Abstract
Context Marine macroalgae contain several bioactive molecules that may be developed as functional foods, but information about their neuroprotective potential is scarce.
Objective The objective of this study is to determine the in vitro antioxidant and neuroprotective features of marine algae from the southern coast of Portugal and to assess the total content of different types of bioactives.
Materials and methods Methanol extracts from 21 macroalgal species from the southern Portugal were evaluated for in vitro antioxidant and acetylcholinesterase (AChE) inhibition. Active extracts were further evaluated for inhibitory activity against butyrylcholinesterase (BuChE) and tyrosinase (TYRO), and for their ability to attenuate hydrogen peroxide (H2O2)-induced toxicity in SH-SY5Y cells. The total contents of different phenolic groups were determined for the most active extracts.
Results Cystoseira tamariscifolia (Hudson) Papenfuss (Sargassaceae) had the highest antiradical activity (92%, 1 mg/mL). Cystoseira nodicaulis (Withering) M. Roberts (Sargassaceae) (75%) and Cystoseira humilis Schousboe ex Kützing (Sargassaceae) (70%) had the highest iron-chelating activity at 10 mg/mL. Cystoseira baccata (S.G. Gmelin) P.C. Silva (Sargassaceae) was more active towards copper (66%, 10 mg/mL). Cystoseira tamariscifolia had the highest AChE inhibitory capacity (85%, 10 mg/mL). Cystoseira tamariscifolia and C. nodicaulis were also active against BuChE and TYRO, and were able to protect SH-SY5Y cells against oxidative stress induced by H2O2. Cystoseira tamariscifolia had the highest content of all the groups of phenolics, and was particularly enriched in hydroxycinnamic acids (106 mg CAE/g DW).
Discussion and conclusion Results indicate that C. tamariscifolia and C. nodicaulis are important sources of nutraceutical compounds and may be considered functional foods that could improve cognitive functions.
Introduction
Marine macroalgae are considered important reservoirs of several bioactive compounds, such as sulphated polysaccharides, peptides and phlorotannins. These molecules display important biological activities, which may be relevant for the improvement of human health, including antioxidant, anti-inflammatory and antitumour activities (Andrade et al. Citation2013).
Macroalgae also contain compounds with recognised neuroprotective features, with high potential in the development of functional foods and/or nutraceuticals (Natarajan et al. Citation2009; Pangestuti & Kim Citation2013). The use of products and/or compounds able to decrease the risk of disease and provide relief and/or treatment of cognitive dysfunctions is of particular interest in a society where there is an upsurge in the number of patients diagnosed with neurodegenerative diseases, especially Alzheimer’s (AD) and Parkinson’s (PD) (Chacón-Lee & González-Mariňo Citation2010). At present, more than 10 million people exhibit symptoms related to different forms of dementia, and by 2040, neurodegenerative disorders could be the second leading cause of death in elders, after cancer (Bjarkam et al. Citation2001; Ansari et al. Citation2010).
Marine algae have been eaten and used in traditional medicine in China and in some Western countries for centuries (Fleurence et al. Citation2012). Interestingly, the lower incidence of neurodegenerative diseases in East Asia, as compared with that in Western countries, have been linked to higher consumption of fish and marine algae by East Asian inhabitants (Jorm & Jolley Citation1998; Kannappan et al. Citation2011). However, published information about the neuroprotective potential of marine algae is rather scarce (reviewed in Pangestuti & Kim Citation2011, Citation2013). This information could be particularly useful for future research, development and commercialisation of high-value biocompounds to be used in the pharmaceutical and/or food industries.
The biodiversity of marine macroalgae on the Portuguese coast is high, and includes more than 250, 100 and 60 Rhodophyta, Ochrophyta and Chlorophyta species, respectively (Sousa-Pinto Citation1998). Taking advantage of this diversity, this work reports the antioxidant activity and the acetylcholinesterase (AChE) inhibitory activity of methanol extracts of 21 species of macroalgae from the southern coast of Portugal. Selected extracts were then evaluated for their inhibitory activity against butyrylcholinesterase (BuChE) and tyrosinase (TYRO), capacity to attenuate hydrogen peroxide-induced toxicity in the human dopaminergic cell line SH-SY5Y and content in different groups of phenolic compounds.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH), AChE (from Electrophorus electricus, Type V-S, EC 3.1.1.7), BuChE (from horse serum, EC 3.1.1.8) and TYRO (from mushroom, EC 1.14.1.8.1, 30 U) were provided from Sigma (Steinheim, Germany). Sodium carbonate was from Fluka (Steinheim, Germany), whereas Merck (Darmstadt, Germany) supplied dimethyl sulphoxide (DMSO), trichloroacetic acid (TCA) and Folin–Ciocalteu (F–C). Methanol was from Fischer Scientific (Loughborough, UK). Additional reagents and solvents were acquired from VWR International (Leuven, Belgium).
