Abstract
Context: Lichens are source of natural bioactive compounds which are traditionally used to cure a variety of ailments.
Objective: The objective of this study is to assess free radical scavenging, prolyl endopeptidase inhibitory (PEPI), and antimicrobial potential of a high altitude lichen species Cetrelia olivetorum (Nyl.) W. L. Culb. & C. F. Culb (Parmeliaceae).
Materials and methods: Lichen C. olivetorum has been cultured in vitro, and optimized culture conditions were implemented in bioreactor to obtain high quantity of biomass for the study of radical scavenging, PEPI, and antimicrobial activities. Radical scavenging activity of methanol extract of Cetrelia olivetorum (MECO) was tested at 100 µg/mL, PEPI activity at 25 and 50 µg/mL, and antimicrobial activity at 5, 25, 50, and 100 µg/mL conc. All the biological activities of natural thallus extract and its derived culture extract were evaluated spectrophotometrically.
Results: Murashige and Skoog medium supplemented with 3% glucose and 100 ppb indole-3-butyric acid (IBA) supported biomass growth at flask level and yielded 5.095 g biomass in bioreactor. MECO of both the cultured and the natural lichen exhibited half inhibiting concentration (IC50) for radical scavenging activities in the range of 50–60 µg/mL, whereas the IC50 value of standard antioxidants was found to be in the range of 12–29 µg/mL. The IC50 value of lichen extract for PEPI activity was 144–288 µg/mL, whereas the IC50 value of standard prolyl endopeptidase inhibitor, Z-pro-prolinal, was 57.73 µg/mL. As far as the antimicrobial activity of MECO is concerned, minimum inhibitory concentration (MIC) value of lichen extracts against tested microorganisms was obtained in the range of 50–104 µg/mL and found to be more effective than commercially available standard erythromycin.
Discussion: Murashige and Skoog medium containing IBA was found to be suitable for maximum biomass production of C. olivetorum under bioreactor conditions. The cultured lichen biomass extract also showed antioxidant, PEPI, and antimicrobial potential.
Conclusion: The present study indicates therapeutic potential of Himalayan lichen C. olivetorum against neurodegenerative diseases owing to its radical scavenging, PEPI, and antimicrobial activities. Further, the result encourages its commercial exploitation through mass culture for production of its bioactive components and their use in pharmaceutical and nutraceutical industries.
Introduction
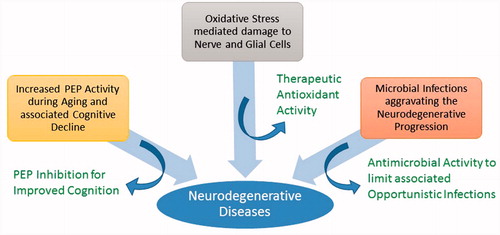
Neurodegenerative diseases are neurological abnormalities, in which primarily nerve cells are affected in terms of loss of structure and function, leading to neuronal death. A large proportion of human population suffer from neurodegenerative diseases worldwide. Currently, more than 35 million of world population are affected by dementia (Prince et al., Citation2013). Alzheimer’s disease (AD) is the most frequent neurodegenerative disease, responsible for the loss of ability to think and reason clearly, leading to profound cognitive impairment (Budson & Solomon, Citation2011; Querfurth & LaFerla, Citation2010; Zhao & Zhao, Citation2013). Occurrence of AD and its accelerated progression during aging is a major reason for deaths in old age people (Greeve et al., Citation2004; Ohsawa et al., Citation2008; Zhao & Zhao, Citation2013). In spite of the tremendous medical advancements in neurosciences in recent years, there are no effective therapeutics for the treatment of dementia (Zhao & Zhao, Citation2013).
Prolyl endopeptidase (PEP) is a serine peptidase, specifically cleaves peptide bonds at the carboxy terminal of proline residues (Amor et al., Citation2004; Diderot et al., Citation2005; Lee & Paik, Citation2003). Some of the cognitively important proline-specific neuropeptides are believed to be degraded by PEP (Amor et al., Citation2004; Diderot et al., Citation2005; Huston & Hasenohrl, Citation1995). Various authors have reported an unusually higher PEP levels in the patients suffering from AD and suggested that PEP might be one of the several factors involved in cognitive decline associated with aging (Lee & Paik, Citation2003; Tezuka et al., Citation1999). Therefore, PEP inhibition might have a therapeutic value in the treatment of AD and PEP inhibitors could be a novel anti-amnesic drug (Morain et al., Citation2002; Schneider et al., Citation2002; Toide et al., Citation1997).
