Abstract
Context: Natural products are good sources of natural dietary antioxidants that are believed to protect the body against hepatotoxic effect induced by oxidative stress. Hedyotis diffusa Willd (Rubiaceae) (HDW) is a traditional Chinese medicinal herb that has been shown to possess a variety of antioxidant properties.
Objective: The present study examines and explains the cell protective property of HDW water extract (WEHDW).
Materials and methods: 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH) assay was used to measure the free radical scavenging property of WEHDW (0.001–10 mg/mL). The protective effect of WEHDW (0.3–10 mg/mL 2 h pretreatment) against hydrogen peroxide (H2O2, 200 μM for 6 h) induced cytotoxicity in human hepatic cells, LO2, was evaluated using cell viability assay and nuclear staining. The molecular pathway of WEHDW's effect was investigated by using Western blot assay.
Results: WEHDW had a 50% scavenging concentration (SC50) at 0.153 mg/mL in the DPPH assay. Exposure of LO2 cells to H2O2 resulted in apoptosis which could be markedly attenuated by pre-treating WEHDW in a concentration-dependent manner (0.5, 1, 3, 5, or 10 mg/mL) (all with p < 0.001, versus control). Moreover, Hoechst (nuclear) staining showed that 1 mg/mL WEHDW could protect LO2 cells by attenuating apoptotic cell death mediated by H2O2. It was found that WEHDW reversed H2O2-induced activation of MEK/ERK pathway and H2O2-induced inhibition of P13-K/AKT/GSK3β pathway in LO2 cells.
Discussion and conclusion: WEHDW may help to improve the antioxidant defense system, resulting in prevention of oxidative stress-related fatty liver diseases.
Introduction
In recent years, non-alcoholic fatty liver disease (NAFLD) has been recognized as a major cause of liver disease and hepatocellular carcinoma (HCC). Histopathological features of NAFLD can include a wide spectrum of liver damage, ranging from simple steatosis to steatohepatitis, advanced fibrosis, hepatitis and cirrhosis, cell death by necrosis, and apoptosis (Marrero et al., Citation2002; Ockner, Citation2001; Serviddio et al., Citation2010). In fact, there are multiple factors for the development and progression of NAFLD, and oxidative stress is one of them (Caro & Cederbaum, Citation2004; Roskams et al., Citation2003; Xu et al., Citation2003).
Reactive oxygen species (ROS) generally represents highly reactive molecules, including oxygen radicals such as hydroperoxyl and superoxide radical, and non-radical oxygen derivatives such as hydrogen peroxide (H2O2). Recent studies have demonstrated that decreasing ROS production and increasing amounts of cellular antioxidants might have therapeutic benefits in chronic diseases and diseases that are caused by oxidative stress (Bhattacharya et al., Citation2011; Conde de la Rosa et al., Citation2008; Feng et al., Citation2011; Halliwell & Cross, Citation1994). Therefore, antioxidants are thought to be substances that are capable of protecting against hepatic injury in NAFLD. Apart from cellular antioxidants, dietary food is also an important source of antioxidants. Phytochemicals in plants are potential sources of natural antioxidants such as phenolic acids, flavonoids, and tannins (Moure et al., Citation2000). Recently, antioxidant content and activity in Traditional Chinese Medicine (TCM) has been studied intensively. Many Chinese medicinal herbs were found to contain high quantity of constituents that possess antioxidative properties (Chan, Citation2012; Chan et al., Citation2008).
Hedyotis diffusa Willd (Rubiaceae) (HDW), a well-known TCM herb, is widely distributed throughout the northeast Asia and the southern regions of China (Ko, Citation1999). Phytochemical studies revealed that the major components of HDW are iridoid glycosides, anthraquinones, flavones, steroids, phenylpropanoids, and polysaccharides (Yan et al., Citation2012). HDW is widely used in Chinese medicine formulae for treating inflammation-related diseases such as hepatitis, bronchitis, and urethral infection. HDW has been used for a long time as an adjuvant therapy against various cancers, including HCC and lung cancer (Caro & Cederbaum, Citation2004; Chen et al., Citation2012; Liang et al., Citation2008; Xu et al., Citation2003). Crude HDW polysaccharides isolated using graded ethanol precipitation have also been intensively investigated for their antioxidant and anticancer capacities (Yan et al., Citation2012). Additionally, HDW has been proven to have a high level of lanthanum (La) that can help preventing and treating hyperlipidemia caused by trace element deficiency (Moure et al., Citation2000), a disease that is well known to be related to NAFLD. Clinical and experimental research has also reported the hepatoprotective effects of HDW extract combined with other TCM (Xu & Xu, Citation2009). It has been demonstrated that HDW could alleviate the degree of liver damage induced by intraperitoneal administration of hepatotoxins (carbon tetrachloride or d-galactosamine) in rats (Lin et al., Citation2002).