Sample collection and processing
Biomass was collected in the middle/lower intertidal areas, during the low tide in July–November of 2009 and in May 2010 at beaches throughout the Algarve coast, namely Vila Real de Santo António, Faro and Albufeira. A total of 21 macroalgal species were screened: 10 species belonging to the Ochrophyta phylum [Cystoseira tamariscifolia (Hudson) Papenfuss (Sargassaceae), C. usneoides (Linnaeus) M. Roberts (Sargassaceae), C. nodicaulis (Withering) M. Roberts (Sargassaceae), C. baccata (S.G. Gmelin) P.C. Silva (Sargassaceae), C. humilis Schousboe ex Kützing (Sargassaceae), Dictyota dichotoma (Hudson) J.V. Lamouroux (Dictyotaceae), Dictyota spiralis Montagne (Dictyotaceae), Sargassum vulgare C. Agardh (Sargassaceae), Sargassum muticum (Yendo) Fensholt (Sargassaceae) and Taonia atomaria (Woodward) J. Agardh (Dictyotaceae)], five rhodophytes [Asparagopsis armata Harvey (Bonnemaisoniaceae), Peyssonnelia sp. (Peyssonneliaceae), Peyssonnelia squamaria (S.G. Gmelin) Decaisne (Peyssonneliaceae), Pterocladiella capillacea (S.G. Gmelin) Santelices & Hommersand (Pterocladiaceae) and Jania sp. (Corallinaceae)] and six chlorophytes [Chaetomorpha sp. (Cladophoraceae), Codium fragile (Suringar) Hariot, (Codiaceae), Codium sp. (Codiaceae), Enteromorpha sp. (Ulvaceae), Ulva sp. (Ulvaceae) and Cladophora albida (Nees) Kutzing, (Cladophoraceae)]. Identification of specimens was made by Dr. Aschwin Engelen (Centre of Marine Sciences, University of Algarve, Portugal). Samples were washed with seawater, kept cold until arrival to the laboratory, washed with tap water, freeze dried at 40 °C for 2–3 d, ground and stored at −20 °C. Voucher specimens (code nos. MB001–MB022) were deposited at the Centre of Marine Sciences, University of Algarve.
Preparation of the extracts
Methanol extracts were prepared by mixing 1 g of milled samples with 40 mL of methanol, followed by cell disruption with an Ultra Turrax T25 (IKA Labortechnik Basic, Staufen, Germany) disperser for 2 min. Samples were then extracted for 16 h at room temperature (20 °C, RT) with stirring. The extracts were centrifuged (5000 g, 15 min, 20 °C), filtered (Whatman no. 4) and dried under vacuum. Dried extracts were resuspended in methanol at the concentration of 10 mg/mL and stored at −20 °C.
Antioxidant activity
Radical-scavenging activity (RSA) on DPPH
The RSA was evaluated by the method of Brand-Williams et al. (Citation1995), as described in Custódio et al. (Citation2015). Briefly, the extracts (22 μL at the concentrations of 1, 5 and 10 mg/mL) were mixed with 200 μL of a methanol DPPH solution (120 μM) in 96-well flat bottom microtitration plates, and incubated in darkness at RT for 30 min. The absorbance was measured at 515 nm and RSA was calculated as the percentage inhibition relative to a blank containing methanol. Butylated hydroxytoluene (BHT, 1 mg/mL) was used as a positive control.
Copper chelating activity (CCA)
The CCA was determined by the method described by Megías et al. (Citation2009). Samples (30 μL at the concentrations of 1, 5 and 10 mg/mL) were mixed in 96-well microplates with 200 μL of 50 mM sodium acetate buffer (pH 6), 6 μL of pyrocatechol violet (4 mM) in the acetate buffer and 100 μL of copper sulphate. The change in colour of the solution was measured at 632 nm using a microplate reader (Biotek Synergy 4, Winooski, VT). The synthetic metal chelator ethylenediamine tetraacetic acid (EDTA) was used as a positive control at the concentration of 1 mg/mL.
Iron chelating activity (ICA)
The ICA chelating activity was determined by measuring the formation of the Fe2+ ferrozine complex according to Megías et al. (Citation2009), with some modifications. Samples (30 μL at the concentration of 1, 5 and 10 mg/mL) were mixed in 96-well microplates with 200 μL of distilled water and 30 μL of an iron(II) chloride solution (0.1 mg/mL in water). After 30 min, 12.5 μL of ferrozine solution (40 mM in water) were added. Change in colour was measured in a microplate reader at 562 nm. EDTA was used as a positive control at the concentration of 1 mg/mL.
In vitro neuroprotective properties: inhibition of enzymes related with neurological diseases
AChE and BuChE inhibition
The inhibitory effect on AChE and BuChE activities was measured by the Ellman method (Ellman et al. Citation1961) as described previously (Custódio et al. Citation2015). Briefly, 20 μL of each extract (1, 5 and 10 mg/mL) were mixed with 140 μL of 0.1 mM sodium phosphate buffer (pH 8.0) and 20 μL of AChE or BuChE solution (0.28 U/mL) in 96-well microplates and incubated at RT for 15 min. The reaction was initiated by adding 10 μL of acetylthiocholine or butyrylthiocholine iodide (4 mg/mL) together with 20 μL of 5,5′-dithio-bis(2-nitrobenzoic acid (DTNB: 1.2 mg/mL). The hydrolysis of acetylthiocholine or butyrylthiocholine iodide was monitored by the formation of the yellow 5-thio-2-nitrobenzoate anion as a result of the reaction of DTNB with thiocholines catalysed by the enzymes, at 412 nm, using a 96-well micro-plate reader (Biotek Synergy 4, Winooski, VT). Results were expressed as AChE and BuChE percentage inhibition relative to a negative control, containing methanol in place of the sample. Galanthamine was used as the positive control at the concentration of 1 mg/mL.
