Abstract
Context: 2,7-Dihydroxy-3-methylanthraquinone (DDMN) is reported to have a remarkable anticancer activity against gastric cancer SGC-7901 cells.
Objective: The objective of this study is to study the anticancer effect and mechanism of DDMN on SGC-7901 cells.
Materials and methods: The MTT assay was used to determine the effect of DDMN on cell viability of SGC-7901 cells, and the cytotoxic effect was evaluated by the IC50 value. After treatment with different doses of DDMN (10, 20, and 40 μM) for 48 h, flow cytometry was used to investigate the apoptosis of SGC-7901 cells induced by DDMN. Further, western blotting was performed to study anticancer mechanism by assaying apoptosis-related proteins containing Mcl-1, Bcl-xl, Bcl-2, Bax, Bak, Bad, cytochrome c, caspase-3, and caspase-9. Finally, xenograft assay was used to further evaluate the effect of DDMN on SGC-7901 cells by determining body weight of nude mice, tumor volumes, and apoptosis-related proteins.
Results: These results suggest that DDMN can significantly inhibit (IC50 value = 20.92 μM) the proliferation of SGC-7901 cells and induce apoptosis of SGC-7901 cells demonstrated by flow cytometry analysis. Additionally, the results of western blotting indicated that DDMN can suppress the expression of anti-apoptotic proteins Bcl-xl and Bcl-2, increase the expression of pro-apoptotic proteins Bax, Bad (40 μM), caspase-3 and caspase-9, and evidently promote the release of cytochrome c from the mitochondria to the cytoplasm. The xenograft assay further confirmed that DDMN had significant anticancer effects on SGC-7901 cells.
Conclusion: DDMN had significant anticancer effect on SGC-7901 cells in vitro and in vivo related to mitochondria-mediated apoptosis.
Introduction
Hedyotidis diffusae herba, a common folk medicine, is from Hedyotis diffusa Willd. (Rubiaceae), widely used in China. In the past decade, a few anticancer studies of Hedyotidis diffusae herba have been reported (Che et al., Citation2007). Lin et al. (Citation2010) demonstrated that the ethanol extract of Hedyotidis diffusae herba can induce the apoptosis of HT-29 human colon carcinoma cell line related to mitochondrion-medicated apoptosis and further investigation (Lin et al., Citation2011a) showed that the anticancer mechanism of Hedyotidis diffusae herba may be related to inhibiting tumor angiogenesis. Liu et al. (Citation2010) suggested that methylanthraquinone from Hedyotidis diffusae herba can induce MCF-7 human breast cancer cells apoptosis via Ca2+/calpain/caspase-4 pathway. Lin et al. (Citation2011b) indicated that Hedyotis diffusa had an antiproliferative-activity on Murine leukemia WEHI-3 cells, and could promote T- and B-cell proliferation in leukemic mice. In addition, there were several further reports about the effect of Hedyotidis diffusae herba on different cancer cells (Chen et al., Citation2012; Gupta et al., Citation2004; Lee et al., Citation2011; Shi et al., Citation2008; Wang et al., Citation2011; Willimott et al., Citation2007). Due to the broad anticancer activity of Hedyotidis diffusae herba, its chemical components have drawn our attention.
Although there have been reports of the antitumor activity of Hedyotidis diffusae herba, with the discovery of 2,7-dihydroxy-3-methylanthraquinone (DDMN) (Yu et al., Citation2008), the antitumor activity of Hedyotidis diffusae herba still draws the attention of researchers. According to our team’s preliminary study, DDMN can inhibit proliferation of human gastric cancer SGC-7901 cells demonstrated by MTT assay. Continuing the study of the anticancer effect and mechanism of DDMN on human gastric cancer SGC-7901 cells, we report the results of further MTT assays, flow cytometry analyses, western blotting, and xenograft assay in this work.