However, there are few studies on the mechanism of the protective effects of HDW on liver. Therefore, aim of this study was to investigate the hepatoprotective effect of HDW against H2O2-induced liver damage in vitro. The mechanisms of HDW's hepatoprotective effects were studied using Western blot assay. Normal liver cells (LO2) were used in the experiment in order to evaluate its hepatoprotective effect. HDW was extracted with boiling water to mimic the process of preparation TCM decoction. This study aimed at providing scientific evidence for suggesting the use of HDW as an effective and economical herb for human consumption to prevent liver diseases.
Materials and methods
Chemical and reagents
RPMI 1640 medium, fetal bovine serum (FBS), phosphate buffer saline (PBS), penicillin/streptomycin, trypsin, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), and Hoechst 33342 were purchased from Invitrogen (Grand Island, NY). H2O2, PD98059 were obtained from Calbiochem (San Diego, CA). 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl (DPPH), ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO). ECL plus kit were purchased from Amersham Bioscience (Aylesbury, UK). Antibodies against phospho-MEK 1/2, phospho-ERK 1/2, phospho-AKT, and phospho-GSK3β were bought from Cell Signaling Technology (Beverly, MA). Antibody against β-actin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of water extract of H. diffusa Willd (WEHDW)
HDW was weighed and 50 g of the dried herb was first extracted with 300 mL of boiling ultra-filtered water for 1.5 h. The residues were re-extracted with 300 mL of boiling ultra-filtered water for another hour. The extract was centrifuged at 5000 rpm for 15 min to remove any suspended solid. The supernatant was freeze-dried for 48–72 h to obtain a dry powder. Samples were stored at −20 °C before use. They were dissolved in MilliQ water and sequentially passed through 0.22 μm filters for sterilization and then further diluted in MilliQ water before use.
Cell culture and treatment
Human normal hepatocytes (LO2 cells) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). LO2 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (100 µg/mL) in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. They were routinely sub-cultured with trypsin–EDTA every 3–4 d.
Cell viability assay
The viability of LO2 cells was assessed by using MTT assay. Briefly, the medium was removed and replaced with the medium (100 µL/well) containing 10% FBS after incubation. Each well was added with 10 µL of a 5 mg/mL MTT solution prepared in PBS. After incubation for 4 h at 37 °C, the cell supernatants were discarded and MTT crystals were dissolved in 100 µL DMSO. The absorbance of the samples was measured at a wavelength of 570 nm with 655 nm as a reference wavelength. All assays were performed in triplicate. Unless otherwise indicated, the extent of MTT conversion in cells exposed to H2O2 is expressed as a percentage of the control.
DPPH free radical scavenging activity
DPPH is a stable free radical. It appears as red-purple in methanol and has a maximal absorption at 515 nm. Various concentrations of selected samples were prepared by dissolving the dried extract powder with MilliQ water. Test solutions (50 μL) were added to 1.95 mL of DPPH methanol solution (24 mg/L) and allowed to react for 1 h. After 1 h of incubation, the absorbance value of the sample (Asample) was determined at 515 nm. The free radical scavenging capacity (SR%) was calculated by the following equation:
Acontrol: absorbance value of DPPH• solution with 50 μL of water.
Asample: absorbance value of DPPH• solution with 50 μL of herbal extract.
The 50% scavenging concentration (SC50) was calculated by the software GraphPad Prism (GraphPad Software, San Diego, CA).
Hoechst staining assay
Chromatin condensation was detected by nucleus staining with Hoechst 33342. LO2 (2 × 106 cells/well) (in a 12-well plate) were washed with ice-cold PBS. Cells were then stained with Hoechst 33342 (5 μg/mL) for 5 min. Nuclei were visualized using a fluorescence microscope at × 400 magnification.