TYRO inhibition
The inhibitory activity against mushroom TYRO was determined by the method reported by Nerya et al. (Citation2003) using l-tyrosine as a substrate. Samples (70 μL) were mixed in 96-well microplates with 30 μL of TYRO (333 units/mL in phosphate buffer, pH 6.5) and incubated for 5 min. Then, 110 μL of substrate (l-tyrosine, 2 mM in water) were added to each well and further incubated for 30 min at RT. The optical densities of the wells were read at 492 nm using a 96-well micro-plate reader (Biotek Synergy 4, Winooski, VT). The extracts were tested at the concentrations of 1, 5 and 10 mg/mL and results were expressed as TYRO percentage inhibition relative to a negative control, containing methanol. Arbutin was used as the positive control at the concentration of 1 mg/mL.
In vitro neuroprotective properties: cell-based assay
Protective effect of selected extracts on hydrogen peroxide-induced cytotoxicity
SH-SY5Y cells were kindly provided by Dr. Eduardo Soriano (Barcelona Science Park, Barcelona, Spain). Cells were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with glucose (4500 mg/mL), 10% heat inactivated foetal bovine serum (FBS), l-glutamine (2 mM), penicillin (50 U/mL) and streptomycin (50 μg/mL), and were grown at 37 °C in an incubator with 5.1% CO2, in humidified atmosphere. To determine the effect of the extracts on the viability of SH-SY5Y cells, they were seeded in 96-well plates at a density of 2 × 104 cell per well, incubated for 24 h and exposed to the extracts from C. tamariscifolia and C. nodicaulis at different concentrations (2–125 μg/ml). Cells were then incubated for 24 h and cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann Citation1983) as described elsewhere (Custódio et al. Citation2015).
To evaluate the protective effect of selected extracts on hydrogen peroxide-induced cytotoxicity, SH-SY5Y cells were seeded in 96-well culture plates at a density of 20 × 104 cells per well, left to attach for 16 h and treated with non-toxic concentrations of methanol extracts of C. tamariscifolia and C. nodicaulis in culture medium. After 24 h of incubation, the extracts were removed and cells were treated with H2O2 (100 μM) for a period of 30 min (Kim et al. Citation2005; Custódio et al. Citation2013, Citation2015). To avoid the formation of artefacts, the stock solution of H2O2 was prepared on phosphate-buffered saline (PBS, pH 7.4) and diluted with culture medium without FBS immediately before use in the assays. Negative control cells were treated with DMSO at the highest concentration used in test wells (0.5%, v/v), and cell viability was determined by the MTT assay.
Bioactive constituents
Total phenolics content (TPC)
TPC was determined by the F–C assay according to Velioglu et al. (Citation1998). The extracts (5 μL at the concentration of 10 mg/mL) were mixed with 10-fold diluted F–C reagent in distilled water (100 μL) and incubated at RT for 5 min. Afterwards, 100 μL of sodium carbonate (75 g/L, w/v) were added, samples were incubated for 90 min at RT, and absorbance was measured at 725 nm on a microplate reader (Biotek Synergy 4, Winooski, VT). Results were expressed as gallic acid equivalents in milligrams per gram of extract (mg GAE/g dry weight, DW) using a calibration curve of gallic acid standard solutions.
Hydroxycinnamic acids
Hydroxycinnamic acids were detected by spectrophotometry according to Mazza et al. (Citation1999), modified to 96-well microplates. The extracts (20 μL at the concentration of 1 mg/mL) were placed in 96-well plates, diluted with 20 μL of an aqueous ethanol solution (95%, v/v) containing 0.1% hydrochloric acid and mixed with 160 μL of 2% hydrochloric acid. The absorbance was measured at 320 nm to determine hydroxycinnamic acids using caffeic acid as standard. Results were expressed as milligram of caffeic acid equivalents (CAE) per gram of extract DW.
Flavone and flavonols
Flavone and flavonol contents were quantified according to the method described by Ahn et al. (Citation2007), with modifications. Briefly, 50 μL of 2% aluminium chloride–ethanol solution was added to 50 μL of the extracts at the concentration of 1 mg mL − 1. After 1 h at RT, the absorbance was measured at 420 nm. Quercetin was used as standard, and results were expressed as milligram of quercetin equivalents (QE) per gram of extract (DW).
Total condensed tannins content (CTC)
The CTC of the extracts was evaluated by the 4-dimethylaminocinnamaldehyde–hydrochloric acid (DMACA-HCl) colorimetric method (Li et al. Citation1996) adapted to 96-well microplates (Zou et al. Citation2011). The extracts (10 μL) were mixed with 200 μL of a methanol solution of DMACA (1% in methanol) and 100 μL of 37% hydrochloric acid. After 15 min of incubation, the absorbance was measured at 640 nm in a microplate reader (Biotek Synergy 4, Winooski, VT). CTC was calculated based in a standard curve of different concentrations of catechin and the results were expressed as milligram of catechin equivalents per gram of extract DW (mg CE/g DW).
Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM), and experiments were conducted at least in triplicate. Significant differences were assessed by analysis of variance (ANOVA) or using Duncan’s New Multiple Range Test when parametricity of data did not prevail. SPSS statistical package for Windows (release 15.0, SPSS Inc., Chicago, IL) was used.
