Abstract
Context: This is the first study on the phytochemistry, antioxidant, anticholinesterase, and antibacterial activities of Sedum caeruleum L. (Crassulaceae).
Objective: The objective of this study is to isolate the secondary metabolites and determine the antioxidant, anticholinesterase, and antibacterial activities of S. caeruleum.
Materials and methods: Six compounds (1–6) were isolated from the extracts of S. caeruleum and elucidated using UV, 1D-, 2D-NMR, and MS techniques. Antioxidant activity was investigated using DPPH•, CUPRAC, and ferrous-ions chelating assays. Anticholinesterase activity was determined against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes using the Ellman method. Antibacterial activity was performed according to disc diffusion and minimum inhibitory concentration (MIC) methods.
Results: Isolated compounds were elucidated as ursolic acid (1), daucosterol (2), β-sitosterol-3-O-β-d-galactopyranoside (3), apigenin (4), apigetrin (5), and apiin (6). The butanol extract exhibited highest antioxidant activity in all tests (IC50 value: 28.35 ± 1.22 µg/mL in DPPH assay, IC50 value: 40.83 ± 2.24 µg/L in metal chelating activity, and IC50 value: 23.52 ± 0.44 µg/L in CUPRAC), and the highest BChE inhibitory activity (IC50 value: 36.89 ± 0.15 µg/L). Moreover, the chloroform extract mildly inhibited (MIC value: 80 µg/mL) the growth of all the tested bacterial strains.
Discussion and conclusion: Ursolic acid (1), daucosterol (2), β-sitosterol-3-O-β-d-galactopyranoside (3), apigenin (4), apigetrin (5), and apiin (6) were isolated from Sedum caeruleum for the first time. In addition, a correlation was observed between antioxidant and anticholinesterase activities of bioactive ingredients of this plant.
Introduction
The Crassulaceae family comprises 1250–1500 species divided into six subfamilies and about 33 genera (Berger, Citation1930). Sedum is the largest genus comprising 350–500 species. It is often regarded as the core genus of Crassulaceae containing the least advanced as well as some highly derived taxa (Hart, Citation1982). Sedum species have been used in folk medicine against various symptoms (Niemann et al., Citation1976). They have been reported for their anti-inflammatory (Altavilla et al., Citation2008; De-Melo et al., Citation2005), antinociceptive, antioxidant (Bonina et al., Citation2000; Thuong et al., Citation2007), hepatoprotective, and antitumor effects (Camargo et al., Citation2002; Jung et al., Citation2008; Kang et al., Citation2000; Torres et al., Citation2003). Phenolic acids and flavonoids (Korul’kin, Citation2001; Mulinacci et al., Citation1995; Stevens et al., Citation1994; Wolbis, Citation1987, Citation1989a,Citationb), coumarins (Wolbis & Krolikowska, Citation1988; Zaitsev et al., Citation1983), terpenes (He et al., Citation1998; Wollenweber et al., Citation1999), and alkaloids (Colau & Hootele, Citation1983; Kim et al., Citation1996) have been isolated from Sedum species.
After publication of some research data on synthetic antioxidants that may cause cancer (Grice, Citation1988), there is a growing trend to find naturally occurring safe and effective antioxidants (Scalbert et al., Citation2005) to use in food and pharmaceutical industries. According to Atta-ur-Rahman and Choudhary (Citation2001), regular antioxidants may stop or slow down the neuronal degeneration that can prevent Alzheimer’s disease progression. Alzheimer's disease is the severe form of dementia, and the acetylcholinesterase (AChE) inhibitor drugs are used to treat Alzheimer’s disease. Most of these drugs cause bradycardia and liver, stomach, and intestine toxicities (Dökmeci, Citation2000). For these reasons, the improvement and handling of safe anticholinesterases and antioxidants from nature are desired.
With regard to medicinal uses of some Sedum species, this study investigates the secondary metabolites of aerial parts of S. caeruleum L. as well as its in vitro antioxidant, anticholinesterase, and antibacterial activities. We report the first phytochemical investigations on Sedum caeruleum, also known as Sky Stone-crop, Baby-blue, and Red-leaf in Algeria.