The human body is constantly exposed to free radicals; these radicals are the unpaired electron-containing reactive molecules, known for their lethal toxicity by causing oxidative damage to cellular organelles, particularly nerve and glial cells (Gilgun-Sherki et al., Citation2001; Uttara et al., Citation2009; Wang & Michaelis, Citation2010). This oxidative stress to biomolecules significantly increases neuronal apoptosis and leads to various pathological changes during aging (Uttara et al., Citation2009). Antioxidants are known to suppress the deleterious effects of free radicals by neutralizing them and are believed to hold a promising neuroprotective role by combating oxidative stress (Shi et al., Citation2013; Zbarsky et al., Citation2005; Zhao & Zhao, Citation2013).
Involvement of microbial infections as well as the role of their byproducts in the pathogenesis of neurodegeneration has already been reported (Chiara et al., Citation2012; Nicolson, Citation2008). Neurodegenerative disease accompanied by the immunocompromised condition of the host in old age is likely to attract opportunistic infections that could be involved in the progression of disease (Nicolson, Citation2008). An effective radical scavenging drug supplement with neuroprotective and antimicrobial effects could be of immense help to limit the various pathological conditions in old age (Nicolson & Haier, Citation2010) ().
Lichen is a symbiotic association of a fungal (mycobiont) and an algal partner (photobiont). They have long been used for treating a variety of diseases, in many ancient cultures and medical practices, throughout the world. As a natural bioresource, lichens are reported to have several novel biomolecules that are yet to be explored for various biological activities (Molnar & Farkas, Citation2010; Srivastava et al., Citation2013; Toma et al., Citation2001). Currently, 1050 secondary metabolites are known which are unique to lichens (Stocker-Worgotter, Citation2008; Verma & Behera, Citation2015). Bioactivity studies on lichen metabolites reported in recent years indicate their therapeutic potential in terms of natural antioxidants, antimicrobial, anticancer, probiotic, anti-angiogenic, and cardiovascular protective agents (Behera et al., Citation2012; Gaikwad et al., Citation2014; Koparal et al., Citation2010; Mahadik et al., Citation2011; Srivastava et al., Citation2013).
Lichen species Cetrelia olivetorum (Nyl.) W. L. Culb. & C. F. Culb. belongs to the Parmeliaceae family of lichenized fungi, which is widely distributed over the Himalayan region. The characteristic harsh abiotic environmental conditions of this region, particularly low temperature, high altitudes, extremities in wind speed, snowfall, and UV radiation contribute to slow growth and development of unique lichen substances (Huneck & Yoshimura, Citation1996).
In view of the above-mentioned challenges and considering the scope in terms of potential of lichens, the present study was undertaken with an objective to establish in vitro culture of lichen C. olivetorum and to study its radical scavenging, PEPI and antimicrobial potential.
Materials and methods
Chemicals
Prolyl endopeptidase, Z-gly-pro-4-nitroanilide, Z-pro-prolinal, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), quercetin, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO). Folin–Ciocalteu reagent, polysaccharide solution, linoleic acid, and 1,1-diphenyl-2-picryl hydrazil (DPPH) were procured from HiMedia (Mumbai, India). Other routine chemicals and components of culture media used were of analytical grade.
Lichen material
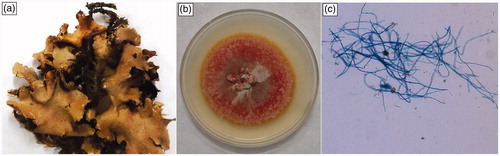
Fresh thallus of lichen species C. olivetorum () producing olivetoric acid under natural condition was collected from the Himalayan region of Chamoli District of Uttarakhand State, India. GPS coordinates were N 30°31′08.83″ E 79°34′03.95″ and the elevation was 2911 m. Collected lichen species was identified by morphological, anatomical, and chemical analysis, with medullary reactions as K−, C + pink, P− as well as with routinely used standardized procedure of thin-layer chromatography (TLC) (Culberson & Kristinsson, Citation1972; Elix, Citation2014). A part of the lichen material used for the study has been preserved as dry specimen (Voucher no. 11-017408) at the Lichen Herbarium (LWG) of National Botanical Research Institute (CSIR), Lucknow, India.
Microorganisms and media
The microorganisms used in the study for antimicrobial activity of lichen were the clinical isolates of Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus epidermidis, Candida albicans, and obtained from B. J. Medical College, Pune, India. The respective bacterial and fungal cultures were maintained on the recommended Nutrient Agar and Sabourad Dextrose Agar medium, respectively, and were stored at 4 °C.
In vitro culture and optimization of nutritional requirements of Cetrelia olivetorum
The natural thallus of lichen C. olivetorum was cultured in vitro according to the methodology described by Yamamoto et al. (Citation1985) with some minor modifications reported by Verma et al. (Citation2012). A number of nutrient media were used: Bold’s Basal (BB) (Deason & Bold, Citation1960), Lilly Barnett (LB) (Lilly & Barnett, Citation1951), Murashige Skoog (MS) (Murashige & Skoog, Citation1962), and Malt Yeast Extract (MYE) (Ahmadjian, Citation1993). The inoculated Petri dishes were incubated in a culture room at 18 °C with an alternating photo period of 8 h light (400 lux)/16 h dark and 50–80% of relative humidity for a period of 2 months.