Results
Antioxidant activity
The DPPH scavenging activity followed the trend Ochrophyta > Rhodophyta > Chlorophyta (). Ochrophytes belonging to the Cystoseira genus had the highest scavenging potential, especially C. tamariscifolia (92% at 1 mg/mL; ). When tested at the concentration of 10 mg/mL other species had also a high RSA, namely C. usneoides (109.0%), S. vulgare (98.2%), C. nodicaulis (95.1%) and C. baccata (90.4%; ). At the concentration of 10 mg/mL rhodophytes had moderate activities, ranging from 4.7% for Peyssonnelia squamaria to 40.5% for Peyssonnelia sp., whereas, in chlorophytes, the RSA values went from 10.8% in Codium fragile to 41.2% in Cladophora albida (). None of the species had relevant iron chelation activity (ICA) at the concentration of 1 mg/mL, although at 10 mg/mL ICA was detected, with C. nodicaulis (75.0%) and C. humilis (70.4%) showing the highest levels (). Samples had low capacity to chelate copper when tested at 1 mg/mL, and at the highest concentration, the highest activities were detected in C. baccata (66.3%), Pterocladiella capillacea (62.0%) and C. albida (62.0%) extracts ().
Table 1. Radical scavenging activity (RSA) on DPPH radicals of methanol extracts of different species of macroalgae.
Table 2. Metal chelating activity on iron and copper of methanol extracts of different species of macroalgae.
Inhibition of enzymes related with neurological diseases (AChE, BuChE and TYRO)
Concerning the extent of enzyme inhibitory activity (%), the effect was classified as potent (>50%), moderate (30–50%), low (<30%) or nil (<5%) according to Vinutha et al. (Citation2007). Ochrophytes had the highest inhibitory capacity on AChE, particularly C. tamariscifolia with potent inhibition at 1 mg/mL (70.1%), and 10 mg/mL (85.3%), and C. nodicaulis at 10 mg/mL (64.4%; ). Regarding the rhodophytes analysed, A. armata had potent inhibitory capacity (58.4% at 10 mg/mL), whereas chlorophytes had low or nil activity (). The species with the highest capacity to inhibit AChE, i.e., C. tamariscifolia, C. nodicaulis and A. armata were further evaluated for BuChE and TYRO inhibition, and results are summarised in . The highest BuChE inhibition was obtained with C. nodicaulis (1 mg/mL: 84.5%) and C. tamariscifolia (1 mg/mL: 83.2%), similar to the values obtained with the application of galanthamine (80.3%) used as a positive control. Cystoseira nodicaulis had potent activity on TYRO at 1 mg/mL (85.6%), higher than the result obtained with the positive control, arbutin (78.0%).
Table 3. Acetylcholinesterase (AChE) inhibitory activity of methanol extracts of different species of macroalgae.
Table 4. Butyrylcholinesterase (BuChE) and tyrosinase (TYRO) inhibitory activity of methanol extracts of selected species of macroalgae.
Neuroprotective effect against H2O2-induced cytotoxicity on SH-SY5Y cells
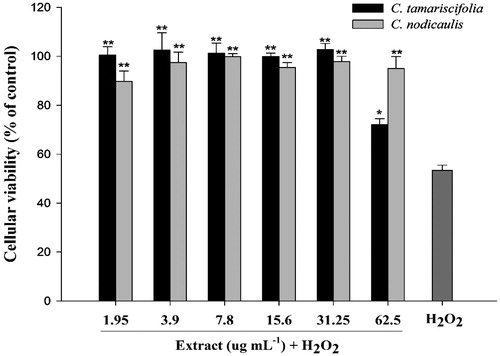
Exposure of SH-SY5Y cells to H2O2 concentrations ranging from 1.5 to 25 mM for 30 min resulted in a significant loss of cell viability when compared with untreated control cells (p < 0.001), and decreased to 13.3% after exposure to 25 mM (data not shown). The concentration decreasing cell viability by 50%, i.e., 3.12 μM, was selected and used to induce oxidative stress. Non-toxic concentrations of the extracts from the most promising species, i.e., C. tamariscifolia and C. nodicaulis, were selected by exposing cells to samples at different concentrations (2–125 μg mL − 1) for 24 h. No significant decrease in cell viability was observed on treatment with samples up to the concentration of 62.5 μg/mL (data not shown). Thus, those concentrations were used to evaluate the neuroprotective effect of the algal extracts. Pre-treatment of cells with the extract from C. tamariscifolia and C. nodicaulis enhanced cell survival to almost 100% from viability of 53.4% of H2O2-treated cells without algal extract addition ().
Figure 1. Protective effect of methanol extracts of C. tamariscifolia and C. nodicaulis in SH-SY5Y cells. Cells were pre-treated with different concentrations of the extracts for 24 h, and exposed to 3.12 μM of H2O2. Each value represents the mean ± SEM of three replicates. Significantly different than that of the H2O2-treated group: *p < 0.05; **p < 0.01.
Bioactive constituents
The extracts from C. tamariscifolia and C. nodicaulis were evaluated for their total contents in phenolics, hydroxycinnamic acids, flavone and flavonols and condensed tannins, and the results are summarised in . Cystoseira tamariscifolia had higher contents of phenolic compounds of all groups as compared with those of C. nodicaulis. In particular, the former contained high levels of hydroxycinnamic acids (106 mg CAE/g DW).