Materials and methods
Chemicals and drugs
Melting points were determined on the Barnstead Electrothermal apparatus (Thermo Scientific, Waltham, MA) and are uncorrected. 1H-NMR and 13C-NMR spectra (δ, ppm) were recorded on a Bruker ALS 400 MHz spectrometer using tetramethylsilane (E. Merck, Darmstadt, Germany) as the internal reference. Silica gel (35–70 mm), TLC reagents, and Sephadex LH-20 were purchased from E. Merck (Darmstadt, Germany) and Fluka (Newport News, VA).
Bioactivity measurements were carried out on a 96-well microplate reader, SpectraMax 340PC384, Molecular (Newark, DE), at the Department of Chemistry, Muğla Sıtkı Koçman University (Turkey). The measurements and calculations of the activity results were evaluated using Softmax PRO v5.2 software (Molecular, Newark, DE). Ethanol, ammonium acetate, copper (II) chloride, and potassium persulfate were obtained from E. Merck (Darmstadt, Germany). Polyoxyethylenesorbitan–monopalmitate (Tween 40), neocuproine, α-tocopherol, butylatedhydroxytoluene (BHT), 1,1-diphenyl-2-picrylhydrazyl (DPPH), electric eel AChE (Type-VI-S, EC 3.1.1.7, 425.84 U/mg, Sigma, St. Louis, MO), horse serum butyrylcholinesterase (BChE, EC 3.1.1.8, 11.4 U/mg, Sigma, St. Louis, MO), 5,5′-dithiobis (2-nitrobenzoic) acid (DTNB), acetylthiocholine iodide, butyrylthiocholine chloride, and galantamine were obtained from Sigma-Aldrich GmbH (Sternheim, Germany). All other chemicals and solvents were of analytical grade.
Plant material
The aerial parts of Sedum caeruleum were collected on May 2010 at Constantine (North Eastern Algerian) and identified by Professor Gérard De Bélair, Faculty of Sciences University of Annaba, Algeria. A voucher specimen was deposited at the Herbarium of the Laboratory of Therapeutic Substances (LOST), University of Constantine (Herbarium number: LOST.Sc.05.10).
Extraction and isolation of compounds
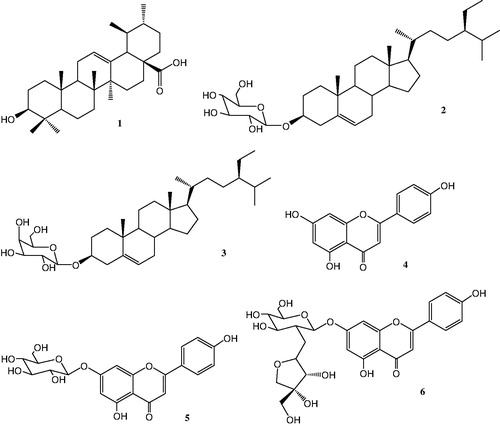
Air-dried and powdered aerial parts (1500 g) of S. caeruleum were extracted with 80% MeOH. After evaporating the methanol under vacuum, the residue was dissolved in water and extracted with petroleum ether, chloroform, ethyl acetate, and butanol, successively. The chloroform extract (4.5 g) was subjected to silica gel column chromatography and was eluted with dichloromethane, followed by gradient of methanol up to 100%. The fractions eluted with CHCl3–MeOH (9:1) and (8:2) were further rechromatographed on silica gel column. This afforded three compounds (1–3). Ethyl acetate and butanol extracts were combined because they were in less amount. The combined extract (9.64 g) was chromatographed on silica gel column, eluted with dichloromethane, followed by gradients of methanol. Three main fractions (1–3) were collected. Fraction 1 (600 mg) was further passed through silica gel column (CH2Cl2–MeOH 9.5:0.5) yielding a white precipitate (4, 25 mg), washed with dichloromethane. Fraction 2 (540 mg) was also chromatographed on silica gel, eluted with an isocratic system (EtOAc–MeOH 15:1) that afforded compound 5 (10 mg). Fraction 3 (790 mg) eluted over Sephadex LH-20 with MeOH using flash column chromatography that led to compound 6 (12 mg). Compounds 1–6 () were identified by 1H-NMR,13C-NMR, DEPT, and 2D-NMR techniques such as COSY, HMQC, HMBC, and ROESY, respectively. In order to identify the flavonoids, the NaOCH3, AlCl3/HCl, and NaOAc/H3BO3 UV spectrum were also used (Mabry et al., Citation1970). All these data were in good agreement with the respective literature data (Ahmad et al., Citation1987; Ali et al., Citation2007; Lendl et al., Citation2005; Markham, Citation1982; Markham & Geiger, Citation1993; Mizushina et al., Citation2006; Wang et al., Citation2009).
