Abstract
Context: Medicinal plants encompass a rich source of active compounds that can neutralize snake venoms or toxins. Costus spicatus (Jacq.) Sw. (Costaceae) is used by the Amazonian population to treat inflammation, pain and other pathological manifestations.
Objective: To evaluate the influence of C. spicatus aqueous extract on edema, peritonitis, nociception, coagulation, haemorrhage and indirect haemolytic activity induced by Bothrops atrox venom (BAV).
Materials and methods: Dried and pulverized leaves were extracted with distilled water. Envenoming was induced by administration of B. atrox snake venom in Swiss Webster mice. The experimental groups consisted of BAV (at the minimum dose to induce measurable biological responses) and C. spicatus extract (CSE, 1.25, 2.5, 5.0, 7.5 and 10 mg/kg/25 μl phosphate-buffered saline) administered individually and in combination (BAVCSE). PBS was used as a control. In vitro assays were also conducted in order to evaluate phospholipase A2 coagulant activities (indirect haemolytic method).
Results: CSE significantly reduced the venom-induced edema and nociception at all concentrations tested and inhibited migration of inflammatory cells at the three least concentrations (5.0, 7.5 and 10 mg/kg/25 μl PBS). CSE was not effective in inhibiting coagulant, haemorrhagic and indirect haemolytic activities of the venom.
Discussion and conclusion: The data suggest that CSE could exhibit a central mechanism for pain inhibition, and may also inhibit prostaglandin synthesis. These findings corroborate the traditional administration of C. spicatus decoction to treat inflammatory disorders, including those caused by B. atrox envenomation.
Keywords:
Introduction
Bothrops atrox (Viperidae), the common lancehead, is a terrestrial, generally nocturnal and highly adaptable pit viper found in tropical lowlands and rainforest (Campbell & Lamar Citation2004). Poisoning by B. atrox, as with other species, triggers local and systemic effects in victims (Borges et al. Citation1999). This species is responsible for most snakebites in the Amazon rainforest region, where difficulties in access to healthcare might increase accident severity.
Bothropic envenoming induces prominent local lesions that are caused by the combined action of venom proteases, haemorrhagic factors, phospholipases A2 (PLA2) and from the release of endogenous mediators (Rothschild & Rothschild Citation1979; Gutiérrez & Lomonte Citation1989; Trebien & Calixto Citation1989; Farsky et al. Citation1997; Carneiro et al. Citation2002; Laing et al. Citation2003; Teixeira et al. Citation2003). Adequate treatment of snakebite envenoming depends on the ability of antivenoms to reverse systemic signs, such as venom-induced coagulopathy, haemorrhage, hypotensive shock, among others (Calvete et al. Citation2009; Núñez et al. Citation2009). Animal models and clinical studies have demonstrated that local reactions are not effectively neutralized by conventional antivenom serum therapy (Avila-Agüero et al. Citation2001; Lomonte et al. Citation2009). In severe cases, local effects of envenoming may lead to permanent tissue loss, disability or amputation (Gutiérrez Citation2002). Such adverse complications can be controlled by use of anti-inflammatory remedies concomitant with antivenom to reduce local pain and inflammation.
Use of medicinal plants has been an elected practise throughout human history, whose knowledge, gathered through the experience of many generations, represents millennia of popular wisdom, since the times when the only available medicinal resources were obtained from the vegetal kingdom (Calixto et al. Citation2000). Medicinal plants still play a key role in world health as they are a rich source of many natural inhibitors and pharmacologically active compounds. Many of these substances structurally resemble biological compounds, and this similarity is the basis of their physiological action (Havsteen Citation1983). Some medicinal plants are used in the Amazon and elsewhere against snakebites or as anti-inflammatory agents, and these plants may prove valuable adjuvants to antivenom therapies (da Silva et al. Citation2005; Soares et al. Citation2005; Bittencourt et al. Citation2014). Hence, it is crucial to undertake an in-depth scientific investigation on the pharmacological and toxicological actions of these plants.