Table 5. Bioactive constituents (mg/g, dry weight) of methanol extracts of C. tamariscifolia and C. nodicaulis.
Discussion
This work aimed to identify macroalgal species from the Portuguese coast with potential interest as functional foods and/or as sources of compounds with neuroprotective features. For that purpose, 21 macroalgae species were first evaluated for their RSA and iron and copper chelation ability. Our results suggest that some species included in this work, especially those belonging to the Ochrophyta phylum, and more specifically to the Cystoseira genus, could be sources of molecules with RSA and metal chelation potential, with possible interest in the management of oxidative stress-linked neurological diseases. In fact, antioxidants can protect the central nervous system (CNS) against free radical-mediated oxidative damage, and constitute a major component of clinical and experimental drugs that are currently under consideration for the prevention and treatment of different neurodegenerative diseases (Moosmann & Behl Citation2002; Andersen Citation2004; Konishi Citation2009). In addition, the use of iron and copper chelators is often considered a valuable strategy for the management of oxidative stress-related neurological disorders, since the accumulation of those metals has a significant impact on reactive oxygen species (ROS) generation through the promotion of the Haber–Weiss/Fenton reaction (Gaeta & Hider Citation2005, Weinreb et al. Citation2011). Moreover, iron may promote the deposition of plasma amyloid beta peptide (Aβ) in senile plaques, which is one of the pathological characteristics of AD along with the intracellular formation of neurofibrillary tangles and loss of neuronal synapses and pyramidal neurons (Weinreb et al. Citation2011). Although the role of copper in AD is controversial, there is direct evidence that an increase in the concentration of that metal in AD patients is related with ROS generation and neuronal cell death (Gaeta & Hider Citation2005).
All the extracts were then tested for their inhibitory capacity on AChE, and the most active ones, i.e., C. tamariscifolia, C. nodicaulis and A. armata, were further evaluated for BuChE and TYRO inhibition. AChE and BChE hydrolyse the neurotransmitter acetylcholine (ACh) and a decrease in the levels of ACh is usually associated with the onset of AD and Parkinson’s (PD), the most common forms of dementia (Filho et al. Citation2006; Prince et al. Citation2012). Thus the use of cholinesterases inhibitors (ChEI) is considered to be a useful therapeutic approach for the symptomatic treatment of AD (Filho et al. Citation2006; Pangestuti & Kim Citation2010). In this sense, our results suggest a possible therapeutic value of the compounds present in C. tamariscifolia and C. nodicaulis as inhibitors of AChE and BuChE. In fact, the most prescribed drugs for the treatment of AD, such as donepezil and galanthamine, are selective inhibitors of AChE (Orhan et al. Citation2007; Dall’Acqua Citation2013). Furthermore, there are reports suggesting that the use of compounds with dual ChE inhibition can increase the efficacy of the treatment, and broaden their application to other disorders affecting the CNS, namely Down syndrome and patients suffering from traumatic brain injuries (Giacobini Citation2004). There are several reports about the AChE inhibitory activity of different macroalgae species (reviewed in Pangestuti & Kim Citation2011). Recently, Andrade et al. (Citation2013) made a screening of the AChE and BuCHE inhibition of ethanol extracts of different Chlorophyta, Rhodophyta and Ochrophyta macroalgae, and observed potent AChE inhibition in extracts from C. usneoides and Fucus spiralis extracts, but no activity in those of C. tamariscifolia and A. armata. Conversely, those authors only detected moderate activity against BuCHE on the extracts made from C. tamariscifolia and C. usneoides.
Cystoseira nodicaulis strongly inhibited TYRO at the lowest concentration tested. TYRO is a multifunctional copper-containing enzyme involved in neuromelanin formation in the human brain and, due to its oxidase activity, can potentially accelerate the induction of catecholamine quinone derivatives, contributing to the neurodegeneration associated with PD (Khan 2007; Hasegawa Citation2010). In fact, tyrosinase inhibitors have become an attractive target for the treatment of this disease. Methanol extracts and organic fractions of other brown algal species, such as Ecklonia stolonifera were reported to strongly inhibit TYRO (Kang et al. Citation2008). However, as far as we know, there were no reports until now on the TYRO inhibition of C. nodicaulis, C. tamariscifolia and A. armata. Assays are in progress to isolate and identify the compounds responsible for the observed inhibition.
Since C. tamariscifolia and C. nodicaulis had the strongest antioxidant potential, coupled with AChE, BuCHE and TYRO inhibition, they were further explored for their neuroprotective potential. This was accomplished through the evaluation of their in vitro protective effect against hydrogen peroxide (H2O2)-induced cytotoxicity on SH-SY5Y cells, a human neuroblastoma cell line often used as an in vitro model in studies regarding the evaluation of the effect of drugs and natural compounds on neuronal ailments (Kim et al. Citation2005; Custódio et al. Citation2013, Citation2015). H2O2 was chosen as the inducer of oxidative stress, because it is one of the main ROS produced in the brain during the progress of AD (Pan et al. Citation2009). The pre-treatment of the cells with the extract from C. tamariscifolia and C. nodicaulis enhanced cell survival up to almost 100% compared with the H2O2-treated group, which had a viability of 53.4% (). Since the extracts were removed from the cells before the application of H2O2, the increase in cell viability may be more likely due to the activation of intercellular and intracellular excitoprotective signalling pathways, and those related to the maintenance of mitochondrial function, than to direct antioxidant effects (Mattson 2003).