Acid hydrolysis of compounds 2, 3, 5, and 6
Each compound (5 mg) was refluxed with 5% H2SO4 (5 mL) in water for 1 h. The reaction mixture was diluted with water and extracted by ethyl acetate. Thin-layer chromatography (TLC) was used to compare the samples with authentic samples. Each remaining aqueous layer was adjusted to pH 7 with NaHCO3 and filtered. The concentrated filtrate and authentic sugars were developed on a silica gel TLC using acetone:H2O (90:10, V/V) solvent mixture.
Determination of antioxidant activity
DPPH free radical scavenging assay
The free radical-scavenging activity was determined spectrophotometrically by the DPPH assay (Tel et al., Citation2013a). BHT and α-tocopherol were used as antioxidant standards for comparison of the activity. The scavenging capability of DPPH radical was calculated using the following equation. The results were given as IC50 value (µg/mL) corresponding the concentration of 50% inhibition:
Metal chelating activity assay
The chelating activity of S. caeruleum extracts on Fe2+ was measured spectrophotometrically (Tel et al., Citation2013a), and the activity was calculated using the following equation. The results were given as IC50 value (µg/mL) corresponding the concentration indicating 50% inhibition:
Cupric reducing antioxidant capacity assay
The cupric reducing antioxidant capacity (CUPRAC) was determined according to the CUPRAC method (Apak et al., Citation2004). The reducing capacity of the extracts was compared with those of α-tocopherol and BHT. The results were given as A0.5 (µg/mL) corresponding the concentration indicating 0.5000 absorbances.
Determination of the anticholinesterase activity
Acetylcholinesterase (AChE) and BChE inhibitory activities were measured by slightly modifying the spectrophotometric method (Ellman et al., Citation1961). AChE from electric eel and BChE from horse serum were used, while acetylthiocholine iodide and butyrylthiocholine chloride were employed as substrates of the reaction. 5,5′-Dithiobis(2-nitrobenzoic) (DTNB) acid was used for the measurement of the activity. Galantamine was used as a positive reference compound. The results were given as IC50 value (µg/mL) corresponding to the concentration shows 50% inhibition.
Determination of antibacterial activity
The antibacterial activity of the chloroform extract was tested against a range of microorganisms, namely Escherichia coli ATCC 25922, E. coli (HS), Staphylococcus aureus ATCC 43300, S. aureus (HS), Pseudomonas aeruginosa ATCC 27853, P. aeruginosa (HS), Klebsiella pneumonia ATCC 700603, K. pneumonia (HS), and Streptococcus enterococcus (HS). The reference strains (ATCC, American type culture collection) were obtained from the Pasteur Institute (Algiers) whereas the others (HS, hospital strains) were obtained from the laboratory of bacteriology, Benbadis Hospital, Constantine, Algeria, using conventional methods by The National Committee For Clinical Laboratory Standards (NCCLS) and The Clinical and Laboratory Standards Institute (CLSI) (CLSI, Citation2006; NCCLS, Citation1997).
