Abstract
Context: trans-3,4,5,4′-Tetramethoxystilbene (DMU-212), an derivative of resveratrol, shows strong antiproliferative activities against many cancer cells. In our previous study, we demonstrated that DMU-212 possesses potent proapoptosis and antiangiogenesis effects on vascular endothelial cells (VECs), which made it a promising agent for the treatment of angiogenesis-related diseases.
Objective: We studied the gene expression profile of DMU-212-treated VECs to gain further insight into the mechanisms by which DMU-212 exerts its potent pro-apoptosis and antiangiogenesis effects.
Materials and methods: The potential changes in the gene expression of VECs incubated with DMU-212 were identified and analyzed using the Affymetrix HG-U133 Plus 1.0 array. In addition, the gene expression profile was validated by quantitative real-time PCR (qRT-PCR) analysis for seven of those altered genes.
Results and conclusion: DMU-212 was found to regulate a diverse range of genes, including cytokines (IL8, selectin E, MPZL2, EGR1, CCL20, ITGB8, CXCL1, VCAM1, KITLG, and AREG), transport proteins (TRPC4, SLC41A2, SLC17A5, and CREB5), metabolism (CYP1B1, CYP1A1, PDK4, CSNK1G1, MVK, TCEB3C, and CDKN3), enzymes (RAB23, SPHK1, CHSY3, PLAU, PLA2G4C, and MMP10), and genes involved in signal transduction (TMEM217, DUSP8, and SPRY4), chromosome organization (HIST1H2BH and GEM), cell migration and angiogenesis (ERRFI1, HBEGF, and NEDD9), and apoptosis (TNFSF15, TNFRSF9, CD274, BCL2L11, BIRC3, TNFAIP3, and TIFA), as well as other genes with unknown function (PGM5P2, SNORD1142, LOC151760, KRTAP5-2, C1orf110, SNORA14A, MIR31, C2CD4B, SCARNA4, C2orf66, SC4MOL, LOC644714, and LOC283392). This is the first application of microarray technique to investigate and analyze the profile of genes regulated by DMU-212 in VECs. Our results lead to an increased understanding of the signaling pathways involved in DMU-212-induced apoptosis and antiangiogenesis.
Introduction
Resveratrol (trans-3,4′,5-trihydroxystilbene), a naturally polyphenolic phytoalexin found in grapes, cranberries, peanuts, cranberries and other dietary constituents, exerts a variety of pharmacological activities, including antioxidative, antiinflammatory, antitumor, and antiplatelet both in vitro and in vivo (Cong et al., Citation2014; Olas et al., Citation2002; Stakleff et al., Citation2012; Yun et al., Citation2014). In recent years, although resveratrol has been reported to regulate angiogenesis, it possesses either pro- or antiangiogenic properties depending on the situation, applied dosage, or cell type (Wang et al., Citation2010). In addition, its biological effects in vivo appear to be strongly limited because of a poor oral adsorption and metabolic stability (Walle et al., Citation2004). Structural modifications of resveratrol are needed to increase its bioavailability while preserving its beneficial activities.
The trihydroxystilbene scaffold of resveratrol has been the subject of structural alternations to improve the pharmacokinetic properties of this natural compound. The structure–activity studies pointed that the introduction of methoxy groups in place of hydroxy moieties could increase its stability (Piotrowska et al., Citation2014). An especially auspicious finding concerning these derivatives is the fact that trans-3,4,5,4′-tetramethoxystilbene (DMU-212; ) possesses more favorable bioavailability in mouse liver and plasma than resveratrol (Sale et al., Citation2004). DMU-212 also possesses stronger antiproliferative activities in a variety of cells, such as transformed fibroblasts, prostate, cervical, ovarian, breast, hepatoma, and colon cancer cells (Piotrowska et al., Citation2014). DMU-212 exerts superior antitumor activity over resveratrol because this compound induces cell death and antiproliferation events through different mechanisms from those of resveratrol (Ma et al., Citation2008). In addition to the introduction of G2/M arrest, DMU-212 down-regulates the expression of antiapoptotic proteins, including cyclin D1, BCL2-like 1(BCL2L1), and B cell CLL/lymphoma 2(BCL2), in cancer cells (Ma et al., Citation2008). Despite these widely described antitumor properties of DMU-212, very little is known concerning its antiangiogenic potential.