The cultures were ensured to be axenic during incubation by periodic microscopic examination of cell aggregate formation (algae and fungi). In the cell aggregates of C. olivetorum culture grown in MS medium, fungal symbiont hyphae were observed in blue color and algal cells in dark green color. The growth of symbionts was recorded microscopically (Olympus CX41, Olympus Inc., Houston TX) by macro and microphotography (). The production of lichen compounds, olivetoric acid, was analyzed using TLC with standard solvent system toluene:1,4 dioxane:acetic acid 180:45:5 (TDA) ().
Similar to other lichens, the growth of this lichen species in culture is also considerably slow. In order to find out a suitable culture medium for maximum biomass production, nutritional requirements were optimized at three levels. First, freshly cultured symbionts were inoculated into the conical flasks containing 100 mL liquid BB, LB, MS, MYE medium, and incubated under the same culture conditions mentioned above. Next two levels of optimization were carried out by supplementing the media with different sources of carbon and nitrogen. At each level, the nutrient medium with increased biomass production was selected for next level. The carbon sources used were glucose, sucrose, and mannitol of 3% concentration, whereas the nitrogen sources were biotin, glycine, and indole-3-butyric acid (IBA) of 50 and 100 ppb concentration. The MS medium supplemented with 3% glucose, 100 ppb biotin and MS medium with 3% glucose, 100 ppb IBA yielded maximum quantity of biomass (2.280 g and 2.304 g, respectively). Both the supplemented media were continued for further study at the bioreactor level to increase the biomass of C. olivetorum.
Bioreactor study
The culture conditions observed during growth optimization of C. olivetorum at flask level were implemented in the bioreactor. The lichen C. olivetorum was attempted for mass production in a continuously operating stirred tank bioreactor of 5 L working volume (Sartorius Stedim BIOSTAT® B Plus – 5LCC, Sartorius Stedim Biotech, Gottingen, Germany). The bioreactor parameters were set as follows – nutrient medium volume: 3 L, temperature: 18 °C, pH: 6.5, stirrer speed: 50 rpm, pO2: 40%. Inoculum of 100 mL culture suspension was inoculated in the bioreactor vessel containing optimized MS medium supplemented with 3% glucose, 100 ppb biotin or 100 ppb IBA, and cultivated for 7 d. After completion of batch, developed biomass was harvested, dried at 40 °C for 36 h and weighed.
Extraction of culture biomass and natural lichen thallus
The dried biomass of C. olivetorum culture (5.09 g) was extracted in methanol (99.8%) using the Soxhlet apparatus. The extract was filtered using Whatman No. 1 filter paper. The filtrate was concentrated and dried in Heidolph Rotary Evaporator under vacuum. The dry weight of culture extract was recorded as 2.481 g. Similar procedure was followed for the extraction of natural lichen thallus (1 g) and the dry weight of extract was recorded as 0.19 g. Dry extracts of both culture biomass and natural thallus were used to prepare various concentrations in methanol for further experimental work.
Determination of radical scavenging activity
The free radical scavenging potential of methanol extract of Cetrelia olivetorum (MECO) was determined with 1,1-diphenyl-2-picryl-hydrazil (DPPH) according to the method of Blois (Citation1958). Anti-lipid peroxidation (ALP) activity was determined using the method of Liegeois et al. (Citation2000). Trolox equivalent antioxidant capacity (TEAC) of lichen extracts was determined following Miller et al. (Citation1995) with minor modifications reported in Verma et al. (Citation2008). Standard antioxidants butylated hydroxyl anisole (BHA), butylated hydroxytoluene (BHT), quercetin, and water soluble vitamin E analogue trolox were used as the positive control.
Determination of prolyl endopeptidase inhibitory (PEPI) activity
The PEPI activity of C. olivetorum extracts was determined using the method established by Yoshimoto et al. (Citation1980) and Tezuka et al. (Citation1999) with slight modifications. Briefly, a mixture of 1.68 mL of Tris-HCl (0.1 M, pH 7.0), 100 µL of prolyl endopeptidase (0.1 unit/mL), and 20 µL of lichen extract of 5, 10, 25, and 50 µg/mL concentration was preincubated for 5 min at 30 °C. The reaction was started by adding 200 µL Z-gly-pro-4-nitroanilide (2 mM in 100% 1,4-dioxane) as a substrate and further incubated for 30 min at 30 °C. The amount of p-nitroaniline produced was determined spectrophotometrically at 405 nm in a spectrophotometer (UV–vis 1601 Shimadzu Corp, Kyoto, Japan).