Statistical analysis
All data on both antioxidant and anticholinesterase activity tests were the average of triplicate analyses. The data were recorded as mean ± standard error meaning (SEM). Significant differences between means were determined by Student’s t-test, and p values <0.05 were regarded as significant.
Results and discussion
Identification of compounds 1–6
Ursolic acid (1) (20 mg), daucosterol (2) (7.2 mg), and β-sitosterol-3-O-β-d-galactopyranoside (3) (8 mg) were isolated from the chloroform extract whereas apigenin (4) (25 mg), apigetrin (5) (10 mg), and apiin (6) (12 mg) were the combined from ethyl acetate and butanol extracts.
β-Sitosterol-3-O-β-d-glucopyranoside (Daucosterol) (2)
C35H60O6, white powder, 1H-NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 5.30 (1H, d, J = 4.64, H-6), 4.21 (1H, J = 7.8, H-3), 0.65 (3H, s, H-18), 0.95 (3H, s, H-19), 0.90 (3H, d, J = 6.4, H-21), 0.78 (3H, m, H-26), 0.81 (3H, d, J = 6.4, H-27), 0.84 (3H, m, J = 6.5, H-29), 4.84 (1H, d, H-1′), 4.41 (1H, m, H-2′), 3.64 (1H, m, H-3′). 13C-NMR (100 MHz, CD3OD, δ): 141.67 (C-5), 121.66 (C-6), 76.66 (C-3), 50.0 (C-9), 44.16 (C-25), 42.50 (C-13), 38.34 (C-4), 37.50 (C-1), 11.66 (C-29), 20.0 (C-11), 23.33 (C-28), 30.0 (C-7), (C sugar) 100.83 (C-1′), 61.66 (C-6′), 70.0 (C-4′), 77.0 (C-3′), 73.68 (C-2′), 76.84 (C-5′). Acid hydrolysis afforded β-sitosterol and d-glucopyranose.
β-Sitosterol-3-O-β-d-galactopyranoside (3)
C35H60O6, white powder, 1H-NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 5.40 (1H, s H-6), 4.24 (1H, d, H-3), 0.75 (3H, s, H-18), 1.06 (3H, s, H-19), 0.98 (3H, d, J = 7.0, H-21), 0.84 (3H, m, J = 6.4, H-26), 0.87 (3H, d, J = 6.4, H-27), 0.90 (3H, m, J = 6.5, H-29), 4.42 (1H, d, J = 7.7, H-1′), 13C-NMR (100 MHz, CD3OD, δ): (Me):11.0 (C-19), 11.20 (C-29), 17.9 (C-21), 18.2 (C-26), 18.5 (C-18), 19.0 (C-27), (CH2): 20.1 (C-11), 22.1 (C-28), 23.4 (C-15), 25.0 (C-23), 28.8 (C-2), 30.9 (C-7), 33.0 (C-22), 35.8 (C-16), 36.4 (C-12), 37.9 (C-1), 38.6 (C-4), (CH): 31.0 (C-8), 44.8 (C-25), 49.2 (C-9), 55.0 (C-17), 55.8 (C-14), 75.1 (C-3), 121.0 (C-6), (C quaternary) 41.4 (C-13), 139.6 (C-5), 35.2 (C-10), (C sugar) 61.5 (C-6′), 70.1 (C-4′), 72.8 (C-2′), 76.24 (C-5′), 77.6 (C-3′), 100.4 (C-1′). Acid hydrolysis gave β-sitosterol and d-galactopyranose.
Antioxidant properties
Three methods were selected to determine the antioxidant capacity of the extracts. DPPH free radical scavenging activity measures the ability of electron transfer to the media. The CUPRAC method also measures electron transferring of the antioxidant. In case of bulky compounds, CUPRAC gives better and accurate results. Since transition metals can accelerate the lipid peroxidation via the Fenton reaction (Tel et al., Citation2013b), the metal chelating activity was selected to measure the binding capacity of iron of the extracts.