Western blot assay
Briefly, cells were harvested in cell lysis buffer (Cell Signaling Technology, Beverly, MA) with 1 mM PMSF. After incubation on ice for 15 min and centrifugation at 14 000 rpm at 4 °C for 10 min, whole protein concentrations were determined by the BSA assay (Pierce, Rockford, IL) using bovine serum albumin as standard. Cell lysates were diluted in the SDS sample buffer, and the mixture was boiled for 5 min. The protein (30 µg) was separated on a 10% SDS-polyacrylamide gel. Blocking was performed onto polyvinyldifluoride membranes with a blocking buffer and detected using primary antibodies. After incubation overnight at 4 °C, signals were obtained by binding a secondary horseradish peroxidase-conjugated antibody. The membrane was probed with primary antibody (1:1000) followed by secondary antibody and visualized using an ECL plus kit (Amersham Bioscience, Aylesbury, UK) and exposed to FujiFilm autoradiographic films according to the protocol of the manufacturer.
To determine whether WEHDW's anti-apoptotic effect involves ERKs (extracellular signal-regulated kinases) and PI3-K/AKT/GSK3β pathways, LO2 cells were pre-treated with various specific inhibitors, such as PD98059 (a specific inhibitor of MEK) or SB415286 (a specific inhibitor of GSK3β) for 2 h before challenging with H2O2. LY29400 (a specific P13-K inhibitor) pretreatment was 30 min before challenging with H2O2. The treated LO2 cells were harvested for subsequent Western blot assay.
Statistical analysis
All data were expressed as means ± SEM. Differences in the means among groups were assessed for significance by one-way ANOVA combined with the Bonferroni test. All statistical analyses were performed by GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, CA).
Results
Assessment of the antioxidant properties of WEHDW
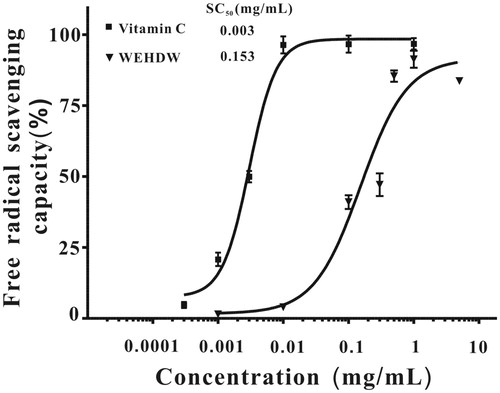
To study the antioxidant properties of WEHDW, DPPH radicals were used to interact with WEHDW (0.001–10 mg/mL) chemically at different concentrations. The result is shown in . Vitamin C was used as a standard. shows that vitamin C could scavenge almost all DPPH radicals starting at 0.01 mg/mL while WEHDW could scavenge around 90% DPPH radicals at 1 mg/mL. Vitamin C had SC50 at 0.003 ± 0.001 mg/mL; WEHDW had SC50 at 0.150 ± 0.006 mg/mL.
WEHDW attenuates H2O2-induced cell death in LO2 cells
The effect of H2O2 (50, 100, 150, 200, 250, 300, 400, or 500 μM) on LO2 cell viability was evaluated. Cell viability was measured by MTT assay. As shown in , the cell viability was significantly reduced in a concentration-dependent manner. Based on these results, challenging the cells with 200 μM H2O2 for 6 h was selected as an optimal condition for subsequent experiments as cell viability reduced to 52.80% (p < 0.001 when compared with the non-treated group).
Figure 2. Examination of the cytotoxicity of H2O2 and the protective effect of WEHDW against H2O2-induced damage on LO2 cells. (A) Cells were treated with different concentrations (50, 100, 150, 200, 250, 300, 400, or 500 μM) of H2O2 for 6 h. Data were expressed as mean ± SEM of three separate experiments; ###p < 0.001 versus control. (B) LO2 cells were pretreated with WEHDW (0.3, 0.5, 1, 3, 5, or 10 mg/mL, 2 h) before challenging with H2O2 (200 μM, 6 h). Data were expressed as the means ± SEM of three separate experiments; ###p < 0.001 versus control and ***p < 0.001 versus the H2O2 alone group.
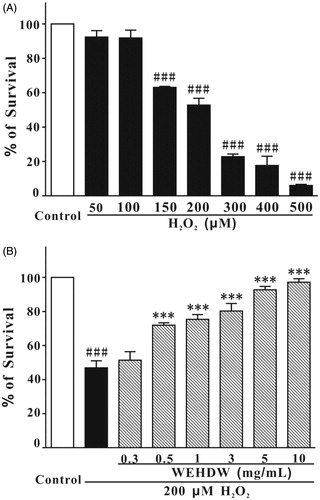
To investigate the effects of WEHDW on H2O2-induced toxicity, LO2 cells were pre-incubated with WEHDW at concentrations of 0.3, 0.5, 1, 3, 5, or 10 mg/mL for 2 h before incubation with H2O2 (200 μM for 6 h). WEHDW could suppress H2O2-induced cell death significantly (0.5 mg/mL = 71.96 ± 1.38%, p < 0.001; 1 mg/mL = 75.35 ± 2.84%, p < 0.001; 3 mg/mL = 80.25 ± 4.42%, p < 0.001; 5 mg/mL = 92.75 ± 1.97%, p < 0.001; and 10 mg/mL = 97.15 ± 2.10%, p < 0.001) in a concentration-dependent manner as compared with the group treated with 200 μM H2O2 alone (46.87 ± 4.17%) (). Adding different concentrations of WEHDW (0.3, 0.5, 1, 3, 5, or 10 mg/mL) alone to the LO2 cells had no significant effect on cell proliferation or on toxicity (data not shown).