Materials and methods
Plant material
Hedyotidis diffusae herba was purchased from www.ZYCTD.com in 2012, and was identified by Liu Yang, Liaoning Cancer Hospital & Institute’s pharmacy department, Shenyang Liaoning, China. A voucher specimen (voucher no. 2012354/LCHI) was stored in dispensary of traditional Chinese medicine of our hospital for future reference.
Chemicals and reagents
RPMI-1640 media and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA). MTT Cell Proliferation and Cytotoxicity Assay Kits were purchased from Beyotime Institute of Biotechnology (Shanghai, China). Annexin V-FITC/PI apoptosis assay kits were obtained from STANDARDS (Shanghai, China). Primary antibodies for Mcl-1, Bcl-xl, Bcl-2, Bax, Bak, Bad, cytochrome, caspase 3/9, and β-actin were purchased from Cell Signaling Technology (Beverly, MA) and Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody was purchased from Jackson Immuno Research Laboratories (West Grove, PA). Moreover, silica gel (300–400 mesh), all analytical grade reagents were purchased from Qingdao Haiyang Chemical Co. (Qingdao, China).
Animals
Nude mice 5–6 weeks old were obtained from the SLRC Laboratory Animal Company (Shanghai, China). Animals were appropriately treated, and all actions were consistent with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals. Experiments were performed with the approval of the Animal Experimentation Ethics Committee of Liaoning Cancer Hospital & Institute (LNCHI AEEC 2013).
Preparation of DDMN
Hedyotidis diffusae herba (30 kg) was extracted by 90% ethanol. The extract was concentrated under vacuum, and the concentrate (280 mL) was diluted with water (500 mL) and solution was extracted with chloroform. The chloroform layer was condensed and then chromatographed over silica-gel column (300–400 mesh), and eluted with chloroform–methanol (7:3, v/v). Fractions containing DDMN were combined and concentrated to yield 34 g. These fractions were purified by silica-gel column and eluted with gradient of petroleum ether-ethyl acetate (9:1, 7:3, 5:5, 3:7) to give DDMN (0.6 g, purity > 95%), identified by nuclear magnetic resonance (NMR) and high-performance liquid chromatography (HPLC).
Cell culture
Human gastric cancer SGC-7901 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). SGC-7901 cells were cultured in the RPMI-1640 medium containing 10% FBS, 100 U/mL streptomycin, and 100 U/mL penicillin at 37 °C in 5% CO2 and 95% air. Cells were sub-cultured till reaching logarithmic growth phase, and then experiments were immediately carried out on the re-cultured SGC-7901 cells.
MTT assay
In this work, MTT assay was used to evaluate the antiproliferation effect of DDMN on human gastric cancer SGC-7901 cells. According to the instructions of MTT Cell Proliferation and Cytotoxicity Assay Kits, the antiproliferation effect of different doses of DDMN on SGC-7901 cells, treated with DDMN for 48 h, was assessed by the optical density (OD) with a microplate reader (Bio Rad, Hercules, CA, Model 680) at 570 nm. The inhibition rate was used to evaluate the cell viability and its algorithm was the following formula: (ODcontrol − ODtreatment)/ODcontrol × 100% (Liu et al., Citation2013).
Assessment of apoptosis
After treatment with different doses of DDMN for 48 h, SGC-7901 cells were collected, and then were washed thrice with phosphate buffer solution (PBS). According to the instructions of the manufacturer, the washed SGC-7901 cells were resuspended in staining buffer and stained with Annexin V-FITC/PI, and then SGC-7901 cells were analyzed by flow cytometry.
Western blot analysis
After treatment with different doses of DDMN, total proteins of SGC-7901 cells were extracted, and the concentration of total proteins was determined using Enhanced BCA Protein Assay Reagent (Beyotime, Shanghai, China). Then, equal amounts of protein (40 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to a PVDF membrane. After PVDF membrane was blocked with 5% fat-free milk, the membrane was incubated with homologous primary antibodies overnight, and then washed with Tris buffered saline-Tween (TBS-T). The washed membrane was further incubated with HRP-conjugated goat anti-rabbit antibody in TBS-T. Straightway, pro- or anti-apoptotic-related proteins were detected by chemiluminescence. To assess protein loading, β-actin was selected as an internal reference.