Costus spicatus Swartz (Costaceae), commonly called ‘cana-do-brejo’, ‘canafístula’ or ‘canarana’ in Brazil, is a medicinal plant found in wet coastal forests (Silva Citation2002; da Silva & Parente Citation2003). Ethnopharmacological surveys have established that C. spicatus leaf and stem decoctions as well as aqueous or alcoholic infusions are commonly used for the treatment of renal calculi, cutaneous ulcers, infections, inflammation, urethritis, gonorrhoea and leucorrhoea (Carriconde et al. Citation1996; Araújo Viel et al. Citation1999; da Silva et al. Citation2000; Silva Citation2002). Studies have reported the isolation and chemical characterization of three neutral polysaccharides with anti-inflammatory and immunomodulatory activities (da Silva & Parente Citation2003). Other work has demonstrated that methanol extract obtained from the leaves possessed analgesic and anti-inflammatory properties (Quintans Júnior et al. Citation2010). Owing to the presumed anti-inflammatory potential of C. spicatus aqueous extract, we evaluated its ability to reduce edema, peritonitis, nociception, coagulation, haemorrhage and indirect haemolytic activity induced by B. atrox venom (BAV).
Materials and methods
Venom
BAV pool was obtained from four adult specimens captured at Laranjal do Jari, Amapa State, Brazil (0°38′ 12.42″ S, 52°30′ 15.87″ O). The sampling location was marked by a global position measuring (GPS Garmin – modelo nüvi 40). Venom was collected after anesthetizing the snakes with carbon dioxide gas. The venom samples were lyophilized and kept at −20 °C in the freezer until further use. The venom was diluted in phosphate-buffered saline (PBS) immediately prior to its use.
Plant material
Costus spicatus leaves were collected from Macapá in Amapá State, Brazil, in the month of February 2012. The sampling location (00°2'41.821″ S, 51°5′57.253″ W) was deduced by a global position measuring (GPS Garmin Nüvi 40). The plant was identified by Prof. Wegliane Campelo da Silva Aparício, a taxonomist at the Department of Biology, Federal University of Amapá, Macapa, AP, Brazil. A voucher specimen (N° 460) was deposited at the Herbarium of the Federal University of Amapá for future reference.
Preparation of plant extract
Dry and pulverized leaves were extracted with distilled water for 24 h at room temperature. Insoluble material was removed by filtration. The aqueous extract (CSE) was lyophilized and kept at –20 °C in a freezer until further use. Prior to use, the lyophilized extracts were weighed and dissolved in PBS.
Animals
Animal care was performed in accordance with the guidelines of the Brazilian College for Animal Experimentation. Male Swiss Webster mice weighing 20–25 g were used for the experiments and were randomly divided into groups of five animals. The mice were kept in plastic cages with access to water and food ad libitum and were maintained under controlled temperature (18–20 °C) on a 12 h light/dark cycle.
Groups and experimental protocols
The experimental groups were BAV alone, C. spicatus extract alone (CSE), BAV + CSE in various concentrations (BAVCSE) or PBS alone. The venom doses used were selected from previous dose-response experiments. This initial study evaluated different dosages on the ability to induce measurable biological responses. The appropriate doses are specified in each bioassay description below.
Paw edema induction
The minimum dose was defined as the lowest venom dose required for the formation of 30% paw edema (Rocha & Furtado Citation2007). This minimum dose was evaluated as 0.20 mg/kg by subplantar injection of venom, in the right footpad of mice. Inhibition studies were performed with CSE at several concentrations (1.25; 2.5; 5.0; 7.5 and 10 mg CSE/kg). CSE and venom diluted in 50 μl PBS were injected together. Control animals received PBS (50 μl), venom (0.20 mg/kg/50 μl PBS) or CSE only (10 mg/kg/50 μl PBS). The progression of edema was evaluated with a low-pressure pachymeter (Mitutoyo America Corporation, Kanagawa, Japan) 0, 0.5, 1, 2, 3 and 4 h after injection and was expressed in mm of directly induced edema.