The percent inhibition of PEP enzyme was calculated by using the formula given below:
where A is the amount of p-nitroaniline produced due to enzymatic action in the absence of inhibitor, whereas B is the amount of p-nitroaniline formed in the presence of inhibitor. Z-pro-prolinal, a standard PEP inhibitor, was used as a positive control.
Antimicrobial activity
The antimicrobial activity of lichen extract of C. olivetorum towards test microorganisms was evaluated by determining the minimum inhibitory concentration (MIC) using broth dilution method (Verma et al., Citation2011). Inoculum was prepared in Muller–Hinton broth to approximately 106 colony-forming units per mL (CFU/mL). Lichen extracts with a series of concentrations ranging from 5 to 100 µg/mL were introduced. Erythromycin, streptomycin, and tetracycline were used as positive control for bacterial growth inhibition; whereas nystatin, ketoconazole, and fluconazole were used for the inhibition of fungal growth. The test microorganism inoculated in the broth without lichen extract was used as a negative control. All the test tubes were incubated for 24 h at 37 °C. The turbidity was measured at 600 nm using a spectrophotometer (UV–vis 1601, Shimadzu Corp., Kyoto, Japan). The MIC value was expressed as the lowest concentration of lichen extract that completely inhibited microbial growth.
Determination of phytochemical content
In order to know the compounds contributing towards observed biological activities, the phytochemical contents; total polyphenol, total polysaccharide, and protein content in lichen extracts were estimated. The polyphenol content was estimated using the Folin–Ciocalteu method described by Slinkard and Singleton (Citation1977). Total polysaccharide content was determined using the phenol–sulfuric acid method of Dubois et al. (Citation1956). The protein content was estimated according to Lowry et al. (Citation1951). Detailed procedure and modifications related to phytochemical content estimation were adopted from Verma et al. (Citation2008).
Results
In vitro culture and optimization of nutritional requirements
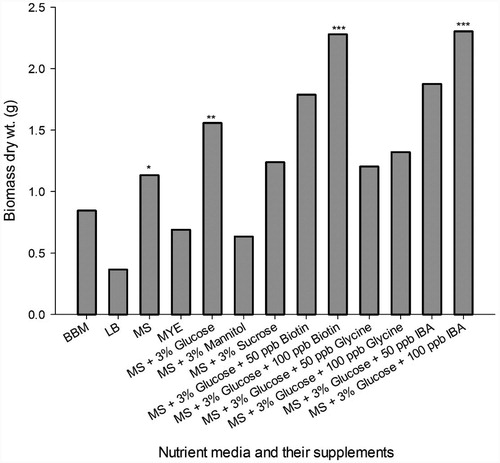
The results of culture biomass of C. olivetorum obtained in different nutrient media are presented in . The maximum biomass production of C. olivetorum was achieved in MS broth medium with 1.135 g/flask; whereas in BB, LB, and MYE medium, the biomass yield was 0.367–0.846 g/flask. Further, the enhancement in biomass production was tested by supplementing the MS medium with various carbon and nitrogen sources. Among the carbon sources tested, 3% glucose was the most effective carbon source yielding 1.559 g/flask of biomass, while 3% mannitol and 3% sucrose yielded 0.636 g and 1.240 g/flask of biomass, respectively. Out of the three nitrogen sources incorporated along with 3% glucose in MS medium, 100 ppb biotin and 100 ppb IBA yielded greater biomass as 2.280 g and 2.304 g/flask, respectively. Glycine as a nitrogen source, yielded least biomass as 1.206 g and 1.322 g/flask at 50 ppb and 100 ppb concentration, respectively. These high-biomass yielding medium compositions were employed in the bioreactor for mass production over a period of 7 d. The lichen biomass obtained from the MS medium containing 3% glucose and 100 ppb IBA was 5.095 g. However, as visually observed, MS medium containing 100 ppb biotin did not promote the growth of lichen biomass in the bioreactor.
Radical scavenging potential
The radical scavenging potential of MECO was evaluated in terms of DPPH radical scavenging activity (DRS), anti-lipid peroxidation (ALP), and trolox equivalent antioxidant capacity (TEAC), and the results are presented in . Methanol extract of cultured biomass and natural lichen showed DRS activity as 93.05% and 98.98%, respectively, at 100 µg/mL conc., whereas the standard antioxidants BHA, BHT, and quercetin exhibited 63.0–85.4% inhibition at 20 µg/mL conc. As far as the ALP activity of cultured lichen extract is concerned, it was found to be more than 89% for both natural and cultured lichen at 100 µg/mL conc., considerably greater than the inhibition showed by the standard antioxidants (35–47%) at 20 µg/mL. In case of TEAC, MECO of cultured lichen showed 7.99 mM TEAC at 100 µg/mL, which was moreover equivalent to TEAC shown by methanol extract of natural lichen (7.21 mM) at 100 µg/mL. Both the lichen extracts exhibited strong TEAC activity as compared with the standard water soluble vitamin E analogue trolox (3.6 mM) at 20 µg/mL.