Xenograft assay in vivo
Nude mice were randomly divided into two groups, control and DDMN groups (n = 10). SGC-7901 cells (2 × 106 cells/nude mouse) were subcutaneously injected in the right flank of nude mice. When the SGC-7901 cells-caused tumors grew to about 2–3 mm in diameter, the control and DDMN groups were separately treated with 0.5% DMSO and DDMN (40 mg/kg, dissolved in 0.5% DMSO) for 20 d by intraperitoneal injection. Then, the tumor sizes (width and length) and body weight of nude mice were measured on the fifth, 10th, and 15th days by a vernier caliper, and the tumor volumes were calculated according to the existing formula: volume = (width2 × length)/2 (Ma et al., Citation2012). Finally, nude mice were killed immediately, and the tumor tissues were collected for western blotting.
Statistical analysis
All data were presented as mean ± standard deviation (SD). A one-way ANOVA and two-tailed Student’s t-test were used to analyze the differences among 3 or more groups and between two groups separately. When p values were less than 0.05, the differences were recognized as statistically significant. All data were analyzed on SPSS 21.0 (SPSS Inc., Chicago, IL).
Results
Identification and purity of DDMN
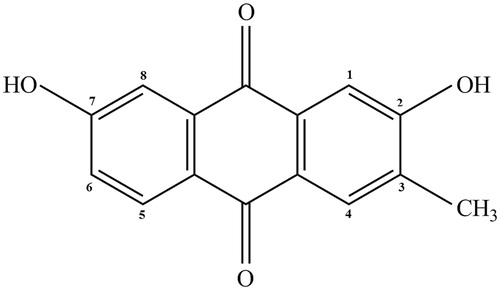
According to 13C-NMR data of target analyte, target analyte can be identified as DDMN when compared with the existing literature (Yu et al., Citation2008). The structure of DDMN is presented in . The purity of DDMN was more than 95% determined by area normalization method of HPLC.
Effect of DDMN on SGC-7901 cells proliferation
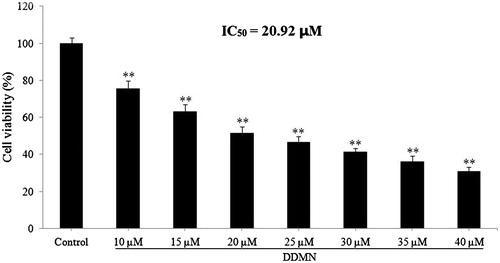
In this study, the effect of different doses of DDMN on SGC-7901 cell proliferation and cytotoxicity was accessed by MTT assay. The results suggested that DDMN had significant antiproliferation and cytotoxicity activities against SGC-7901 cells at doses of 10–40 μM (p < 0.01), and the IC50 value was 20.92 μM. The results are shown in .
Result of the flow cytometry analysis
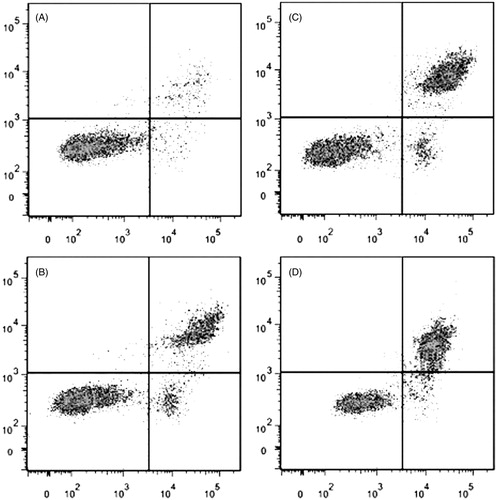
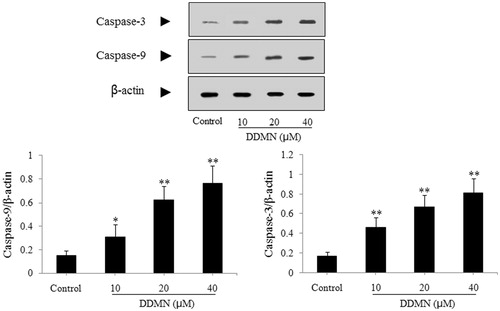
From the result of MTT assay, it is obvious that DDMN possessed the antiproliferation activity on SGC-7901 cells. Then, the flow cytometry analysis was used to confirm whether the antiproliferation effect of DDMN on SGC-7901 cells was related to apoptosis. As depicted in , after treatment with DDMN at doses of 10, 20, and 40 μM for 48 h, significant apoptosis of SGC-7901 cells was observed. Therefore, the antiproliferation activity of DDMN on SGC-7901 cells was related to apoptosis.