Figure 1. The chemical structure of trans-3,4,5,4′-tetramethoxystilbene (DMU-212). DMU-212 has the molecular formula C18H20O4 and a molecular weight of 300.3490 g/mol.
Our previous study showed that DMU-212 could induce apoptosis and antiangiogenesis effects in vascular endothelial cells (VECs) both in vitro and in vivo (Chen et al., Citation2013). However, the mechanism by which DMU-212 exerts its proapoptosis and antiangiogenesis effects are complex and remains unclear. In the present study, we performed DNA microarrays on cultured human umbilical VECs (HUVECs) treated with DMU-212 to examine the potential changes in the gene expression. A network extension of the signaling pathways that participate in apoptosis and antiangiogenesis mediated by DMU-212 was then created.
Materials and methods
Cell culture
HUVECs were obtained in our laboratory as described (Jaffe et al., Citation1973). Cells were cultured on gelatin-coated plastic dishes in MCDB31 medium (Sigma, St Louis, MO) supplemented with 20% fetal bovine serum (FBS, HyClone Laboratory, Logan, UT) and 70 ng/mL fibroblast growth factor 2 in a humidified incubator at 37 °C with 5% CO2. Cells were used at or before passage 8. The identity of HUVECs was confirmed by their cobblestone morphology and strong positive immunoreactivity to CD31.
RNA isolation, microarray experiment, and analysis
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer from the HUVECs treated with DMSO or DMU-212 (20 μM) for 6 h. The quality of the samples was assessed by spectrophotometry (Nanodrop 2000, Thermo Fisher Scientific, Waltham, MA), and samples of high quality were transferred to the Gene Tech (Shanghai, China) Co., Ltd for labeling and hybridization to the Affymetrix Human GeneChip® Gene 1.0 ST Arrays according to the protocol of the manufacturer. The microarray data analysis was also performed by Gene Tech (Shanghai, China) Co., Ltd. Genes with >2-fold changes and both the false discovery rate (FDR, q) and the Wilcoxon Rank-Sum test significance level of (p) < 0.05 were considered significantly regulated.
Quantitative real-time PCR (qRT-PCR)
After treatment, total RNA was extracted from cells with use of TriZol Reagent (Invitrogen Life Technologies, Waltham, MA). RNA was reverse-transcribed by use of M-MLV reverse transcriptase (Promega, Madison, WI). Forward (F) and reverse (R) primers designed for each genes were as follows: interleukin 8(IL8)-F 5′-GGTGCAGTTTTGCCAAGGAG-3′, IL8-R 5′-TTCCTTGGGGTCCAGACAGA-3′; early growth response 1 (EGR1)-F 5′-AGTCCCATTTACTCAGCGGC-3′, EGR1-R 5′-GTTCATCGCTCCTGGCAAAC-3′; ERBB receptor feedback inhibitor 1 (ERRFI1)-F 5′-GGGCATGCTTCCAAATCTGC-3′, ERRFI1-R 5′-ATCTTCGGTGGGTCTGAGGT-3′; transient receptor potential cation channel (TRPC4)-F 5′-TCTGCAAATATCTCTGGGAAGAATGC-3′, TRPC4-R 5′-AAGCTTTGTTCGTGCAAATTTCCATTC-3′; baculoviral IAP repeat-containing 3(BIRC3)-F 5′-GGAGACAGAGTGGCTTGCTT-3′, BIRC3-R 5′-TCATCTCCTGGGCTGTCTGA-3′; cytochrome P450, family 1, subfamily B, polypeptide 1(CYP1B1)-F 5′-GTCATGAGTGCCGTGTGTTTC-3′, CYP1B1-R 5′-TGGTCACCCATACAAGGCAG-3′; mevalonate kinase (MVK)-F 5′-GTGTGCGAGGAGATCCCAAA-3′, MVK-R 5′-CGAGGGACTTTGGTGTTGGT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-F 5′-CTTTGGTATCGTGGAAGGACTC-3′, GAPDH-R 5′-TCTTCCTCTTGTGCTCTTGCTG-3′. The qRT-PCR reactions were performed with the Eppendorf PCR System (Eppendorf AG, Hamburg, Germany). The level of each gene was calculated by comparing the Ct value in the samples to a standard curve generated from serially diluted cDNA from a reference sample and normalization to the level of GAPDH.