To gain further knowledge regarding the phytochemical composition of the extracts from C. tamariscifolia and C. nodicaulis, they were evaluated for their total contents in phenolics, hydroxycinnamic acids, flavone and flavonols and condensed tannins. Cystoseira tamariscifolia had a higher content of all the groups of phenolic compounds than C. nodicaulis. The TPC of the former species was also higher than those reported for other species of the Sargassaceae family, such as S. vulgare and S. muticum, and also than other Ochrophyta, as for example D. dichotoma and D. spiralis (Zubia et al. Citation2009; Airanthi et al. Citation2011). Biophenols are believed to shield the algal thallus against photodestruction caused by UV radiation, herbivores and pathogens (Abdala-Díaz et al. Citation2006). Moreover, phenolic compounds are considered as one the most potent and therapeutically useful molecules due to their capacity to prevent oxidative stress-mediated disorders, such as cancer and neurodegenerative disorders (Soobrattee et al. Citation2005). Cystoseira tamariscifolia was particularly enriched in hydroxycinnamic acids. Hydroxycinnamic acids including ferulic, caffeic, sinapic and p-coumaric acids are phenolic compounds widely distributed in plants and also present in algae (Shahidi & Chandrasekara Citation2010; Novoa et al. Citation2011). These compounds display a strong anti-radical activity, which is related to their capacity to donate hydrogen or electron, and to delocalise/stabilise the resultant phenoxyl radical (Teixeira et al. Citation2013). The highest hydroxycinnamic acids content in C. tamariscifolia could thus explain its higher RSA, when compared with C. nodicaulis. Additionally, there is evidence that different hydroxycinnamic acids can protect neuronal cells against oxidative damage in in vitro models (Pavlica & Gebhardt Citation2005; Nakajima et al. Citation2009; Jeong et al. Citation2011; Garrido et al. Citation2012). Moreover, in vivo studies in rats and mice suggest that those compounds can be effective neuroprotective agents (Cheng et al. Citation2008; Tsai et al. Citation2011). In this sense, it is possible that these compounds are involved in the observed protective effect of the extract of C. tamariscifolia against oxidative stress imposed by H2O2 treatment on of SH-SY5Y, but not in C. nodicaulis, since in this species hydroxycinnamic acids were detected in significantly lower amounts.
Different Cystoseira species contain different classes of biocompounds with recognised health promoting potential, such as polyunsaturated fatty acids (PUFA), phlorotannins, tetraprenyltoluquinols, sesquiterpene derivates and diterpenoids (Koivikko et al. Citation2005; Colombo et al. Citation2006; Gouveia et al. Citation2013; Silva et al. Citation2013). Specifically, the species that yielded the best results in this work, i.e., C. tamariscifolia and C. nodicaulis, have a high PUFA content, with values of 44.3 and 43.6%, respectively, with predominance of arachidonic (AA, C20:4n-6) and eicosapentaenoic acids (C20:5n-3, EPA; Vizetto-Duarte et al. Citation2015). The incorporation of AA and EPA in the diet is generally encouraged since they often lead to the reduction of coronary risk and aid in the prevention and/or treatment of neurological pathologies such as depression (Chowdhury et al. Citation2014). Additionally, C. tamariscifolia and C. nodicaulis have a low ratio PUFA/saturated fatty acids (SFA) (Vizetto-Duarte et al. Citation2015), which is considered appropriate for human consumption (HMSO Citation1994). Moreover, the n-6 PUFA/n-3 PUFA ratios found for these species by Vizetto-Duarte et al. (Citation2015) are within the values recommended by WHO to prevent neurological diseases (Kumari et al. Citation2013). In addition, C. tamariscifolia and C. nodicaulis contain different types of phlorotannins, such as fucophloroethol, fucodiphloroethol, fucotriphloroethol, 7-phloroeckol, phlorofucofuroeckol and bieckol/dieckol (Ferreres et al. Citation2012). Those compounds could be associated with the radical scavenging activity detected in this work in both species, since they are known to have strong antioxidant properties (Kim et al. Citation2009; Ferreres et al. Citation2012), and thus, useful for the prevention/treatment of disorders associated with oxidative stress, such as those affecting the CNS.
Conclusions
Our results suggest that the brown seaweeds C. tamariscifolia and C. nodicaulis contain molecules appropriate for nutraceuticals and may constitute functional foods aiming the improvement of cognitive functions, through their capacity to scavenge free radicals, chelate metals, protect neuronal cells from oxidative stress and inhibit enzymes involved in the degradation of the neurotransmitter ACh. Assays are already in progress aiming to isolate and identify the bioactive compounds in those extracts.
Acknowledgements
The authors would like to acknowledge Dr. Aschwin Engelen (Centre of Marine Sciences, University of Algarve) for his support during the collection and morphological identification of algal samples.