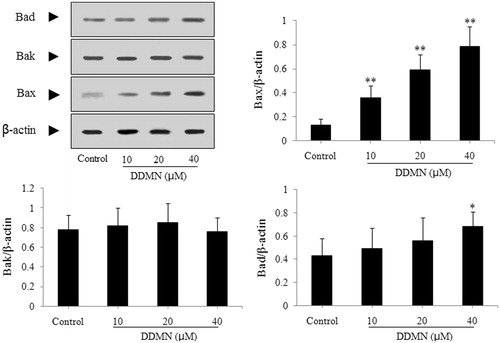
Effects of DDMN on anti- and pro-apoptotic proteins
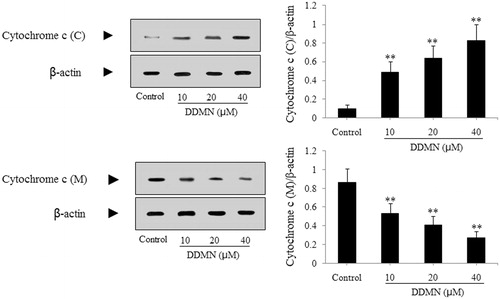
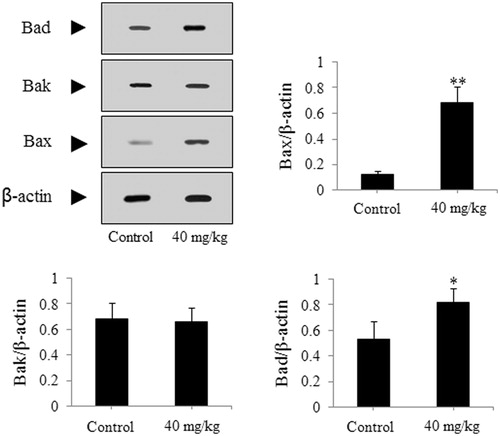
According to the result of the flow cytometry analysis, DDMN can induce the apoptosis of SGC-7901 cells. To study the mechanism that DDMN induced SGC-7901 cells apoptosis, anti-apoptotic proteins (Mcl-1, Bcl-xl, and Bcl-2) and pro-apoptotic proteins (Bax, Bak, Bad, cyctochrome c, caspase-3, and caspase-9) were investigated by western blotting. As shown in , DDMN (10, 20, and 40 μM) can inhibit the expression of anti-apoptotic Bcl-xl and Bcl-2, and increase the expression of pro-apoptotic Bax, caspase-3, and caspase-9; and significantly promote the release of cytochrome c from the mitochondria to the cytoplasm (p < 0.05 or 0.01). Moreover, when DDMN was 40 μM, DDMN can significantly upregulate the expression of pro-apoptotic Bad (p < 0.05). However, the expression level of other proteins including anti-apoptotic Mcl-1 and pro-apoptotic Bak had no significant changes.
Figure 4. Effect of DDMN on expression of anti-apoptotic protein including Mcl-1, Bcl-xl, and Bcl-2 in SGC-7901 cells. *p < 0.05, **p < 0.01, compared with the control.