Statistical analyses
All experiments were repeated at least three times. The data are expressed as the means ± standard error of the mean (SE). The differences between groups were analyzed by one-way variance (ANOVA), and the means of two groups were compared using Student’s t-test with SPSS 17.0 (SPSS Inc., Chicago, IL) version. Differences were considered statistically significant at p < 0.05.
Results
Microarray analysis of DMU-212-regulated gene expression profile
The differential expressed genes in VECs treated with DMU-212 were detected and analyzed using the Affymetrix HG-U133 Plus 1.0 array. Microarray profiling data revealed a total of 56 altered genes (genes that were up or down-regulated > 2-fold), including 44 up-regulated genes and 12 down-regulated genes in DMU-212-treated cells compared with its matched control cells. Although most of these genes were found to encode proteins with known function, the function of a few of genes is as yet unknown. Categorizing these genes based on their molecular functions revealed that they consisted of genes that encode products involved in the regulation of cell apoptosis, migration, inflammation, signal transduction, and metabolism ().
Table 1. Genes whose average expression differed by greater than 2-fold are grouped by functional category: labeled cRNA prepared from HUVECs at 6 h after infection with DMU-212 use Affymetrix Human GeneChip® Gene 1.0 ST Arrays.
Verification of microarray analysis by qRT-PCR
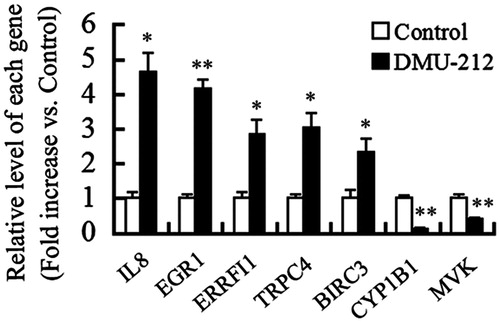
To validate the data of microarray, we performed qRT-PCR analysis for seven randomly selected genes (IL8, EGR1, ERRFI1, TRPC4, BIRC3, CYP1B1, and MVK). The changes of all the selected genes were in accordance with corresponding microarray data. All these expression pattern variations were statistically significant ().
Pathway analysis of DMU-212-induced apoptosis and antiangiogenesis in VECs
Functional analysis of the Gene Ontology (GO) terms associated with the differential expressed genes showed significant enrichment for metabolic process, immune system process, apoptotic process, and various kinds of cellular process (). BCL2 is an important antiapoptosis factor. Two BCL2 inhibitors, BCL2-like 11(BCL2L11) and CD274 molecule (CD274), were up-regulated by DMU-212, suggesting that these proapoptotic factors may be involved in DMU-212-induced apoptosis in VECs. In addition, tumor necrosis factor (ligand) superfamily, member 15 (TNFSF15) and tumor necrosis factor receptor superfamily, member 9 (TNFRSF9), members of tumor necrosis factor (TNF) superfamily, may contribute to VEC apoptosis and antiangiogenesis induced by DMU-212. Notably, several kinds of proinflammatory cytokines, such as interleukin-8 (IL-8), selectin E (SELE), chemokine (C-X-C motif) ligand 1 (CXCL1), and vascular cell adhesion molecule 1 (VCAM1) may also participate in DMU-212-induced endothelial dysfunction and apoptosis. Except for the induction of apoptosis in VECs, genes associated with cell proliferation, such as ERRFI1, were altered by DMU-212. Furthermore, we used the KEGG orthology-based annotation system (KOBAS) (http://kobas.cbi.pku.edu.cn), which predicted the biochemical pathways in which molecules are involved, to analyze the signaling pathways linked to DMU-212-induced apoptosis and antiangiogenesis in VECs (). The results showed that the alter genes enriched were related with TNF signaling pathway and NF-κB signaling pathway, which was similar with gene ontology (GO) analysis. Collectively, the differentially expressed genes were collated into a comprehensive signaling network associated with apoptosis and angiogenesis in the present study ().