Declaration of interest
Financial support for this work was provided by the SEABIOMED (PTDC/MAR/103957/2008) and XtremeBio projects (PTDC/MAR-EST/4346/2012) funded by the Foun-dation for Science and Technology (FCT) and the Portuguese National Budget. This work also received national funds through FCT project CCMAR/Multi/04326/2013. Catarina Vizetto-Duarte is a recipient of a doctoral fellowship (SFRH/BD/81425/2011), as well as Hugo Pereira (SFRH/BD/105541/2014). Luísa Custódio was supported by FCT Investigator Programme (IF/00049/2012).
References
- Abdala-Díaz RT, Cabello-Pasini A, Pérez-Rodríguez E, Conde Álvarez RM, Figueroa FL. 2006. Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar Biol. 148:459–465.
- Airanthi MKWA, Hosokawa M, Miyasita K. 2011. Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci. 76:104–111.
- Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P. 2013. Valuable compounds in macroalgae extracts. Food Chem. 138:1819–1828.
- Ahn M-R, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, Nakayama T. 2007. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 101:1383–1392.
- Andersen J. 2004. Oxidative stress in neurodegeneration: cause or consequence? Nat Rev Neurosci. 5:S18–S25.
- Ansari J, Siraj A, Inamdar N. 2010. Pharmacotherapeutic approaches of Parkinson’s disease. Int J Pharmacol. 6:584–590.
- Bjarkam CR, Sørensen JC, Sunde NÅ. 2001. New strategies for the treatment of Parkinson's disease hold considerable promise for the future management of neurodegenerative disorders. Biogerontology 2:193–207.
- Brand-Williams W, Cuvelier M, Berset C. 1995. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 28:25–30.
- Chacón-Lee TL, González-Mariňo GE. 2010. Microalgae for “healthy” foods – possibilities and challenges. Comp Rev Food Sci F. 9:655–675.
- Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. 2008. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 36:1105–1119.
- Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. 2014. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 160:398–406.
- Colombo ML, Risé P, Giavarini F, de Angelis L, Galli C, Bolis CL. 2006. Marine macroalgae as sources of polyunsaturated fatty acids. Plant Foods Hum Nutr. 61:67–72.
- Custódio L, Patarra J, Alberício F, Neng NR, Nogueira JMF, Romano A. 2013. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind Crops Prod. 45:114–120.
- Custódio L, Soares F, Pereira H, Rodrigues MJ, Barreira L, Rauter AP, Albericio F, Varela J. 2015. Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J Appl Phycol. 27:839–848.
- Dall’Acqua S. 2013. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Bot Target Ther. 3:19–28.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95.
- Ferreres F, Lopes G, Gil-Izquierdo A, Andrade PB, Sousa C, Mouga T, Valentão P. 2012. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MS: approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar Drugs 10:2766–2781.
- Filho J, Medeiros K, Diniz M, Batista L, Athayde-Filho P, Silva M, da-Cunha E. 2006. Natural products inhibitors of the enzyme acetylcholinesterase. Br J Pharmacogn. 16:258–285.
- Fleurence J, Morançais M, Dumay J, Decottignies P, Turpin V, Munier M, Garcia-Bueno N, Jaouen P. 2012. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci Technol. 27:57–61.
- Gaeta A, Hider RC. 2005. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol. 146:1041–1059.
- Garrido J, Gaspar A, Garrido EM, Miri R, Tavakkoli M, Pourali S, Saso L, Borges F, Firuzi O. 2012. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 94:961–967.
- Giacobini E. 2004. Cholinesterases: new roles in brain function and in Alzheimer's disease. Neurochem Res. 28:515–522.
- Gouveia V, Seca AML, Barreto MC, Pinto DCGA. 2013. Di- and sesquiterpenoids from Cystoseira genus: structure, intra-molecular transformations and biological activity. Mini-Rev Med Chem. 13:1150–1159.
- Hasegawa T. 2010. Tyrosinase-expressing neuronal cell line as in vitro model of Parkinson's disease. Int J Mol Sci. 11:1082–1089.
- HMSO UK. 1994. Nutritional aspects of cardiovascular disease (report on health and social subjects No. 46). London: HMSO.
- Jeong CH, Jeong HR, Choi GN, Kim DO, Lee U, Heo HJ. 2011. Neuroprotective and anti-oxidant effects of caffeic acid isolated from Erigeron annuus leaf. Chin Med. 6:25.
- Jorm AF, Jolley D. 1998. The incidence of dementia: a meta-analysis. Neurology 51:728–733.
- Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, Lee IK, Kim BJ, Shin T, Park JW, Lee LH, et al. 2008. Inhibitory effects of triphlorethol-A on MMP-1 induced by oxidative stress in human keratinocytes via ERK and AP-1 inhibition. J Toxicol Environ Health Part A. 71:992–999.
- Kannappan R, Gupta S, Kim J, Reuter S, Aggarwal B. 2011. Neuroprotection by spice-derived nutraceuticals: you are what you eat! Mol Neurobiol. 44:142–159.
- Kim AR, Shin TS, Lee MS, Park JY, Park KE, Yoon NY, Kim JS, Choi JS, Jang BC, Byun DS. 2009, et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J Agric Food Chem. 57:3483–3489.
- Kim S-S, Park R-Y, Jeon H-J, Kwon Y-S, Chun W. 2005. Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SHSY5Y cells. Phytother Res. 19:243–245.
- Koivikko R, Loponen J, Honkanen T, Jormalainen V. 2005. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J Chem Ecol. 31:195–212.