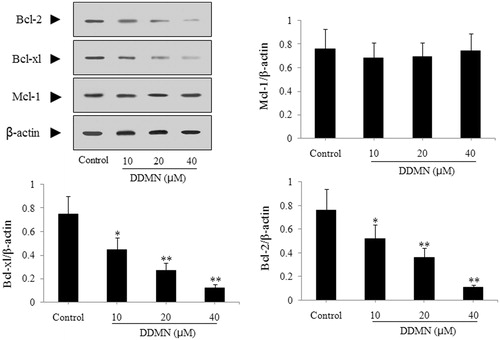
Figure 5. Effect of DDMN on expression of pro-apoptotic protein including Bax, Bak, and Bad in SGC-7901 cells. *p < 0.05, **p < 0.01, compared with the control.
Anticancer effect of DDMN ON SGC-7901-caused xenograft model
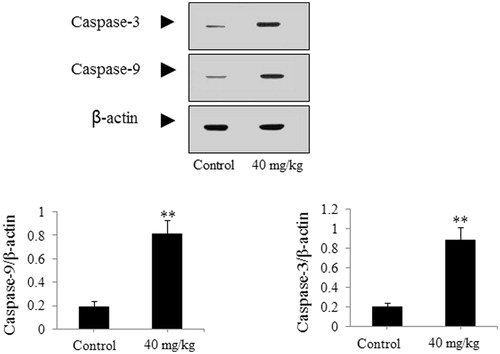
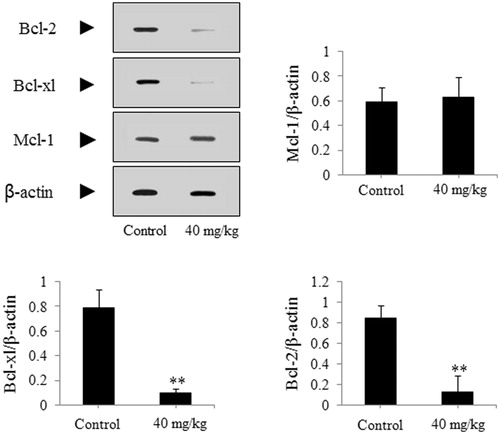
The SGC-7901-caused xenograft model was used to further authenticate the effect of DDMN on SGC-7901 cells. As presented in , after treatment with DDMN for 20 d (40 mg/kg/d), tumor growth on nude mice could be significantly inhibited during the 20-d observation period (p < 0.05 or 0.01), compared with the control group. However, body weight of nude mice did not have significant difference between the DDMN group and the control group. In addition, the results of western blotting indicated that the expression of anti-apoptotic Bcl-xl and Bcl-2 was significantly down-regulated (p < 0.01), and the expression of pro-apoptotic Bax, Bad, caspase-3, and caspase-9 was significantly up-regulated (p < 0.05 or 0.01) in the tumor tissues of the SGC-7901 cells-caused xenograft model, compared with control group. The results are shown in .
Figure 8. Effect of DDMN on tumor volumes and body weight of nude mice in the SGC-7901 cells-caused xenograft model. *p < 0.05, **p < 0.01, compared with the control.
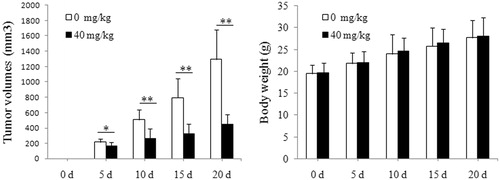
Figure 9. Effect of DDMN on expression of anti-apoptotic protein including Mcl-1, Bcl-xl, and Bcl-2 in the SGC-7901 cells-caused xenograft model. **p < 0.01, compared with the control.
Discussion
In this study, MTT assay, flow cytimetry analysis, western blotting, and xenograft assay were used to study the effect of DDMN on SGC-7901 cells, and it was demonstrated that DDMN possessed antigastric cancer activity for the first time.