WEHDW decreases the apoptosis induced by H2O2 in LO2 cells
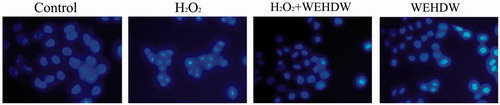
To determine whether H2O2 induces LO2 cells death through apoptosis, DNA staining with Hoechst 33342 was used to evaluate nuclear condensation. WEHDW (1 mg/mL) was added to the LO2 cells for 2 h and then the LO2 cells were exposed to 200 μM H2O2 for 6 h. Hoechst 33342 staining assay showed that 1 mg/mL WEHDW decreased the number of cells with brightly staining condensed chromatin and nuclear fragmentation significantly (). Adding WEHDW alone had no effect on Hoechst 33342 staining assay as compared with the control group.
WEHDW attenuates H2O2-induced apoptosis in LO2 cells by inhibiting ERK pathway
Both 30 and 50 μM PD98059 significantly suppressed H2O2-induced cell death (30 μM = 78.55 ± 3.53%, p < 0.01; 50 μM = 75.90 ± 4.52%, p < 0.01) in a concentration-dependent manner as compared with the group treated with 200 μM H2O2 alone (53.00 ± 2.14%) ().
Figure 4. WEHDW attenuated the phosphorylation of MEK and ERK caused by H2O2. (A) PD98059 prevented H2O2-induced cell death in a concentration-dependent manner. After pre-treatment with PD98059 at different concentrations as indicated, LO2 cells were exposed to 200 μM H2O2 for 2 h. Cell viability was measured at 6 h after the H2O2 challenge by MTT assay. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments, ###p < 0.001 versus control and **p < 0.01 versus the H2O2 alone group. (B) H2O2 time-dependently increased the levels of phospho-MEK and phospho-ERK in the first 15 min. LO2 cells were incubated with 200 μM H2O2 at the indicated time points, and total proteins were detected with the use of specific antibodies. WEHDW attenuated the increase of phospho-MEK and phospho-ERK caused by H2O2. LO2 cells were pre-treated with 1 mg/mL WEHDW for 2 h and then exposed to 200 μM H2O2 for 15 min.
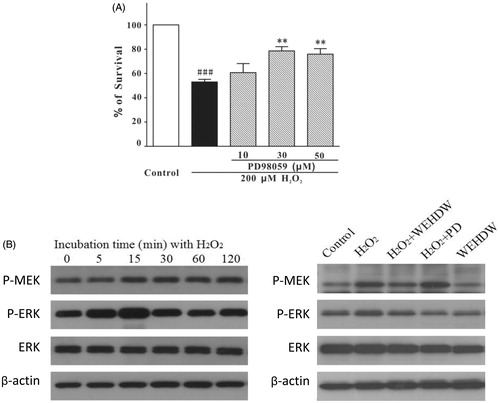
To investigate whether WEHDW could protect LO2 cells through inhibition of the ERK pathway, the levels of phospho-MEK and phospho-ERK were determined by Western blot assay. As shown in , the level of phospho-ERK peaked at 15 min after the challenge of 200 μM H2O2 in a time-dependent manner and WEHDW at 1 mg/mL significantly reversed this event. Adding 1 mg/mL WEHDW for 2 h could prevent the increase of phospho-MEK and phospho-ERK caused by H2O2, indicating that WEHDW may have protective effects against H2O2-induced apoptosis by altering the level of phospho-MEK and phospho-ERK in LO2.