The free radical scavenging activity of the extracts is given in . Radical scavenging activity increased linearly with increasing amount of the extracts. The butanol extract exhibited highest activity (IC50 value: 28.35 ± 1.22 µg/mL). It indicated higher free radical scavenging activity than that of BHT, but lower than α-tocopherol.
Table 1. Antioxidant activity of S. caeruleum by the DPPH•, CUPRAC, and metal chelating assaysa.
The results of CUPRAC of the extracts are compared with those of α-tocopherol and BHT (). Activity (absorbance) increased linearly with the increasing amount of extracts. The butanol extract exhibited highest activity (A0.50 value: 23.52 ± 0.44 µg/mL) among the extracts, followed by chloroform (A0.50 value: 29.40 ± 1.10 µg/mL) and ethyl acetate extract (A0.50 value: 99.36 ± 1.32 µg/mL). However, none of the extracts exhibited higher activity than those of antioxidant standards.
also shows the chelating effects of extracts on ferrous ions, compared with EDTA. Metal chelating activity increased linearly with increasing concentration of the extracts. The butanol extract (IC50 value: 40.83 ± 2.24 µg/mL) also showed the highest metal chelating activity, followed by chloroform (IC50 value: 132.23 ± 2.31 µg/mL) and ethyl acetate extracts (IC50 value: 161.43 ± 2.34 µg/mL).
Anticholinesterase activity
shows the AChE and BChE inhibitory activities of the extracts, compared with that of galantamine used as a standard drug to treat mild Alzheimer’s disease. Chloroform (IC50 value: 180.79 ± 0.47 μg/mL) and butanol extracts (IC50 value: 184.59 ± 2.04 μg/mL) showed weak inhibitory activity against AChE. The ethyl acetate extract, however, was inactive at all concentrations. The butanol extract (IC50 value: 36.89 ± 0.15 μg/mL) exhibited highest inhibitory activity against BChE, even higher than galantamine (IC50 value: 50.81 ± 0.91 μg/mL). The chloroform extract exhibited moderate BChE inhibitory activity (IC50 value: 84.32 ± 0.46 μg/mL). The ethyl acetate extract, however, had a weak inhibitory activity (IC50 value: 174.04 ± 1.42 μg/mL). The butanol and chloroform extracts showed weak AChE and the good BChE inhibitory activities, respectively. The activity of chloroform extract may be due the presence of ursolic acid (Kolak et al., Citation2009) found in large amounts. The presence of flavonoids in the butanol extract may have caused BChE inhibitory activity.
Table 2. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of various extracts of S. caeruleuma.
Antibacterial activity
shows the antibacterial activity (inhibition zones and MIC) of the chloroform extract at 128 µg/mL against six Gram (−) pathogen bacteria; i.e., E. coli ATCC 25922, E. coli (HS), P. aeruginosa ATCC 27853, P. aeruginosa (HS), K. pneumonia (HS), K. pneumoniae (ATCC 700603), and against three Gram (+) pathogen bacteria, namely, S. aureus ATCC 43300, S. aureus (HS), and S. enterococcus (HS). According to the data the chloroform extract mildly inhibited the growth of all bacterial strains with 12–13 mm inhibition zone diameters. The MIC values were found to be 80 µg/mL against all tested bacteria.
Table 3. Antibacterial activity (inhibition zones and MIC) of the chloroform extract of S. caeruleum.
Conclusions
Sedum caeruleum was studied phytochemically for the first time. Ursolic acid (1), daucosterol (2), β-sitosterol-3-O-β-d-galactopyranoside (3), apigenin (4), apigetrin (5), and apiin (6) were isolated from this specie. The butanol extract exhibited highest activity in all tests except AChE assay, followed by chloroform and ethyl acetate extracts. The highest activity of polar extracts may be due to the presence of the flavonoids 4–6. The extracts showing higher antioxidant activity also exhibited the higher BChE inhibitory activity. Similarly, the chloroform extract exhibited weak antioxidant activity; it showed weak activity against BChE and no activity against AChE. Thus it can be said that there is a correlation between antioxidant and AChE inhibitory activities. The difference between the extracts and the control was statistically significant in all antioxidant tests (p < 0.05). Moreover, the chloroform extract showed a mild antibacterial activity against all tested bacterial strains.