Table 1. Radical scavenging, prolyl endopeptidase inhibitory activity and phytochemical content of lichen Cetrelia olivetorum at 25–100 µg/mL concentration.
Prolyl endopeptidase inhibitory (PEPI) potential
PEPI activity of MECO was determined at various concentrations and the results are presented in . The extract of cultured lichen showed 6.66% PEP inhibition at 25 µg/mL, whereas the extract of natural lichen thallus showed 17.33% inhibition at a higher concentration (50 µg/mL). The PEPI activity of natural and cultured lichen extract of C. olivetorum was found to be equivalent or higher than the activity shown by the standard PEP inhibitor, Z-pro-prolinal (8.66%) at 10 µg/mL concentration.
Half-inhibiting concentration (IC50) of lichen extracts
Half-inhibiting concentration (IC50) of lichen extracts was determined as the concentration responsible for 50% of radical scavenging and PEPI activity. The results are presented in . The IC50 value for PEPI activity of cultured lichen C. olivetorum was 187.68 µg/mL, whereas for natural lichen and standard PEP inhibitor, Z-pro-prolinal, the IC50 values were found to be 144.25 and 57.73 µg/mL, respectively. As far as the antioxidant activities are concerned, the IC50 value of methanol extracts of cultured lichen was found in the range of 50–60 µg/mL, which was higher than the standard antioxidants BHA, BHT, and quercetin (12–30 µg/mL) used in the experiment.
Table 2. Half-inhibiting concentration (IC50) of methanol extracts of C. olivetorum for radical scavenging and the prolyl endopeptidase inhibitory activity.
Antimicrobial potential
Antimicrobial efficacy of MECO was estimated in terms of MIC against clinical isolates of human pathogens and the results are shown in . MIC of cultured lichen against E. cloacae and K. pneumoniae was found to be 50.35 µg/mL and 50.11 µg/mL, respectively, whereas the MIC value of natural lichen extract and erythromycin against the same microorganisms was found to be in the range of 101–104 µg/mL. As far as the MIC value of natural lichen extract against P. aeruginosa and S. marcescens is concerned, it was found to be around 51 µg/mL, which was less than the MIC value of erythromycin and cultured lichen extract. The methanol extract of natural lichen showed antimicrobial efficiency against C. albicans at 26.24 µg/mL, significantly lower than the standard ketoconazole and nystatin (54.50 and 68.36 µg/mL, respectively), but higher than fluconazole (7.58 µg/mL). Unlike erythromycin, other standard antibiotics such as streptomycin and tetracycline were more effective than lichen extracts. However, natural lichen extract exhibited MIC values similar to that of streptomycin and tetracycline against S. marcescens.
Table 3. Minimum inhibitory concentration (MIC, µg/mL) of C. olivetorum against clinical isolates.
Phytochemical content analysis
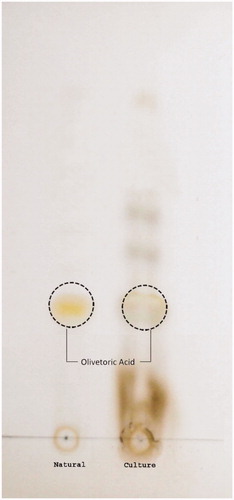
As far as the phytochemical content of lichen C. olivetorum is concerned, extracts of both the cultured and the natural lichen showed variation in their total polyphenol, polysaccharide, and protein contents (). The methanol extract of cultured lichen had 0.22 g of polyphenol content, which was less than the content of natural thallus (1.3 g). A high polysaccharide content (21.30 g) was obtained in cultured lichen, whereas the protein content was found to be 0.97 g and 2.51 g of dried biomass of cultured and natural lichen, respectively. The presence of olivetoric acid was observed on TLC plate of both natural and cultured lichen biomass as a pale orange, grey halo spot with the Rf value of 0.30 and authenticated with the recently published analytical data (Elix, Citation2014) ().
Discussion
Oxidative stress is known to have a key role in onset and progression of neurodegeneration. Production of reactive oxygen species (ROS) is found to be increased in the pathogenesis of AD and damage nerve cells in Parkinson’s disease (Uttara et al., Citation2009). In view of a variety of involvements in neurodegeneration, oxidative stress is one of the preliminary events involved in the onset of AD. Drugs having capacity to scavenge ROS may have the potential to delay its manifestation (Zhao & Zhao, Citation2013) and may prove immensely effective in treating the condition.
Identifying the PEP inhibitors having therapeutic effect against amnesia and dementia highlights the need to analyze novel PEP inhibitors (Fan et al., Citation1999; Diderot et al., Citation2005; Lee & Song, Citation2000). Based on our literature survey on bioactivities of lichens, it was found that lichens and their metabolites are reported for their medicinal value and therapeutic potential in a number of diseases and health problems, except PEP inhibition.
