10) received saline or AMPT (100 mg/kg, i.p.) 24 and 2 h before saline or HEA (800 mg/kg, p.o.); 60 min later, the mice were subjected to the TST. Additional groups of mice (N = 8–10) were treated with saline or PCPA (100 mg/kg, i.p.) once a day for 4 consecutive days; 15 min after the last PCPA treatment, the animals received saline or HEA (800 mg/kg, p.o.) and 60 min later were subjected to the TST.
Investigation of the glutamatergic system in the antidepressant-like effects of HEA
To investigate the involvement of the glutamatergic system in the antidepressant-like effects of HEA, groups of mice (N = 8–10) received saline or NBQX (10 mg/kg, i.p.) 30 min before receiving saline or HEA (800 mg/kg, p.o.). After 60 min, the animals were subjected to the TST.
Tail suspension test
The TST was used as described by Steru et al. (Citation1985). After the treatment described above, mice were suspended 50 cm above the bench using a piece of adhesive tape that was placed approximately 1 cm from the tip of the tail. The mice were observed for 6 min, and the immobility time was recorded on a stopwatch; the mice were considered immobile when hanging passively and motionless. Mice were submitted to the TST 60 min after treatments.
Statistical analysis
The results are expressed as the mean ± SEM. Comparisons between the groups were made by two-way ANOVA followed by Tukey’s post hoc test using SPSS 20.0 (SPSS Inc., Chicago, IL). A value of p < 0.05 was considered to be significant.
Results
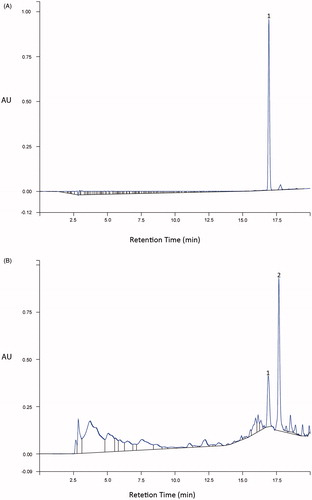
The amount of rutin in the HEA was quantified by HPLC using an analytical curve (r = 0.9997, y = 357771x + 760219) with a retention time of 16.90 min. The HPLC analysis revealed a concentration of 0.0914 g rutin/g total extract of HEA (). Based on a previous study performed under the same conditions (Victório et al., Citation2010), we can infer that the second peak in the sample analysis () corresponds to kaempferol-3-O-glucuronide.
Figure 1. HPLC chromatographic profile of HEA. (A) Rutin standard (RT 16.90 min); (B) EtOAc fraction of HEA (10 mg/mL). (1) Rutin and (2) kaempferol-3-O-glucuronide (according to Victório et al., Citation2010).
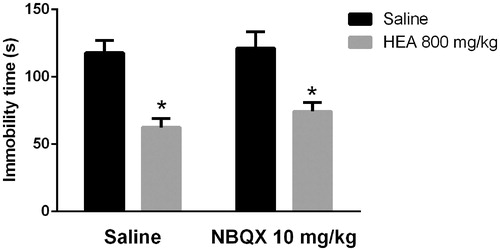
shows the influence of AMPT on the effects of HEA in the TST. A two-way ANOVA revealed a main effect of the pre-treatment (F1,32 = 8.8, p < 0.01), the treatment (F1,32 = 26.1, p < 0.01), and a significant interaction between pre-treatment and treatment (F1,32 = 10.0, p < 0.01). Post hoc analyses indicated that pre-treatment with AMPT prevented the effects of HEA in the TST.
Figure 2. Effects of AMPT (100 mg/kg, i.p.) on the HEA (800 mg/kg, p.o.) actions in the TST. Each column represents the mean ± SEM. N = 8–10. *p < 0.01 × saline–saline. Two-way ANOVA followed by Tukey’s post hoc test.
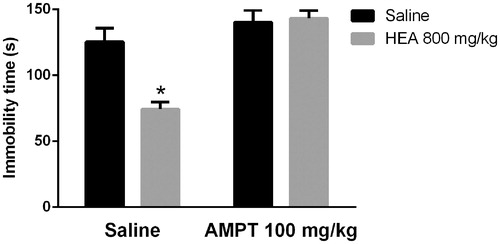
shows the influence of PCPA on the effects of HEA in the TST. A two-way ANOVA revealed a main effect of the pre-treatment (F1,32 = 42.8, p < 0.01) and no significant effects of the treatment (F1,32 = 0.001, p > 0.05) or interaction between pre-treatment and treatment (F1,32 = 0.33, p > 0.05). Post hoc analyses indicated that the pre-treatment with PCPA did not alter the effect of HEA on the TST.
Figure 3. Effects of PCPA (100 mg/kg, i.p.) on the HEA (800 mg/kg, p.o.) actions in the TST. Each column represents the mean ± SEM. N = 8–10. *p < 0.01 × saline–saline. Two-way ANOVA followed by Tukey’s post hoc test.