Human gastric cancer SGC-7901 cells, a common cell line, were used to study antigastric cancer of drugs in vitro (Chen et al., Citation2007; Ji et al., Citation2011; Li & Xu, Citation2003), especially anthraquinones, such as rhein (Li et al., Citation2012) and emodin (Cai et al., Citation2008; Sun & Bu, Citation2012). Metabolism and growth rate of cells could be out of control due to the activation of proto-oncogene of normal cells. For changing the situation of abnormal cells, inducing cells apoptosis was the commonly used treatment. The mitochondria-mediated apoptosis was an important pathway in inducing cancer cells apoptosis (Shi, Citation2001; Tang et al., Citation2006). Bcl-2 family proteins including Mcl-1, Bcl-xl, Bcl-2, Bax, Bak, Bad, etc., cytochrome c, caspase-3, and caspase-9 played a crucial role in mitochondria-mediated apoptosis (Kim et al., Citation2007; Shi, Citation2001). The expression level and the activity of these mitochondria-mediated apoptosis-related proteins would be changed along with the variation of reactive oxygen in cells or proto-oncogene and so on. If cancer cells were treated with anticancer reagents which can induce cells apoptosis related to mitochondria-mediated apoptosis, cancer cells would experience the following process. At first, the expression level and the activity of Bcl-2 family proteins would be regulated (Adams & Cory, Citation1998; Harris & Thompson, Citation2000; Reed, Citation1997, Citation1998), including the decrease of the expression level and the activity of anti-apoptotic proteins, such as Mcl-1, Bcl-xl, and Bcl-2, and the increase of the expression level and the activity of pro-apoptotic proteins, such as Bax, Bak, and Bad. Then the adjustment of expression level and the activity of the Bcl-2 family proteins could result in mitochondrial damage and swelling, which could lead to the increase of outer mitochondrial membrane permeability. Further, cytochrome c was released from the mitochondria to the cytoplasm (Yang et al., Citation2014). The cytochrome c along with apoptotic protease activating factor-1 (Apaf-1), procaspase-9 and ATP could form multiprotein complex (apotosome) in cytoplasm (Zhang, Citation2007). Then the caspase recruitment domain (CARD) of procaspase-9 and Apaf-1 was activated, and effective caspases, such as caspase-3, caspase-6, and caspase-7, were thus activated (Chen et al., Citation2014; Licht et al., Citation2014). Finally, cancer cells apoptosis took place.
The results of MTT assay indicated that DDMN can significantly inhibit (IC50 value = 20.92 μM) the proliferation of SGC-7901 cells. Furthermore, the results of flow cytimetry analysis demonstrated that antiproliferation activity of DDMN on SGC-7901 cells was related to apoptosis. Additionally, the results of western blotting suggested that DDMN can inhibit the expression of anti-apoptotic proteins (Bcl-xl and Bcl-2), increase the expression of pro-apoptotic proteins (Bax, Bad (40 μM), caspase-3, and caspase-9), and evidently promote the release of cytochrome c from the mitochondria to the cytoplasm significantly. Further, xenograft assay confirmed that DDMN had significant antigastric cancer effect. These results suggested that DDMN can induce SGC-7901 cells apoptosis related to mitochondria-mediated apoptosis.