WEHDW reverses the H2O2-induced suppression of PI3-K/AKT/GSK3β pathway in LO2 cells
As shown in , SB415286 at both 30 μM and 50 μΜ significantly protected LO2 cells against H2O2-induced apoptosis (30 μM = 55.56 ± 1.05%, p < 0.001; 50 μM = 57.46 ± 2%, p < 0.001). To further examine whether WEHDW can protect LO2 cells from the inhibition induced by GSK3β activity, the level of phospho-GSK3β was studied. The level of phospho-GSK3β decreased remarkably in a time-dependent manner after LO2 cells were treated with 200 μM H2O2 for the first 30 min (). This inhibition of phospho-GSK3β could be reversed by adding 1 mg/mL WEHDW.
Figure 5. WEHDW activated the phosphorylation of P13-K/AKT/GSK3β pathway. (A) SB415286 prevented H2O2-induced apoptosis in a concentration-dependent manner. LO2 cells were exposed to 200 μM H2O2 at 2 h after pre-treatment with SB415286 at different concentrations as indicated. Cell viability was measured at 6 h after exposure to H2O2 by MTT assay. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments, ###p < 0.001 versus control and ***p < 0.001 versus the H2O2 alone group. (B) Specific P13-K inhibitor abrogated the hepatoprotective effects of WEHDW on H2O2-induced apoptosis. After pre-treatment with 1 mg/mL WEHDW, LO2 cells were incubated with 30 μM or 50 μM LY294002 for 30 min. Then, they were exposed to 200 μM H2O2 at 2 h. Cell viability was measured at 6 h after exposure to H2O2 by MTT assay. Data, expressed as percentage of control, were the mean ± SEM of three separate experiments, ###p < 0.001 versus control and *p < 0.05, **p < 0.01, and ***p < 0.001 versus the H2O2 alone group. (C) H2O2 time-dependently decreased the levels of phospho-AKT and phospho-GSK3β peaked at 30 min without affecting its expression levels. LO2 cells were incubated with 200 μM H2O2 at the indicated time points, and the total proteins were detected with the use of specific antibodies. WEHDW reversed the reduction of phospho-AKT and phospho-GSK3β caused by H2O2. LO2 cells were pre-treated with 1 mg/mL WEHDW for 2 h and then exposed to 200 μM H2O2 for 30 min.
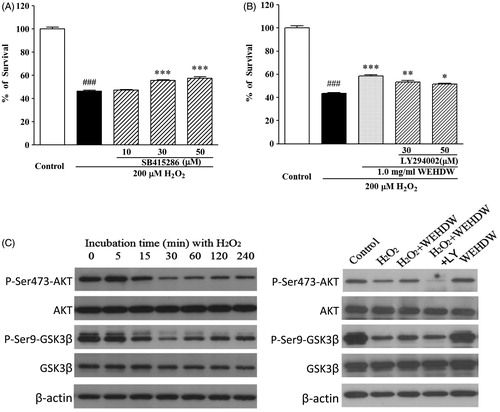
To study whether WEHDW can protect LO2 cells from the H2O2-induced apoptosis by activating PI3-K/AKT pathway, which is supposed to regulate GSK3β activity, the phosphorylation of protein in the P13-K/AKT pathway was tested by Western blot assay. The level of phospho-AKT decreased remarkably in a time-dependent manner after LO2 cells were treated with 200 μM H2O2 for the first 30 min (). This inhibition of phospho-AKT could be reversed by adding 1 mg/mL WEHDW. LY294002, a specific inhibitor of PI3-K, was used to pre-treat LO2 before adding WEHDW and H2O2. As shown in , LY294002 of 30 μΜ and 50 μΜ significantly abolished the hepatoprotective effect of WEHDW against H2O2-induced apoptosis (30 μM = 53.45 ± 1.24%, p < 0.01; 50 μM = 51.54 ± 0.78%, p < 0.05). Also, LY294002 could abolish the reversed effect of WEHDW in both phospho-GSK3β and phospho-AKT.
Discussion
The human liver is a vital organ responsible for many important functions such as detoxification and biochemical metabolisms. Therefore, it is susceptible to oxidative damage. H2O2 is thought to be the major source of highly reactive free radicals and it has been reported to decrease cell viability and induce apoptosis in many different cells such as chondrocytes (Asada et al., Citation2001), tendon fibroblasts, cardiomyocytes, human umbilical vein endothelial cell, and hepatocytes (Novosad et al., Citation2001). Our results demonstrated that H2O2 induced cell death in LO2 cells, and cell viability increased significantly after WEHDW pretreatment, suggesting that WEHDW might have hepatoprotective activity.