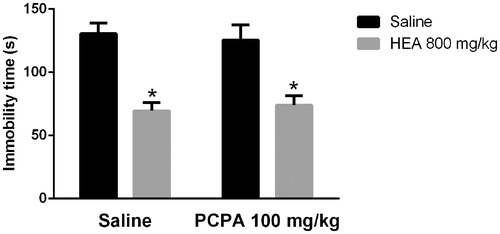
shows the influence of NBQX on the effects of HEA in the TST. A two-way ANOVA revealed a main effect of the pre-treatment (F1,32 = 30.4, p < 0.01) but did not reveal significant effects of the treatment (F1,32 = 0.68, p > 0.05) or interaction between pre-treatment and treatment (F1,32 = 0.21, p > 0.05). Post hoc analyses indicated that the pre-treatment with NBQX did not modify the effect of HEA in the TST.
Discussion
The research of new compounds for the treatment of psychiatric disorders with better efficacy and a reduced incidence of adverse effects is an active field. We can envision the plant kingdom as an immeasurable source of novel compounds that may be useful for the treatment of psychopathology that affects humans. Despite certain limitations, animal models of depression have been used for a long time to screen for new therapeutic compounds and to elucidate the mechanism of action of drug candidates. A previous study showed that HEA (200, 400, and 800 mg/kg) reduced the immobility time of mice in the TST when administered by gavage, an indication of oral bioavailability. Moreover, the effect of HEA was equivalent to that of the tricyclic antidepressant imipramine (Roman Junior et al., Citation2013).
In this study, we examined the involvement of dopamine, noradrenaline, serotonin, and glutamate in the antidepressant-like effects of HEA in mice. The use of synthesis inhibitors and/or antagonists to elucidate the mechanism of action is a pharmacological approach widely used in the literature (Colla et al., Citation2012; Kwon et al., Citation2010; Machado et al., Citation2008). Pre-treatment with AMPT, but not PCPA and NBQX, prevented the antidepressant-like effects of HEA in the TST. AMPT is a monoamine synthesis inhibitor that can reduce the levels of noradrenaline and dopamine in a robust manner without affecting the serotonin levels (Mayorga et al., Citation2001). These results suggest that the effect of HEA in the TST is dependent on the availability of noradrenaline and/or dopamine in the nerve terminal. The effect of AMPT in preventing the anti-immobility activity of antidepressants is well reported in the literature (Cunha et al., Citation2013; Kaster et al., Citation2007). Moreover, these effects have also been shown in clinical experiments (Bremner et al., Citation2003; Delgado et al., Citation2002).
The glutamatergic system seems to be hyperactive in depression, with increased levels of glutamate and glutamine metabolite (Krystal et al., Citation2013; Sanacora et al., Citation2012), while glutamate antagonists have shown antidepressant-like effects in pre-clinical (Zhou et al., Citation2013) and clinical studies (Murrough et al., Citation2013; Zarate et al., Citation2006). Specifically, the modulation of AMPA receptors is associated with the antidepressant effects of compounds such as lithium, ketamine, and N-acetylcysteine (Gould et al., Citation2008; Linck et al., Citation2012; Zhou et al., Citation2013). Our results show that the blockade of AMPA receptors did not modify the effect of HEA in the TST; however, we cannot exclude the participation of NMDA or metabotropic glutamate receptors.
The phytochemical analysis showed that A. zerumbet presents two flavonoids, rutin and kaempferol-3-O-glucoronide. Previous reports indicated that rutin has antidepressant-like effects in both the tail suspension and forced swimming tests (Herrera-Ruiz et al., Citation2011; Machado et al., Citation2008). The data presented here strongly corroborate the results of these studies, suggesting that rutin may contribute to the antidepressant-like effects of this medicinal plant. However, more studies are necessary to better elucidate the participation of other compounds in the antidepressant-like effects of HEA.
Our results present additional evidence that A. zerumbet possesses antidepressant-like effects in a classical animal model of depression. This effect is most likely mediated by the interaction of A. zerumbet with the dopaminergic/noradrenergic system but not with the serotoninergic or glutamatergic systems. HEA may thus act similar to monoaminergic antidepressants but with a better safety profile, as adverse effects that are common to these drugs, such as sedation and anticholinergic effects, have not been reported with the traditional use. Additionally, both the flavonoids that were present in the extract may be related to the behavioral effects. The use of antagonists is a widespread pharmacological approach to evaluate the mechanism of antidepressant-like effect of new drugs, and this method enabled us to determine that a functional noradrenergic and/or dopaminergic system is required for the antidepressant-like effects of HEA. Further studies are necessary to elucidate the exact transporters and receptors possibly involved in this case and to better characterize the antidepressant-like mechanisms of Alpinia zerumbet.