Lichens are known for their characteristic slow growth both under natural conditions and in culture. Therefore, making the biomass available by employing in vitro culture techniques, instead of harvesting this species from nature would be a sustainable approach. Prior to screening of the lichen species C. olivetorum for above-mentioned biological activities, we optimized the culture medium for maximum biomass production of the C. olivetorum culture. MS medium supplemented with 3% glucose and 100 ppb IBA yielded 2.304 g/flask over a period of 60 d. In order to yield biomass sufficient for activity studies, we employed the same nutrient medium and culture conditions at the bioreactor level which yielded 5.095 g of biomass over a period 7 d.
PEPI activity of natural lichen extract found to be 17.33% at 50 µg/mL, which is nearly double as compared with 8.66% of inhibition shown by the standard inhibitor Z-pro-prolinal at 10 µg/mL concentration. Cultured lichen extract showed 6.66% of inhibition at 25 µg/mL concentration and was significantly close to that of the standard inhibitor. The results of PEPI activity imply that crude methanol extracts of both cultured and natural C. olivetorum has the potential to inhibit PEP activity.
Methanol extract of both cultured and natural lichen showed significant antioxidant activities in terms of DPPH radical scavenging (DRS), anti-lipid peroxidation (ALP), and trolox equivalent antioxidant capacity (TEAC). Cultured as well as natural lichen extract exhibited a strong potential for DRS activity, with more than 93% of scavenging of free radicals. In comparison, standard antioxidants showed 63–85.4% of scavenging. Similarly, ALP activity was observed in both the cultured and natural lichen extracts, with at least 89% of inhibition as compared with 47% of inhibition shown by the standard antioxidant BHT. Furthermore, lichen extracts also showed significant Trolox equivalent antioxidant capacity. The ability of lichen extract to inhibit lipid oxidation could be of immense medicinal value in regard to the upraised level of oxidation of fatty acids associated with tau pathology of AD brains (Zhao & Zhao, Citation2013). Many studies have related antioxidant activities to higher polyphenol contents. However, the phytochemical analysis revealed strong antioxidant capacity of lichen extracts due to high polysaccharide content rather than the total polyphenol content. These results are in agreement with those previously reported synergistic effects of polyphenols and polysaccharides towards antioxidant activity (Mahadik et al., Citation2011).
Neurofibrillary tangles composed of hyperphosporylated Tau are hallmarks of AD. A pneumococcal exotoxin (ExoY) has been reported for its potential to cause endothelial Tau hyperphosphorylation (Ochoa et al., Citation2012). While reports related to direct role of ExoY in the onset of AD are lacking, there are reports regarding bacterial and viral involvement in the pathogenesis of neurodegeneration (Braun et al., Citation2007; Chiara et al., Citation2012; Nicolson & Haier, Citation2009). Pseudomonas aeruginosa is known to be an opportunistic human pathogen that causes microbial infection in immunocompromised condition, especially in old age (Gellatly & Hancock, Citation2013). Cetrelia olivetorum has shown varied level of microbial growth inhibition against various clinical isolates. The natural lichen extract was effective against P. aeruginosa with an MIC value of 51.17 µg/mL, as compared with the 114.18 µg/mL of erythromycin. Against remaining bacterial isolates, antimicrobial efficacy of lichen extracts was found to be in the range of 26–106 µg/mL, whereas the MIC value of standard antibiotics was found to be in the range of 4.93–115 µg/mL. Lichen extracts were more effective than erythromycin, except against S. epidermidis. Streptomycin and tetracycline exhibited low MIC values against all bacterial isolates, except S. marcescens, against which the values were equivalent to that of the natural lichen extract. The natural lichen extract was also found to be more effective against C. albicans, in comparison with nystatin and ketoconazole. The observed antimicrobial effect of lichen extract may possibly be due to impaired RNA or DNA synthesis (Maciag-Dorszyńska et al., Citation2014).
Based on the literature survey on PEP inhibitory potential of lichen species, this is the first study on PEPI activity, together with its antioxidant and antimicrobial potential of the lichen C. olivetorum. The study indicates therapeutic potential of Himalayan lichen C. olivetorum against neurodegenerative diseases, encourages its commercial exploitation through mass culture and suggests further research on purification of bioactive components responsible for the observed biological activities. Furthermore, it has also been reported that herpes simplex virus (HSV-1) has considerable involvement in neuronal damage (Braun et al., Citation2007; Chiara et al., Citation2012), hence antiviral potential of bioactive components should also be analyzed.