Figure 3. Biological process classification of the differential expressed genes using the GENEONTOLOGY (The Gene Ontology Consortium, Cambridge, UK) and PANTHER (Evolutionary Systems Biology Group, Menlo Park, CA) database.
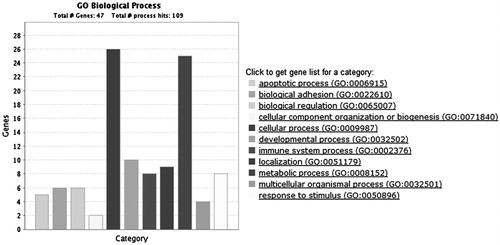
Figure 4. Network extension of the comprehensive signaling pathway collated by the differentially expressed genes that were regulated by DMU-212.
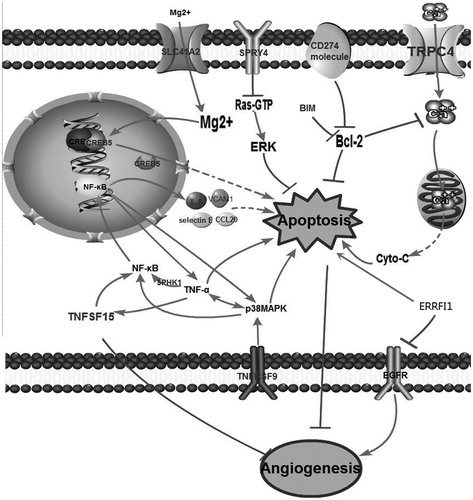
Table 2. Pathway identification analysis using KOBAS.
Discussion
In the present study, we described the genome-wide transcriptional profiling of DMU-212-regulated genes in VECs. Pathway analysis revealed that TNF signaling pathway and NF-κB signaling pathway are remarkably regulated. TNFSF15, a member of the TNF superfamily, has the ability to enforce growth arrest of VECs in G0 and G1 phases of the cell cycle, while inducing apoptosis of proliferating VEC, leading to inhibition of angiogenesis (Yu et al., Citation2001). More specially, the up-regulation of TNFSF15 stimulated by TNF-α is partly but significantly responsible for TNF-α-induced VEC apoptosis (Xu et al., Citation2014). TNFRSF9, a member of the TNF receptor family, could enhance apoptosis by elevating TNF-α production via activation of p38 mitogen-activated protein kinase (MAPK) pathway (Nagila et al., Citation2013). In addition, DMU-212 elevated the gene expression of transmembrane protein 217 (TMEM217), dual specificity phosphatase 8 (DUSP8), and sprouty homolog 4 (Drosophila) (SPRY4), which are participated in p38MAPK signaling pathway. Based on these, we speculated that both TNF signaling and p38MAPK signaling might be participated in DMU-212-induced apoptosis in VECs.
NF-κB also plays an important role in inflammation and apoptosis in VECs. It can be activated by many proinflammatory cytokines, including TNF-α. Data from microarray revealed that sphingosine kinase 1 (SPHK1) was up-regulated by DMU-212. It has been demonstrated that SPHK1 is essential for TNF-α induced NF-κB activation (Alvarez et al., Citation2010; Donnahoo et al., Citation1999; Hait et al., Citation2009). Furthermore, NF-κB regulates a variety of genes, such as p38MAPK, responsible for the generation of TNF-α, thereby providing a positive regulatory loop (Vallabhapurapu & Karin, Citation2009; Valladares et al., Citation2000). Collectively, we speculated that the activation of NF-κB and TNF signaling pathways in response to DMU-212 might lead to a coordinated increase in a wide variety of proinflammatory factors which in turn lead to amplification of the inflammatory response, and subsequently led to excessive cell death in VECs.