Declaration of interest
The authors report that they no conflicts of interests. This work was supported by the Universidade Comunitária da Região de Chapecó [FUMDES-SC No 01/SED/2012].
References
- Almeida ER. (1993). Plantas Medicinais Brasileiras-Conhecimentos Populares e Científicos. São Paulo: Hemus
- Bremner JD, Vythilingam M, Ng CK, et al. (2003). Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: Implications for the neural circuitry of depression. JAMA 289:3125–34
- Calixto JB. (2005). Twenty-five years of research on medicinal plants in Latin America: A personal view. J Ethnopharmacol 100:131–4
- Colla AR, Machado DG, Bettio LE, et al. (2012). Involvement of monoaminergic systems in the antidepressant-like effect of Eugenia brasiliensis Lam. (Myrtaceae) in the tail suspension test in mice. J Ethnopharmacol 143:720–31
- Cunha MP, Pazini FL, Oliveira Á, et al. (2013). The activation of α1-adrenoceptors is implicated in the antidepressant-like effect of creatine in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry 44:39–50
- de Araújo PF, Coelho-de-Souza AN, Morais SM, et al. (2005). Antinociceptive effects of the essential oil of Alpinia zerumbet on mice. Phytomedicine 12:482–6
- de Moura RS, Emiliano AF, de Carvalho LC, et al. (2005). Antihypertensive and endothelium-dependent vasodilator effects of Alpinia zerumbet, a medicinal plant. J Cardiovasc Pharmacol 46:288–94
- Delgado PL, Moreno FA, Onate L, Gelenberg AJ. (2002). Sequential catecholamine and serotonin depletion in mirtazapine-treated depressed patients. Int J Neuropsychopharmacol 5:63–6
- Gould TD, O’Donnell KC, Dow ER, et al. (2008). Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test. Neuropharmacology 54:577–87
- Herrera-Ruiz M, Zamilpa A, González-Cortazar M, et al. (2011). Antidepressant effect and pharmacological evaluation of standardized extract of flavonoids from Byrsonima crassifolia. Phytomedicine 18:1255–61
- Insel TR, Voon V, Nye JS, et al. (2013). Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev 37:2438–44
- Kaster MP, Raupp I, Binfaré RW, et al. (2007). Antidepressant-like effect of lamotrigine in the mouse forced swimming test: Evidence for the involvement of the noradrenergic system. Eur J Pharmacol 565:119–24
- Krishnan V, Nestler EJ. (2010). Linking molecules to mood: New insight into the biology of depression. Am J Psychiatry 167:1305–20
- Krystal JH, Sanacora G, Duman RS. (2013). Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry 73:1133–41
- Kwon S, Lee B, Kim M, et al. (2010). Antidepressant-like effect of the methanolic extract from Bupleurum falcatum in the tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry 34:265–70
- Linck VM, Costa-Campos L, Pilz LK, et al. (2012). AMPA glutamate receptors mediate the antidepressant-like effects of N-acetylcysteine in the mouse tail suspension test. Behav Pharmacol 23:171–7
- Machado DG, Bettio LE, Cunha MP, et al. (2008). Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol 587:163–8
- Mayorga AJ, Dalvi A, Page ME, et al. (2001). Antidepressant-like behavioral effects in 5-hydroxytryptamine (1A) and 5-hydroxytryptamine (1B) receptor mutant mice. J Pharmacol Exp Ther 298:1101–7
- Murrough JW, Iosifescu DV, Chang LC, et al. (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiatry 170:1134–42
- Rodrigues AL, da Silva GL, Mateussi AS, et al. (2002). Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci 70:1347–58
- Rojas-Corrales MO, Berrocoso E, Micó JA. (2005). Role of 5-HT1A and 5-HT1B receptors in the antinociceptive effect of tramadol. Eur J Pharmacol 511:21–6
- Roman Junior WA, Piato AL, Marafiga Conterato GM, et al. (2013). Psychopharmacological and antioxidant effects of hydroethanolic extract of Alpinia zerumbet leaves in mice. Pharmacog J 5:113–18
- Sanacora G, Treccani G, Popoli M. (2012). Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77
- Satou T, Murakami S, Matsuura M, et al. (2010). Anxiolytic effect and tissue distribution of inhaled Alpinia zerumbet essential oil in mice. Nat Prod Commun 5:143–6
- Sobocki P, Jönsson B, Angst J, Rehnberg C. (2006). Cost of depression in Europe. J Ment Health Policy Econ 9:87–8
- Steru L, Chermat R, Thierry B, Simon P. (1985). The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–70
- Victório CP, Arruda R do C de O, Lage CLS, Kuster RM. (2010). Production of flavonoids in organogenic cultures of Alpinia zerumbet. Nat Prod Commun 5:1219–23
- Zarate CA Jr, Singh JB, Carlson PJ, et al. (2006). A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–64
- Zhou W, Wang N, Yang C, et al. (2013). Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 29:419–23