Acknowledgements
The authors thank Dr. D.K. Upreti, National Botanical Research Institute (CSIR), Lucknow, for providing lichen material. The authors also acknowledge the cooperation of Dr. Anju Kagal, B. J. Medical College, Pune, in regard to providing clinical isolates of microorganisms for the study. The authors are also thankful to Director, Agharkar Research Institute, Pune, for necessary research facilities provided. The authors are also thankful to the anonymous reviewers for their critical assessment of the work.
Declaration of interest
The authors report that they have no conflicts of interest. The authors are grateful to the Department of Biotechnology, Science and Engineering Research Board, Government of India, New Delhi, for the financial support.
References
- Ahmadjian V. (1993). The Lichen Symbiosis. New York: Wiley
- Amor EC, Villaseñor IM, Yasin A, Choudhary MI. (2004). Prolyl endopeptidase inhibitors from Syzygium samarangense (Blume) Merr. and L.M. Perry. Z Naturforsch C 59:86–92
- Behera BC, Mahadik N, Morey M. (2012). Antioxidative and cardiovascular protective activities of metabolite usnic acid and psoromic acid produced by lichen species Usnea complanata under submerged fermentation. Pharm Biol 50:968–79
- Blois MS. (1958). Antioxidant determinations by the use of a stable free radical. Nature 181:1199–200
- Braun JS, Hoffmann O, Schickhaus M, et al. (2007). Pneumolysin causes neuronal cell death through mitochondrial damage. Infect Immun 75:4245–54
- Budson AE, Solomon PR. (2011). Memory Loss: A Practical Guide for Clinicians. Edinburgh: Elsevier Saunders
- Chiara GD, Marcocci ME, Sgarbanti R, et al. (2012). Infectious agents and neurodegeneration. Mol Neurobiol 46:614–38
- Culberson CF, Kristinsson HD. (1972). A standardized method for the identification of lichen products. J Chromatogr 45:85–93
- Deason TR, Bold HC. (1960). Phycological studies I. Exploratory studies of Texas soil algae. Publication no. 6022. Austin (TX): University of Texas
- Diderot NT, Silvere N, Yasin A, et al. (2005). Prolyl endopeptidase and thrombin inhibitory diterpenoids from the bark of Xylopia aethiopica. Biosci Biotech Biochem 69:1763–6
- Dubois M, Gilles KA, Hamilton JK, et al. (1956). Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–6
- Elix JA. (2014). A Catalogue of Standardized Chromatographic Data and Biosynthetic Relationships for Lichen Substances. 3rd ed. Canberra: J. A. Elix Publisher
- Fan W, Tezuka Y, Komatsu K, et al. (1999). Prolyl endopeptidase inhibitors from the underground part of Rhodiola sacra. Biol Pharm Bull 22:157–61
- Gaikwad S, Verma N, Sharma BO, Behera BC. (2014). Growth promoting effects of some lichen metabolites on probiotic bacteria. J Food Sci Technol 51:2624–31
- Gellatly SL, Hancock REW. (2013). Pseudomonas aeruginosa: New insights into pathogenesis and host defenses. Pathog Dis 67:159–73
- Gilgun-Sherki Y, Melamed E, Offen, D. (2001). Oxidative stress induced-neurodegerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40:959–75
- Greeve I, Kretzschmar D, Tschäpe JA, et al. (2004). Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. J Neurosci 24:3899–906
- Huneck S, Yoshimura I. (1996). Identification of Lichen Substances. Berlin: Springer
- Huston JP, Hasenohrl RU. (1995). The role of neuropeptides in learning: Focus on the neurokinin substance P. Behav Brain Res 66:117–27
- Koparal AT, Ulus G, Zeytinoglu M, et al. (2010). Angiogenesis inhibition by a lichen compound olivetoric acid. Phytother Res 24:754–8
- Lee HJ, Song KS. (2000). Kynapcin-12, a new p-terphenyl derivative from Polyozellus multiplex, inhibits prolyl endopeptidase. J Antibiotics 53:714–19
- Lee J-H, Paik Y-S. (2003). Prolyl endopeptidase inhibiting principles from raw medicinal materials. Agric Chem Biotechnol 46:165–8
- Liegeois C, Lermusieau G, Collin S. (2000). Measuring antioxidant efficiency of wort, malt, and hops against the 2, 2′-azobis (2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem 48:1129–34
- Lilly VG, Barnett HL. (1951). Physiology of the Fungi. New York: McGraw-Hill, 1–464
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the folin phenol reagent. Biol Chem 193:265–75
- Maciag-Dorszyńska M, Węgrzyn G, Guzow-Krzemińska B. (2014). Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol Lett 353:57–62
- Mahadik N, Morey MV, Behera BC, et al. (2011). Cardiovascularprotective, antioxidative and antimicrobial properties of natural thallus of lichen Usnea complanata. Lat Am J Pharm 30:220–8