Acknowledgements
The authors thank The Muğla Sitki Koçman University, Department of Chemistry.
Declaration of interest
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper. The authors are grateful to ANDRS and DG-RSDT (MESRS. Algeria) for financial support.
References
- Ahmad ZA, Tripathi GS, Begum S. (1987). Sitosterol-β-d-galactoside from Murraya exotica. Planta Med 53:578–9
- Ali SM, Ibrahim SA, Jalil S, Choudhary MI. (2007). Ursolic acid. A potent inhibitor of superoxides produced in the cellular system. Phytother Res 21:558–61
- Altavilla D, Polito F, Bitto A, et al. (2008). Anti-inflammatory effects of the methanol extract of Sedum telephium ssp. maximum in lipopolysaccharide-stimulated rat peritoneal macrophages. Pharmacology 82:250–6
- Apak R, Güçlü K, Özyürek M, Karademir SE. (2004). Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52:7970–81
- Atta-ur-Rahman A, Choudhary MI. (2001). Bioactive natural products as a potential source of new pharmacophores: A theory of memory. Pure Appl Chem 73:555–60
- Berger A. (1930). Crassulaceae. In: Engler A, Prantl K, eds. Die Natiirlichen Pflanzen familien. 2nd ed., vol. 18a. Leipzig: W. Engelmann, 352–483
- Bonina F, Puglia C, Tomaino A, et al. (2000). In-vitro antioxidant and in-vivo photoprotective effect of three lyophilized extracts of Sedum telephium L. leaves. J Pharm Pharmacol 52:1279–85
- Camargo MEM, Romero MB, Zamora DR, et al. (2002). Study of the anti-inflammatory effect of Sedum praealtum (Siempreviva) in the rat: Dose-dependent response. Proc Western Pharmacol Soc 45:129–30
- Colau B, Hootele C. (1983). Sedum alkaloids. V. Sedacrine, the major alkaloid of Sedum acre, and related bases. Can J Chem 61:470–72
- CLSI. (2006). Quality Control Minimal Inhibitory Concentration (MIC) Limits for Broth Dilution and MIC Interpretative Breakpoints (M27-S2). Wayne, PA: Clinical and Laboratory Standards Institute
- De-Melo GO, Malvar DDC, Vanderlinde FA, et al. (2005). Phytochemical and pharmacological study of Sedum dendroideum leaf juice. J Ethnopharmacol 102:217–20
- Dökmeci I. (2000). Fundamentals of Pharmacology. Istanbul, Turkey: Nobel Tıp Kitabevi Press
- Ellman GL, Courtney KD, Andres V, Featherston RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
- Grice HP. (1988). Enhanced tumour development by butylated hydroxyanisole (BHA) from the prospective of effect on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 26:717–23
- Hart T. (1982). The white-flowered Sedum species. 1. Principles of a phylogenetic of the Sedoideae (Crassulaceae) and the position of the white-flowered Sedum species. Proc Konink Nederlands Akad Wetenschappen Ser C 85:663–75
- He A, Hao H, Wang M, et al. (1998). Hepatoprotective triterpenes from Sedum sarmentosum. Phytochemistry 49:2607–10
- Jung HJ, Kang HJ, Song YS, et al. (2008). Anti-inflammatory, anti-angiogenic and anti-nociceptive activities of Sedum sarmentosum extract. J Ethnopharmacol 116:138–43
- Kang TH, Pae HO, Yoo JC, et al. (2000). Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J Ethnopharmacol 70:177–82
- Kim JH, T’Hart H, Stevens JF. (1996). Alkaloids of some Asian Sedum species. Phytochemistry 41:1319–24