Current scientific evidence supports the view that antioxidant compounds can reduce tissue damage caused by oxidative stress through reducing ROS level (Binukumar et al., Citation2010; Guo et al., Citation2013; Milić et al., Citation1998). In the current study, WEHDW scavenged the free radicals produced by DPPH solution with a SC50 value equal to 0.153 ± 0.006 mg/mL. WEHDW showed a significant cytoprotective effect against H2O2-induced cell death in LO2 cells. The protective effect might be responsible for the chemical antioxidant activity of WEHDW.
Apoptosis plays a major role in the progression of H2O2-induced liver injury (Shimamatsu & Wanless, Citation1997). It is characterized by biochemical and morphological features of cells including chromatin condensation, nucleic acid fragmentation, membrane blabbing, and cell shrinkage (Guicciardi & Gores, Citation2010; Ravandi et al., Citation2001). The Hoechst staining assay was conducted to study apoptosis through morphological features of cells. Hoechst stains belong to a family of blue fluorescent dyes used to stain DNA (Latt et al., Citation1975). Apoptotic LO2 cells were detected by staining with DNA-binding fluorescent dye. Condensed chromatin would be stained brighter in apoptotic cells than in normal healthy cells (Kuetemeyer et al., Citation2010). It was found that H2O2-induced LO2 cells death through apoptosis. WEHDW (1 mg/mL) significantly decreased the number of cells with brightly stained condensed chromatin or nuclear fragmentation. It suggested that the cytoproetctive effect of WEHDW may relate to its anti-apoptotic effect.
ROS-induced intracellular signal molecules, such as mitogen-activated protein kinases (MAPKs), tumor necrosis factor-alpha (TNF-α) and nuclear factor (NF-κB), can contribute to cell death (Cohen et al., Citation2009; Schlatter et al., Citation2011). The MAPKs pathway is one of the fundamental signalling systems that exists in all eukaryotic organisms (Pearson et al., Citation2001). MAP kinase cascades, including ERK1/2, the p38 MAP kinases, and c-jun N-terminal kinase and stress-activated protein kinases (JNK/SAPK), have been identified. Oxidative stress-induced p42/44 MAPK (also called extracellular signal regulated kinases 1 and 2: ERK1/2) activation is a curial signaling transduction pathway in mitogen-activated protein kinases (MAPKs) and has been reported in a variety of cell types (McCubrey et al., Citation2007). ERK activation protects against cell injury by different mechanisms. In differentiated cells, ERK has different roles and are involved in different responses (McCubrey et al., Citation2007; Wang et al., Citation2008). ROS such as superoxide anions activate ERK (McCubrey et al., Citation2007) by inducing ERK phosphorylation only at concentrations that induce apoptosis (Conde de la Rosa et al., Citation2006). Previous studies have demonstrated that treatment with PD98059 blocks oxidative stress-induced ERK activation (Xing et al., Citation2011). This indicates that activating actions of oxidative stress do not occur directly on ERK but are localized at upstream targets (McCubrey et al., Citation2007).
It has been widely reported that the MEK/ERK pathways is involved in H2O2-induced apoptosis in LO2 cells (Aggeli et al., Citation2010; Conde de la Rosa et al., Citation2006). Since H2O2 is defined as one of the ROS that induces ERK phosphorylation, it was important to find out that H2O2-induced activation of ERK in a time-dependent manner (Conde de la Rosa et al., Citation2006). The phosphorylation of ERK was induced in a time-dependent manner after H2O2 treatment. It was observed that PD98059 eliminated H2O2-induced toxicity in our model, indicating that the activated ERK pathway might be the key to cell death in LO2 cells. Based on the MTT results, it was speculated that the protective effects of WEHDW might be mediated by the MEK/ERK pathways. The Western blot results indicated that WEHDW attenuated the phosphorylated levels of MEK and ERK, suggesting that the MEK/ERK pathway might be involved in the protective effect of WEHDW.
It has been widely reported that the P13-K/AKT/GSK3β pathway is involved in oxidative stress-induced apoptotic cells (Choi et al., Citation2012; Dal-Cim et al., Citation2012; Han et al., Citation2010). Oxidative stress suppresses the P13-K/AKT/GSK3β pathway and lowers the phosphorylation levels of AKT and GSK3β. The phosphorylation levels of AKT and GSK3β decreased in a time-dependent manner after treatment of H2O2. It was shown that P13-K/AKT/GSK3β signaling pathway participates in the protective effect of WEHDW on LO2. The inhibition of phospho-AKT and phospho-GSK3β in oxidative stress-induced LO2 could be reversed by the treatment of WEHDW. WEHDW could protect LO2 cells through activation of the P13-K/AKT/GSK3β pathway as shown in the Western blot assay. Adding 1 mg/mL WEHDW for 2 h could prevent the decrease of phospho-AKT and phospho-GSK3β caused by H2O2, indicating that WEHDW might have protective effects against H2O2-induced apoptosis by altering the level of phospho-AKT and phospho-GSK3β in LO2. WEHDW had protective effects on LO2 against H2O2-induced apoptosis by reversing the suppression of phosphorylation of AKT and GSK3β. Therefore, WEHDW had protective effects on LO2 against H2O2-induced apoptosis by reversing the suppression of P13-K/AKT/GSK3β pathway.