Conclusion
According to these results, DDMN had significant anticancer effect on human gastric cancer SGC-7901 cells in vitro and in vivo, related to mitochondria-mediated apoptosis. However, researches about further antigastric cancer mechanism of DDMN were needed to be elucidated in the future work.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Adams JM, Cory S. (1998). The Bcl-2 protein family: Arbiters of cell survival. Science 281:1322–6
- Cai J, Niu X, Chen YY, et al. (2008). Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia 10:41–51
- Che JC, Xin N, Fen J. (2007). Research progress of pharmacological functions of Hedyotis diffusa. J Anhui Agric Sci 35:6162–7
- Chen XJ, Deng CS, Tang SL, et al. (2007). Mitochondria-dependent apoptosis induced by nanoscale hydroxyapatite in human gastric cancer SGC-7901 cells. Bio Pharm Bull 30:128–32
- Chen XZ, Cao ZY, Chen TS, et al. (2012). Water extract of Hedyotis diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol Rep 28:742–8
- Chen Y, Yang SH, Hueng DY, et al. (2014). Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem-Biol Interact 216:17–25
- Gupta S, Zhang D, Yi JZ, et al. (2004). Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother 4:21–33
- Harris MH, Thompson CB. (2000). The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ 7:1182–91
- Ji YB, Qu ZY, Zou X. (2011). Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway. Exp Toxicol Pathol 63:69–78
- Kim YA, Xiao D, Xiao H, et al. (2007). Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol Cancer Ther 6:1599–609
- Lee HZ, Bau DT, Kuo CL, et al. (2011). Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am J Clin Med 39:201–13
- Li S, Xu W. (2003). Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J Gastroenterol 9:1930–4
- Li YW, Xu YQ, Lei B, et al. (2012). Rhein induces apoptosis of human gastric cancer SGC-7901 cells via an intrinsic mitochondrial pathway. Braz J Med Biol Res 45:1052–9
- Licht V, Noack K, Schlott B, et al. (2014). Caspase-3 and caspase-6 cleave STAT1 in leukemic cells. Oncotarget 5:2305–17
- Lin CC, Kuo CL, Lee MH, et al. (2011b). Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T-and B-cell proliferation in leukemic mice. In Vivo 25:633–40
- Lin JM, Chen YQ, Wei LH, et al. (2010). Hedyotis diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int J Oncol 37:1331–8
- Lin JM, Wei LH, Xu W, et al. (2011a). Effect of Hedyotis diffusa Willd extract on tumor angiogenesis. Mol Med Rep 4:1283–8
- Liu HY, Zhu YJ, Zhang T, et al. (2013). Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 18:13357–68
- Liu Z, Liu M, Liu M, et al. (2010). Methylanthraquinone from Hedyotis diffusa Willd induces Ca2+-medicated apoptosis in human breast cancer cells. Toxicol In Vitro 24:142–7
- Ma YS, Weng SW, Lin MW, et al. (2012). Antitumor effects of emodin on LS1034 human colon cancer cells in vitro and in vivo: Roles of apoptotic cell death and LS1034 tumor xenografts model. Food Chem Toxicol 50:1271–8
- Reed JC. (1997). Bcl-2 family proteins: Regulators of apoptosis and chemoresistance in hematologic malignancies. Semi Hematol 34:9–19
- Reed JC. (1998). Bcl-2 family proteins. Oncogene 17:3225–36
- Shi Y, Wang CH, Gong XG. (2008). Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa Willd. Biol Pharm Bull 31:1075–8
- Shi YG. (2001). A structural view of mitochondria-mediated apoptosis. Nat Struct Biol 8:394–401
- Sun ZH, Bu P. (2012). Downregulation of phosphatase of regenerating liver-3 is involved in the inhibition of proliferation and apoptosis induced by emodin in the SGC-7901 human gastric carcinoma cell line. Exp Ther Med 3:1077–81
- Tang W, Liu JW, Zhao WM, et al. (2006). Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci 80:205–11
- Wang JH, Shu LH, Yang LL, et al. (2011). 2-Hydro-3-methylanthraquinone from Hedyotis diffusa Willd induces apoptosis alteration of fas/fasl and activation of caspase-8 in human leukemic TPH-1 cells. Arch Med Res 42:577–83
- Willimott S, Barker J, Jones LA, et al. (2007). Apoptosis effect of Oldenlandia diffusa on the leukaemic cell line HL60 and human lymphocytes. J Ethnopharmacol 114:290–9
- Yang XK, Xu MY, Xu GS, et al. (2014). In vitro and in vivo antitumor activity of scutebarbatine A on human lung carcinoma A549 cell lines. Molecules 19:8470–751
- Yu L, Li JM, Jiang Z, et al. (2008). A new anthraquinone from Hedyotis diffusa. Chin J Med Chem 18:298–9
- Zhang QZ. (2007). Molecular Pharmacology. Beijing, China: Higher Education Press