Formalin test
The method used was based on the protocol previously described by Hunskaar and Hole (Citation1987) with modifications recommended by Soares et al. (Citation2009) and De Sousa et al. (Citation2012). As a further modification, formalin used for pain induction was substituted by BAV at 0.20 mg/kg. Symptoms of pain such as intense licking and biting of the paw was recorded in two phases. The first period (first phase) was recorded between 0 and 5 min and the second period (second phase) between 20 and 30 min after the injection. The time (in seconds) spent licking and biting the injected paw was taken as an indicator of pain intensity. The test was performed at ambient temperature (22–26 °C) and care was taken to exclude environmental disturbances (high temperature, noise and excessive movement) that might influence animal behaviour. The animals were examined by the same observer who was responsible for all tests. Due to the number of animals, tests were performed during two consecutive days. CSE nociception inhibition was evaluated at five doses (1.25; 2.5; 5.0; 7.5 and 10 mg/kg). Accordingly, CSE and BAV (0.20 mg/kg) were diluted in PBS (50 μl) and each preparation was injected subcutaneously into the right hind paw of five mice. Control animals received PBS (50 μl), venom (0.20 mg/kg/50 μl PBS) or CSE only (10 mg/kg/50 μl PBS). Mice were then placed under glass funnel individually with mirrors around them to facilitate observation.
Peritonitis induced by B. atrox venom
Peritonitis assays were performed as previously described by Souza and Ferreira (Citation1985) and Souza (Citation2006). The optimal venom dose required to induce cellular migration without causing significant local haemorrhage was 0.20 mg/kg. In the inhibition assays, mixtures of BAV (0.20 mg/kg) and CSE (1.25; 2.5; 5.0; 7.5 and 10 mg/kg) in PBS (50 μl) were administered intraperitoneally. Control animals received PBS (50 μl), venom (0.20 mg/kg/50 μl PBS) or CSE (10 mg/kg/50 μl PBS) by intraperitoneal route. After 4 h, the animals were euthanized in a CO2 chamber and the peritoneal exudates were collected with a plastic Pasteur pipette by abdominal laparoscopy. To facilitate the collection, all the animals received an injection of 2.0 ml of heparinized PBS (1/1000 ml de PBS), and their abdomens were massaged to recover all leukocytes present. The peritoneal wash samples were diluted in Türk’s solution (1:20), and the cells were counted in a Neubauer chamber. The results were expressed as the total number of cells per peritoneal cavity.
Coagulant activity
The minimum coagulant dose, defined as the amount of venom that causes clotting of 200 μl human plasma in 60 s was 20 μg (Theakston & Reid Citation1983). For the inhibition tests, several doses of CSE (26; 52; 104; 208; 416 μg) and BAV (20 μg) diluted in PBS (50 μl) were added immediately to citrated human plasma (200 μl) maintained at 37 °C. Clotting times were recorded. In control assays, PBS (50 μl), BAV (20 μg/50 μl PBS) and CSE (416 μg/50 μl PBS) were added to citrated human plasma.
Haemorrhagic activity
Haemorrhage was induced by intradermal injections performed on the back of mice. After 2 h, the animals were euthanized in a CO2 chamber. The skin near the injection site was removed and haemorrhagic halo formed was measured in millimetres (mm) according to the method of Kondo et al. (Citation1960). The diameters of the haemorrhage spot where the average of the longest diameter of the spot and the diameter perpendicular to the first measurement. The dose of venom inducing a 10 mm haemorrhagic spot was 0.20 mg/kg. In the inhibition assays, mixtures of BAV (0.20 mg/kg) and CSE at five concentrations (1.25; 2.5; 5.0; 7.5 and 10 mg/kg) were mixed in PBS (50 μl) and were injected intradermally. Control animals received PBS (50 μl), venom (0.20 mg/kg/50 μl PBS) or CSE (10 mg/kg/50 μl PBS).
Indirect haemolytic method
PLA2 activity was determined by an indirect haemolytic method using agarose, Tris (20 mm), CaCl2 and egg yolk gels as substrate (Gutiérrez et al. Citation1988). The amount of BAV that produced a 10 mm halo was 20 μg. Mixtures of BAV (20 μg) and CSE (50 μl) at five concentrations (26; 52; 104; 208 or 416 μg/25 μl PBS) were applied at the centre of the dish. After incubation at 37 °C for 12 h, the PLA2 activity was evaluated measuring the diameter of the translucent halo formed. PBS (50 μl), venom (20 μg/50 μl PBS) or CSE (416 μg/50 μl PBS) were used as controls. Measurements were recorded in triplicates.