Phytochemical studies on HDW have demonstrated that iridoid glycosides, anthraquinones, flavones, steroids, phenylpropanoids, and polysaccharides are major components (Yan et al., Citation2012). Anthraquinones, flavones, and phenylpropanoids are well known for their antioxidant activity (Agbor et al., Citation2014; Guo et al., Citation2013; Tattini et al., Citation2015). It is speculated that the antioxidant activity of WEHDW may mainly contributed by these compounds. However, whether the anti-apoptotic effect of WEHDW is provided by a single compound or the interaction between different ingredients in WEHDW is currently not clear and requires more studies.
Conclusion
In conclusion, the current study showed that WEHDW possessed chemical antioxidant capacity. It demonstrated that WEHDW significantly attenuated the H2O2-induced hepatotoxicity of LO2 cell in vitro. These effects may be mediated in part by the antioxidative functions of WEHDW. Moreover, by Hoechst (nuclear) staining, with the addition of WEHDW had shown to attenuate H2O2-induced apoptotic cell death markedly. Additionally, Western blot assay demonstrated that WEHDW could protect LO2 cells against oxidative stress by attenuating the activation of MEK and ERK caused by H2O2. WEHDW also reversed the H2O2-induced suppression of P13-K/AKT/GSK3β pathway. Therefore, HDW has potent hepatoprotective effect and it is a possible TCM for preventing or treating oxidative stress-related liver injury and liver disease.
Declaration of interest
The authors report that they have no conflicts of interests regarding the publication of this paper. This research was financially supported by the Technological and Higher Education Institute of Hong Kong (Hong Kong, China) and Shenzhen Key Laboratory Advancement Program (Project code: CXB201104220020A).
References
- Agbor GA, Longo F, Makong EA, Tarkang PA. (2014). Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharm Biol 52:1128–33
- Aggeli IK, Beis I, Gaitanaki C. (2010). ERKs and JNKs mediate hydrogen peroxide-induced Egr-1 expression and nuclear accumulation in H9c2 cells. Physiol Res 59:443–54
- Asada S, Fukuda K, Nishisaka F, et al. (2001). Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 50:19–23
- Bhattacharya S, Gachhui R, Sil PC. (2011). Hepatoprotective properties of kombucha tea against TBHP-induced oxidative stress via suppression of mitochondria dependent apoptosis. Pathophysiology 18:221–34
- Binukumar BK, Bal A, Kandimalla R, et al. (2010). Mitochondrial energy metabolism impairment and liver dysfunction following chronic exposure to dichlorvos. Toxicology 270:77–84
- Caro AA, Cederbaum AI. (2004). Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol 44:27–42
- Chan SW. (2012). Panax ginseng, Rhodiola rosea and Schisandra chinensis. Int J Food Sci Nutr 63:75–81
- Chan SW, Li S, Kwok CY, et al. (2008). Antioxidant activity of Chinese medicinal herbs. Pharm Biol 46:587–95
- Chen XZ, Cao ZY, Chen TS, et al. (2012). Water extract of Hedyotis diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol Rep 28:742–8
- Choi H, Park HH, Koh SH, et al. (2012). Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology 33:85–90
- Cohen JI, Roychowdhury S, DiBello PM, et al. (2009). Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology 49:1709–17
- Conde de la Rosa L, Moshage H, Nieto N. (2008). Hepatocyte oxidant stress and alcoholic liver disease. Rev Esp Enferm Dig 100:156–63
- Conde de la Rosa L, Schoemaker MH, et al. (2006). Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: Involvement of JNK and ERK MAP kinases. Hepatology 44:918–29
- Dal-Cim T, Molz S, Egea J, et al. (2012). Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3beta pathway. Neurochem Int 61:397–404
- Feng Y, Wang N, Ye X, et al. (2011). Hepatoprotective effect and its possible mechanism of Coptidis rhizoma aqueous extract on carbon tetrachloride-induced chronic liver hepatotoxicity in rats. J Ethnopharmacol 138:683–690