As we speculated above, DMU-212 induced inflammation characterized by the upregulation of several key inflammatory mediators, including interleukin-8 (IL-8), SELE, vascular cell adhesion molecule 1 (VCAM-1), and so on, which might consequently result in endothelial dysfunction and cytotoxicity. It is noticed that these cytokines also exerted prosurvival and proangiogenic effects. IL-8 has been reported to increase proliferation and survival of VECs and promote angiogenic responses (Lee et al., Citation2014). Down-regulation of vascular selectin E by siRNA profoundly inhibited tumor angiogenesis (Liu et al., Citation2011). In addition, Kang et al. (Citation2014) reported that VCAM-1 was involved in Curculigoside A-induced angiogenesis. Based on these observations, the role of these elevated cytokines on DMU-212-treated HUVECs needs to be addressed in more detail in our future study.
SPRY4, a glycoprotein can block the binding of Ras-GTP (Sasaki et al., Citation2003), is also up-regulated by DMU-212. Ras-GTP activates ERK pathway through receptor-mediated activation of the small G-protein (Macdonald et al., Citation1993). ERK1/2-mediated signaling plays a significant role in cell survival (Xia et al., Citation1995). In our previous study, we detected that the level of phosphorylation ERK1/2 is decreased by DMU-212 (Chen et al., Citation2013). From these observations, we speculated that the inactivation of ERK might be involved in DMU-212-induced VEC apoptosis.
BCL2 is an important anti-apoptotic protein. Our result showed that the mRNA levels of BCL2L11 and CD274 molecule, two BCL2 inhibitors, were elevated. BCL2L11 protein containing BCL2 homology domain 3 (BH3), which could interact with the members of BCL2 family, including BCL2, BCL2L1, and myeloid cell leukemia 1 (MCL1), acts as an apoptotic activator (Huang & Strasser, Citation2000; Wilson-Annan et al., Citation2003; Zong et al., Citation2001). CD274 is a type 1 transmembrane protein that has been speculated to play a major role in suppressing the immune system during particular events such as pregnancy, tissue allografts, autoimmune disease, and other diseases (Francisco et al., Citation2010). In addition, it has been reported that this molecule could control the accumulation of foreign antigen specific T cells in the lymph nodes through regulating BCL2 and apoptosis (Chemnitz et al., Citation2004). cAMP responsive element binding protein 5 (CREB5) belongs to the CRE-binding protein family. It binds to certain DNA sequences called cAMP responsive element (CRE), which leads to the activation of CREB. CREB activation regulates the transcription of several downstream apoptotic-associated genes, and subsequently, apoptosis occurred (Craig et al., Citation2001; Zhu et al., Citation2012). More interestingly, Mg2+ is essential for the binding of CREB and CRE (Lin et al., Citation2009). Data from genechip showed that the mRNA level of solute carrier family 41, member 2 (SLC41A2), a Mg2+ channel, was elevated by DMU-212. These results promoted us to speculate that DMU-212 might increase intracellular Mg2+ through SLC41A2, which participated in CREB5 activation and VEC apoptosis.
Besides Mg2+ channel, one of the transient receptor potential canonical channels (TRPCs), TRPC4 was also up-regulated. TRPC4 is widely expressed in the vasculature and has been suggested as a key determinant of endothelial functions, such as endothelial permeability (Tiruppathi et al., Citation2002), nitric oxide (NO)-dependent vasorelaxation (Freichel et al., Citation2001), barrier stability (Graziani et al., Citation2010), endothelial proliferation (Abdullaev et al., Citation2008), and tube formation (Antigny et al., Citation2012). Although there is no direct evidence that TRPC4 is involved in VEC apoptosis, this channel is important for intracellular Ca2+ maintenance in VECs (Bair et al., Citation2009). The high level of intracellular Ca2+ can affect the permeability of mitochondria membrane and cause the release of cytochrome C, leading to mitochondria-initiated apoptosis (Andreyev & Fiskum, Citation1999; Schild et al., Citation2001).
Enhanced mRNA levels of ERRFI1 also attracted our attention. ERRFI1 has been identified as an immediate early response gene encoding a scaffolding adaptor protein and a negative regulatory protein that inhibits the kinase domains of epidermal growth factor receptor (EGFR) (Yoon et al., Citation2012). Activated EGFR pathway may promote cell proliferation, differentiation, angiogenesis, and antiapoptosis (Wang et al., Citation2015). ERRFI1 might exert its antiapoptosis and antiangiogenic effects through inhibiting EGFR signaling in DMU-212-treated VECs.