- Miller NJ, Diplock AT, Rice-Evans CA. (1995). Evaluation of the total antioxidant as a marker of the deterioration of apple juice on storage. J Agric Food Chem 43:1794–801
- Molnar K, Farkas E. (2010). Current results on biological activities of lichen secondary metabolites: A review. Z Natureforch C 65:157–73
- Morain P, Lestage P, De Nanteuil G, et al. (2002). S 17092: A prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment: Preclinical and clinical studies. CNS drug Rev 8:31–52
- Murashige T, Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–97
- Nicolson GL. (2008). Chronic bacterial and viral infections in neurodegenerative and neurobehavioral diseases. Lab Med 39:291–9
- Nicolson GL, Haier J. (2009). Role of chronic bacterial and viral infections in neurodegenerative, neurobehavioral, psychiatric, autoimmune and fatiguing illness Part – 1. Br J Med Pract 2:20–28
- Nicolson GL, Haier J. (2010). Role of chronic bacterial and viral infections in neurodegenerative, neurobehavioral, psychiatric, autoimmune and fatiguing illness Part – 2. Br J Med Pract 3:301
- Ochoa CD, Alexeyev M, Pastukh V, et al. (2012). Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial Tau phosphorylation and permeability. J Biol Chem 287:25407–18
- Ohsawa I, Nishimaki K, Murakami Y, et al. (2008). Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J Neurosci 28:6239–49
- Prince M, Prina M, Guerchet, M. (2013). World Alzheimer Report: Journey of Caring; An Analysis of Long-Term Care for Dementia. London: Global Observatory for Ageing and Dementia Care, Health Service and Publication Research Department, King’s College London. Alzheimer’s Disease International (ADI) Publishers
- Querfurth HW, LaFerla FM. (2010). Alzheimer’s disease. N Engl J Med 362:329–44
- Schneider JS, Giardiniere M, Morain P. (2002). Effects of the prolyl endopeptidase inhibitor S 17092 on cognitive deficits in chronic low dose MPTP-treated monkeys. Neuropsychopharmacology 26:176–82
- Shi GF, Wang GY, Chen XF. (2013). Screening of radical-scavenging natural neuroprotective antioxidants from Swertia chirayita. Acta Biol Hung 64:267–78
- Slinkard K, Singleton VL. (1977). Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic 28:49–55
- Srivastava P, Upreti DK, Dhole TN, et al. (2013). Antimicrobial property of Indian lichen against human pathogenic bacteria. Interdiscip Perspect Infect Dis 2013:1–6
- Stocker-Worgotter E. (2008). Metabolic diversity of lichen-forming ascomycetous fungi: Culturing, polyketide and shikimate metabolite production, and PKS genes. Nat Prod Rep 25:188–200
- Tezuka Y, Fan W, Kasimu R, Kadota, S. (1999). Screening of crude drug extracts for prolyl endopeptidase inhibitory activity. Phytomedicine 6:197–203
- Toide K, Shinoda M, Iwamoto Y, et al. (1997). A novel prolyl endopeptidase inhibitor, JTP-4819, with potential for treating Alzheimer's disease. Behav Brain Res 83:147–51
- Toma N, Ghetea L, Nitu R, Corol DI. (2001). Progress and perspectives in the biotechnology of lichens. Rom Biotech Lett 6:1–15
- Uttara B, Singh AV, Zamboni P, Mahajan RT. (2009). Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
- Verma N, Behera BC. (2015). Future directions in the study of pharmaceutical potential of lichens. In: Rankovic B, ed. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential. Switzerland: Springer International, 179–202
- Verma N, Behera BC, Joshi A. (2012). Studies on nutritional requirement for the culture of lichen Ramalina nervulosa and Ramalina pacifica to enhance the production of antioxidant metabolites. Folia Microbiol 57:107–14
- Verma N, Behera BC, Makhija U. (2008). Antioxidant and hepatoprotective activity of a lichen Usnea ghattensis in-vitro. Appl Biochem Biotechnol 151:167–81
- Verma N, Behera BC, Parizadeh H, Sharma BO. (2011). Bactericidal activity of some lichen secondary compounds of Cladonia ochrochlora, Parmotrema nilgherrensis & Parmotrema sancti-angelii. Int J Drug Dev Res 3:222–32
- Wang X, Michaelis EK. (2010). Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2:1–13
- Yamamoto Y, Mizuguchi R, Yamada Y. (1985). Tissue cultures of Usnea rubescens and Ramalina yasudae and production of usnic acid in their cultures. Agric 49:3347–8
- Yoshimoto T, Walter R, Tsuru D. (1980). Proline-specific endopeptidase from Flavobacterium purification and properties. J Biol Chem 255:4786–92
- Zbarsky V, Datla KP, Parkar S, et al. (2005). Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free Radic Res 39:1119–25
- Zhao Y, Zhao B. (2013). Oxidative stress and pathogenesis of Alzheimer’s disease. Oxid Med Cell Long 2013:316523