- Kolak U, Hacibekiroğlu I, Öztürk M, et al. (2009). Antioxidant and anticholinesterase constituents of Salvia poculata. Turk J Chem 33:813–23
- Korul’kin DY. (2001). Chemical composition of certain Sedum species of Kazakhstan. Chem Nat Compds 37:219–23
- Lendl A, Werner I, Glasl S, et al. (2005). Phenolic and terpenoid compounds from Chionevenosa (SW.) Urban var. venosa (Bois Bandé). Phytochemistry 66:2381–7
- Mabry TJ, Markham KR, Thomas MB. (1970). The Systematic Identification of Flavonoids. Berlin, Heidelberg, New York: Springer-Verlag
- Markham KR. (1982). Techniques of Flavonoid Identification. London, England: Academic Press
- Markham KR, Geiger H. (1993). 1H Nuclear magnetic resonance spectroscopy of flavonoids and their glycosides in hexadeuterodimethylsulfoxide. In: Harborne JB, ed. The Flavonoids: Advances in Research since 1986. London: Chapman and Hall, 441–7
- Mulinacci N, Vincieri FF, Baldi A, et al. (1995). Flavonol glycosides from Sedum telephium subspecies maximum leaves. Phytochemistry 38:531–3
- Mizushina Y, Nakanishi R, Kuriyama I, et al. (2006). β-Sitosterol-3-O-β-d-glucopyranoside: A eukaryotic DNA polymerase inhibitor. J Steroid Biochem Mol Biol 99:100–7
- NCCLS (National Committee for Clinical Laboratory Standards). (1997). Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. Wayne, PA: National Committee for Clinical Laboratory Standards
- Niemann GJ, Visser-Simons JM, Hart H. (1976). Flavonoids of some species of Sedum. Planta Med 30:384–7
- Scalbert A, Manach C, Morand C, Remesy C. (2005). Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci 45:287–306
- Stevens JF, Elema ET, T’Hart H. (1994). Myricetin 3-O-arabinofuranoside from Sedum montanum ssp. orientale. Biochem Syst Ecol 22:861–2
- Tel G, Deveci E, Küçükaydın S, et al. (2013a). Evaluation of antioxidant activity of Armillaria tabescens, Leucopaxillus gentianeus and Suillus granulatus: The mushroom species from Anatolia. Eurasian J Anal Chem 8:136–47
- Tel G, Öztürk M, Duru ME, et al. (2013b). Fatty acid composition, antioxidant, anticholinesterase and tyrosinase inhibitory activities of four Serratula species from Anatolia. Rec Nat Prod 7:86–95
- Thuong PT, Ho JK, Min-Kyun N, et al. (2007). Anti-oxidant constituents from Sedum takesimense. Phytochemistry 68:2432–8
- Torres RS, Rosales HM, Zamora DR, et al. (2003). Spermicidal activity of the crude ethanol extract of Sedum praealtum in mice. J Ethnopharmacol 85:15–17
- Wang Y, Lai D, Zhang Y, et al. (2009). Study of steroidal saponins in Dioscorea zingiberensis C.H. Wright. J Nat Prod (India) 2:123–32
- Wolbis M. (1987). Polyphenolic compounds in certain species of Sedum I. Coumarins and phenolic acids in Sedum acre, Sedum album and Sedum maximum. Acta Pol Pharm 44:563–6
- Wolbis M. (1989a). Polyphenolic compounds in Sedum species. III: Sedum reflexum. Acta Pol Pharm 46:500–5
- Wolbis M. (1989b). Flavonol glycosides from Sedum album. Phytochemistry 28:2187–9
- Wolbis M, Krolikowska M. (1988). Flavonol glycosides from Sedum acre. Phytochemistry 27:3941–3
- Wollenweber EM, Doerr K, Siems R, et al. (1999). Triterpenoids in lipophilic leaf and stem coatings. Biochem Syst Ecol 27:103–5
- Zaitsev VG, Fursa NS, Belyaeva LE. (1983). Hydroxycoumarins and flavonol 7-monorhamnosides of Sedum caucasicum. Chem Nat Compd 4:527–52