- Konishi T. 2009. Brain oxidative stress as basic target of antioxidant traditional oriental medicines. Neurochem Res. 34:711–716.
- Kumari P, Bijo AJ, Mantri VA, Reddy CR, Jha B. 2013. Fatty acid profiling of tropical marine macroalgae: an analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 86:44–56.
- Li YG, Tanner G, Larkin P. 1996. The DMACA-HC1 protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J Sci Food Agric. 70:89–101.
- Mazza G, Fukumoto L, Delaquis P, Girard B, Ewert B. 1999. Anthocyanins, phenolics, and color of cabernet franc, merlot, and pinot noir wines from British Columbia. J Agric Food Chem. 47:4009–4017.
- Megías C, Pastor-Cavada E, Torres-Fuentes C, Girón-Calle J, Alaiz M, Jua R, Julio P, Javier V. 2009. Chelating, antioxidant and antiproliferative activity of Vicia sativa polyphenol extracts. Eur Food Res Technol. 230:353–359.
- Moosmann B, Behl C. 2002. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs 11:1407–1435.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63.
- Nakajima Y, Nishida H, Nakamura Y, Konishi T. 2009. Prevention of hydrogen peroxide-induced oxidative stress in PC12 cells by 3,4-dihydroxybenzalacetone isolated from chaga (Inonotus obliquus (persoon) Pilat). Free Radic Biol Med. 47:1154–1161.
- Natarajan S, Shanmugiahthevar KP, Kasi PD. 2009. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar). Nat Prod Res. 23:355–369.
- Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. 2003. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem. 51:1201–1207.
- Novoa AV, Andrade-Wartha ERS, Linares AF, Silva AMO, Genovese MI, González AEB, Vuorela P, Costa A, Mancini-Filho J. 2011. Antioxidant activity and possible bioactive components in hydrophilic and lipophilic fractions from the seaweed Halimeda incrassate. Braz J Pharmacogn. 21:53–57.
- Orhan I, Kartal M, Naz Q, Ejaz A, Yilmaz G, Kan YKB, Sener B, Choudhary MI. 2007. Antioxidant and anticholinesterase evaluation of selected Turkish Salvia species. Food Chem. 103:1247–1254.
- Pan W, Dancik CM, Nelson VM, Jiang Z-G, Lebowitz MS, Ghanbari HA. 2009. A novel neuroprotectant PAN-811 protects neurons from oxidative stress. Cent Eur J Biol. 4:34–40.
- Pangestuti R, Kim S-K. 2010. Neuroprotective properties of chitosan and its derivatives. Mar Drugs 8:2117–2128.
- Pangestuti R, Kim S-K. 2011. Neuroprotective effects of marine algae. Mar Drugs 9:803–818.
- Pangestuti R, Kim S-K. 2013. Marine-derived bioactive materials for neuroprotection. Food Sci Biotechnol. 22:1175–1186.
- Pavlica S, Gebhardt R. 2005. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic Res. 39:1377–1390.
- Prince M, Bryce R, Ferri C. 2012. World Alzheimer report 2011. London, UK: Alzheimer’s Disease International (ADI).
- Ragan MA. 1985. Brown algal polyphenols: synthesis of “fucophlorethol A” octamethyl ether (2,2′,4,6,6′-pentamethoxy-4′-(2,4,6-trimethoxyphenoxy)biphenyl). Can J Chem. 63:294–303.
- Shahidi F, Chandrasekara A. 2010. Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev. 9:147–170.
- Silva G, Pereira RB, Valentão P, Andrade PB, Sousa C. 2013. Distinct fatty acid profile of ten brown macroalgae. Braz J Pharmacogn. 23:608–613.
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. 2005. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 579:200–213.
- Sousa-Pinto I. 1998. The seaweed resources of Portugal. In: Critchley AT, Ohno M, editors. Seaweed resources of the world. Yokosuka: Japan International Cooperation Agency. p. 176–184.
- Teixeira J, Gaspar A, Garrido EM, Garrido J, Borges F. 2013. Hydroxycinnamic acid antioxidants: an electrochemical overview. Biol Med Res Int. Article ID 251754. 11 pages.
- Tsai SJ, Chao CY, Yin MC. 2011. Preventive and therapeutic effects of caffeic acid against inflammatory injury in striatum of MPTP-treated mice. Eur J Pharmacol. 670:441–447.
- Velioglu YS, Mazza G, Gao L, Oomah BD. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem. 46:4113–4117.
- Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, Radhika S, Amit A, Venkateshwarlu K, Deepak M. 2007. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 109:359–363.
- Vizetto-Duarte C, Pereira H, Bruno de Sousa C, Rauter AP, Albericio F, Custódio L, Barreira L, Varela J. 2015. Fatty acid profile of different species of algae of the Cystoseira genus: a nutraceutical perspective. Nat Prod Res. 2:1–7.
- Weinreb O, Mandel S, Bar-Am O, Amit T. 2011. Iron-chelating backbone coupled with monoamine oxidase inhibitory moiety as novel pluripotential therapeutic agents for Alzheimer’s disease: a tribute to Moussa Youdim. J Neural Transm. 118:479–492.
- Zou JY, Chang SKC, Gu Y, Qian SY. 2011. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J Agric Food Chem. 59:2268–2276.
- Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvrea V, Fauchon M, Deslandes E. 2009. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 116:693–701.