Conclusions
In summary, a combination of microarray data and pathway analysis first introduces many possible biological pathways to explain the mechanisms of DMU-212-mediated VEC apoptosis and antiangiogenesis. Although the corresponding mechanisms are complex, the activation of NF-κB and TNF signaling pathways, which led to an amplification of proapoptotic events and proinflammatory responses, might be the main reason for the excessive cell death in DMU-212-treated VECs. These findings help us to further understand the function of DMU-212 and might provide several novel potential molecular targets for antiangiogenesis therapy. Further studies will focus particularly on the roles of SPRY4, CREB5, SLC41A2, TRPC4, and ERRFI1 as potentially important regulators of VEC apoptosis and angiogenesis.
Acknowledgements
The authors thank Prof. Bo Zhou (State Key Laboratory of Applied Organic Chemistry, Lanzhou University) for kindly providing DMU-212 (purity was at least 99% by HPLC analysis).
Declaration of interest
The authors report no declarations of interest. This study was financially supported by the National Natural Science Foundation of China (Nos. 31471296 and 31101001), the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 15HASTIT031), the Key Project of Henan Educational Committee (No. 14A180028), and the Foundation for University Key Teacher by Henan Educational Committee (No. 2013GGJS-078).
References
- Abdullaev IF, Bisaillon JM, Potier M, et al. (2008). Stim1 and Orai1 mediate CRAC currents and store-operated calcium entry important for endothelial cell proliferation. Circ Res 103:1289–99
- Alvarez SE, Harikumar KB, Hait NC, et al. (2010). Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 24:1084–8
- Andreyev A, Fiskum G. (1999). Calcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver. Cell Death Differ 6:825–932
- Antigny F, Girardin N, Frieden M. (2012). Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J Biol Chem 287:5917–27
- Bair AM, Thippegowda PB, Freichel M, et al. (2009). Tiruppathi C. Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-kappaB activation in endothelial cells through AMP-activated protein kinase and protein kinase. Cdelta. J Biol Chem 2:563–74
- Chemnitz JM, Parry RV, Nichols KE, et al. (2004). SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173:945–54
- Chen LK, Qiang PF, Xu QP, et al. (2013). trans-3,4,5,4′-Tetramethoxystilbene, a resveratrol analog, potently inhibits angiogenesis in vitro and in vivo. Acta Pharmacol Sin 34:1174–82
- Cong X, Li Y, Lu N, et al. (2014). Resveratrol attenuates the inflammatory reaction induced by ischemia/reperfusion in the rat heart. Mol Med Rep 9:2528–32
- Craig JC, Schumacher MA, Mansoor SE, et al. (2001). Consensus and variant cAMP-regulated enhancers have distinct CREB-binding properties. J Biol Chem 276:11719–28
- Donnahoo KK, Shames BD, Harken AH, et al. (1999). Review article: The role of tumor necrosis factor in renal ischemia–reperfusion injury. J Urol 162:196–203
- Francisco LM, Sage PT, Sharpe AH. (2010). The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236:219–42
- Freichel M, Suh SH, Pfeifer A, et al. (2001). Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol 3:121–7
- Graziani A, Poteser M, Heupel WM, et al. (2010). Cell–cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: Evidence for a regulatory TRPC4-beta-catenin interaction. J Biol Chem 285:4213–23
- Hait NC, Allegood J, Maceyka M, et al. (2009). Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 4:1254–7
- Huang DC, Strasser A. (2000). BH3 – Only proteins-essential initiators of apoptotic cell death. Cell 103:839–42
- Jaffe EA, Nachman RL, Becker CG, et al. (1973). Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–56
- Kang Z, Zhu H, Luan H, et al. (2014). Curculigoside A induces angiogenesis through VCAM-1/Egr-3/CREB/VEGF signaling pathway. Neuroscience 267:232–40