- Guicciardi ME, Gores GJ. (2010). Apoptosis as a mechanism for liver disease progression. Semin Liver Dis 30:402–410
- Guo DJ, Li F, Yu PH, Chan SW. (2013). Neuroprotective effects of luteolin against apoptosis induced by 6-hydroxydopamine on rat pheochromocytoma PC12 cells. Pharm Biol 51:190–6
- Halliwell B, Cross CE. (1994). Oxygen-derived species: Their relation to human disease and environmental stress. Environ Health Perspect 102:5–12
- Han JW, Zhan XR, Li XY, et al. (2010). Impaired PI3K/Akt signal pathway and hepatocellular injury in high-fat fed rats. World J Gastroenterol 16:6111–18
- Ko WC. (1999). Flora republicase popularis sinicae. Vol. 71. Beijing: Science Press, 75–6
- Kuetemeyer K, Rezgui R, Lubatschowski H, Heisterkamp A. (2010). Influence of laser parameters and staining on femtosecond laser-based intracellular nanosurgery. Biomed Opt Express 1:587–97
- Latt SA, Stetten G, Juergens LA, et al. (1975). Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J Histochem Cytochem 23:493–505
- Liang Z, He M, Fong W, et al. (2008). A comparable, chemical and pharmacological analysis of the traditional Chinese medicinal herbs Oldenlandia diffusa and O. corymbosa and a new valuation of their biological potential. Phytomedicine 15:259–67
- Lin CC, Ng LT, Yang JJ, Hsu YF. (2002). Anti-inflammatory and hepatoprotective activity of peh-hue-juwa-chi-cao in male rats. Am J Chin Med 30:225–34
- Marrero JA, Fontana RJ, Su GL, et al. (2002). NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 36:1349–1354
- McCubrey JA, Steelman LS, Chappell WH, et al. (2007). Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773:1263–84
- Milić BL, Djilas SM, Čanadanović-Brunet JM. (1998). Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chem 61:443–7
- Moure A, Franco D, Sineiro J, et al. (2000). Evaluation of extracts from Gevuina avellana hulls as antioxidants. J Agric Food Chem 48:3890–7
- Novosad J, Kodydkova K, Krejsek J. (2001). Apoptosis, its mechanisms and medical significance. I. Definition of apoptosis and its progression at the cellular level. Vnitr Lek 47:381–6
- Ockner RK. (2001). Apoptosis and liver diseases: Recent concepts of mechanism and significance. J Gastroenterol Hepatol 16:248–60
- Pearson G, Robinson F, Beers Gibson T, et al. (2001). Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 22:153–83
- Ravandi F, Kantarjian HM, Talpaz M, et al. (2001). Expression of apoptosis proteins in chronic myelogenous leukemia: Associations and significance. Cancer 91:1964–72
- Roskams T, Yang SQ, Koteish A, et al. (2003). Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol 163:1301–11
- Schlatter R, Schmich K, Lutz A, et al. (2011). Modeling the TNFalpha-induced apoptosis pathway in hepatocytes. PLoS One 6:e18646
- Serviddio G, Bellanti F, Sastre J, et al. (2010). Targeting mitochondria: A new promising approach for the treatment of liver diseases. Curr Med Chem 17:2325–37
- Shimamatsu K, Wanless IR. (1997). Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology 26:343–50
- Tattini M, Loreto F, Fini A, et al. (2015). Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed Platanus × acerifolia plants during Mediterranean summers. New Phytol. [Epub ahead of print]. DOI: 10.1111/nph.13380
- Wang H, Xue Z, Wang Q, et al. (2008). Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesth Analg 107:534–40
- Xing HY, Liu Y, Chen JH, et al. (2011). Hyperoside attenuates hydrogen peroxide-induced L02 cell damage via MAPK-dependent Keap(1)-Nrf(2)-ARE signaling pathway. Biochem Biophys Res Commun 410:759–65
- Xu H, Xu HE. (2009). Analysis of trace elements in Chinese therapeutic foods and herbs. Am J Chin Med 37:625–38
- Xu Y, Leo MA, Lieber CS. (2003). Lycopene attenuates alcoholic apoptosis in HepG2 cells expressing CYP2E1. Biochem Biophys Res Commun 308:614–18
- Yan C, Kong F, Ou X. (2012). Antioxidant and anti-glycated activities of polysaccharides in vitro isolated ftom Hedyotis diffusa Wild. J Med Plants Res 6:2895–900