- Lee CY, Tsai YT, Loh SH, et al. (2014). Urotensin II induces interleukin 8 expression in human umbilical vein endothelial cells. PLoS One 9:e90278
- Lin WY, Chang YC, Lee HT, et al. (2009). CREB activation in the rapid, intermediate, and delayed ischemic preconditioning against hypoxic-ischemia in neonatal rat. J Neurochem 108:847–59
- Liu ZJ, Tian R, Li Y, et al. (2011). Inhibition of tumor angiogenesis and melanoma growth by targeting vascular E-selectin. Ann Surg 254:450–6
- Ma Z, Molavi O, Haddadi A, et al. (2008). Resveratrol analog trans trans-3,4,5,4′-tetramethoxystilbene (DMU-212) mediates anti-tumor effects via mechanism different from that of resveratrol. Cancer Chemother Pharmacol 63:27–35
- Macdonald SG, Crews CM, Wu L, et al. (1993). Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol Cell Biol 13:6615–20
- Nagila A, Netsawang J, Suttitheptumrong A, et al. (2013). Inhibition of p38MAPK and CD137 signaling reduce dengue virus-induced TNF-α secretion andapoptosis. Virol J 4:105
- Olas B, Wachowicz B, Stochmal A, et al. (2002). Anti-platelet effects of different phenolic compounds from Yucca schidigera Roezl. bark. Platelets 13:167–73
- Piotrowska H, Myszkowski K, Abraszek J, et al. (2014). DMU-212 inhibits tumor growth in xenograft model of human ovarian cancer. Biomed Pharmacother 68:397–400
- Sale S, Verschoyle RD, Boocock D, et al. (2004). Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans-3,4,5,4′-tetramethoxystilbene. Br J Cancer 90:736–44
- Sasaki A, Taketomi T, Kato R, et al. (2003). Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat Cell Biol 5:427–32
- Schild L, Keilhoff G, Augustin W, et al. (2001). Distinct Ca2+ thresholds determine cytochrome c release or permeability transition pore opening in brain mitochondria. FASEB J 15:565–7
- Stakleff KS, Sloan T, Blanco D, et al. (2012). Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac J Cancer Prev 13:1333–40
- Tiruppathi M, Freichel SM, Vogel BC, et al. (2002). Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91:70–6
- Vallabhapurapu S, Karin M. (2009). Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733
- Valladares A, Alvarez AM, Ventura JJ, et al. (2000). p38 mitogen-activated protein kinase mediates tumor necrosis factor-alpha-induced apoptosis in rat fetal brown adipocytes. Endocrinology 141:4383–95
- Walle T, Hsieh F, DeLegge MH, et al. (2004). High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–82
- Wang H, Zhou H, Zou Y, et al. (2010). Resveratrol modulates angiogenesis through the GSK3β/β-catenin/TCF-dependent pathway in human endothelial cells. Biochem Pharmacol 80:1386–95
- Wang WM, Zhao ZL, Ma SR, et al. (2015). Epidermal growth factor receptor inhibition reduces angiogenesis via hypoxia-inducible factor-1α and notch1 in head neck squamous cell carcinoma. PLoS One 10:e0119723
- Wilson-Annan J, O'Reilly LA, Crawford SA, et al. (2003). Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J Cell Biol 162:877–87
- Xia Z, Dickens M, Raingeaud J, et al. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–31
- Xu LX, Grimaldo S, Qi JW, et al. (2014). Death receptor 3 mediates TNFSF15- and TNFα-induced endothelial cell apoptosis. Int J Biochem Cell Biol 55:109–18
- Yoon YK, Kim HP, Song SH, et al. (2012). Down-regulation of mitogen-inducible gene 6, a negative regulator of EGFR, enhances resistance to MEK inhibition in KRAS mutant cancer cells. Cancer Lett 316:77–84
- Yu J, Tian S, Metheny-Barlow L, et al. (2001). Modulation of endothelial cell growth arrest and apoptosis by vascular endothelial growth inhibitor. Circ Res 89:1161–7
- Yun H, Park S, Kim MJ, et al. (2014). AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J 281:4421–38
- Zhu J, Nguyen MT, Nakamura E, et al. (2012). Cre-mediated recombination can induce apoptosis in vivo by activating the p53 DNA damage-induced pathway. Genesis 50:102–11
- Zong WX, Lindsten T, Ross AJ, et al. (2001). BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15:1481–6