Statistical analysis
The results are presented as the mean ± SEM. Differences among groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey–Kramer test. The differences were considered to be significant when the associated probability of a null hypothesis (p values) were less than 5% (p < 0.05).
Results
Application of venom-induced edema and the size of the edema measuring the effect of CSE at time 0; 0.5; 1; 2; 3 and 4 h after injection is reported in . CSE significantly reduced edema at all concentrations tested (1.25; 2.5; 5.0; 7.5 and 10 mg/kg/25 μl PBS) when compared with BAV alone. In the nociception assay, the time spent licking and biting over fixed periods of time after subcutaneous injection of 0.20 mg/kg BAV into the right hind paw was recorded as an indicator of pain. In this assay, CSE demonstrated the analgesic effect on the first (0–5 min) and second phases (20–30 min) of BAV-induced pain (. These phases correspond to neurogenic and inflammatory pain, respectively. In the first phase (), CSE significantly reduced neurogenic pain at all concentrations when compared with the BAV group. The analgesic effect was stronger at higher concentration of CSE. In the second phase (), all doses of CSE significantly neutralized inflammatory pain compared with the animals treated with BAV alone. However, in this phase, lower concentrations of CSE exhibited slightly higher analgesic potential.
Figure 1. Effect of CSE on the first (panel A) and the second phase (panel B) of BAV-induced nociception in mice. BAVCSE 1:6: BAV + 1.25 mg CSE/kg/50 μl PBS; BAVCSE 1:12: BAV + 2.5 mg CSE/kg/50 μl PBS; BAVCSE 1:25: BAV + 5.0 mg CSE/kg/50 μl PBS; BAVCSE 1:37: BAV + 7.5 mg CSE/kg/50 μl PBS; BAVCSE 1:50: BAV + 10 mg CSE/kg/50 μl PBS. The results are presented as mean ± SEM for five animals. Differences between BAVCSE groups and BAV group were analyzed by one-way analysis of variance (ANOVA), followed by the Tukey–Kramer test. Differences with an associated probability (p values) of less than 5% (p < 0.05) were considered significant. *p < 0.05; **p < 0.01; ***p < 0.001.
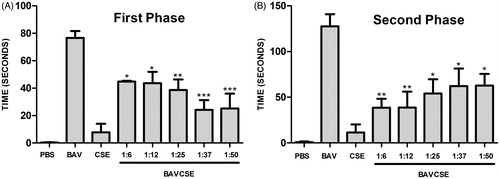
Table 1. Effect of CSE on the edema induced by B. atrox venom.
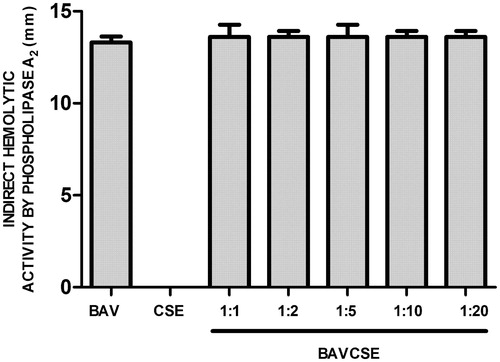
The influx of leukocytes was analyzed by counting the cells in the peritoneal wash (. CSE significantly decreased the number of leukocytes at the three lowest concentrations, which indicated an effect of CSE on the migration of inflammatory cells induced by venom administration. In vitro studies carried out with human plasma demonstrated that CSE concentrations used in the present study did not inhibit venom coagulant activity (. CSE effect on haemorrhagic activity induced by BAV was also tested. As observed in , CSE did not significantly inhibit BAV-induced haemorrhage. Lastly, it was also demonstrated that CSE did not inhibit the PLA2 activity of BAV, as shown in .
Figure 2. Peritonitis induced by B. atrox venom and treated with CSE. BAVCSE 1:6: BAV + 1.25 mg CSE/kg/50 μl PBS; BAVCSE 1:12: BAV + 2.5 mg CSE/kg/50 μl PBS; BAVCSE 1:25: BAV + 5.0 mg CSE/kg/50 μl PBS; BAVCSE 1:37: BAV + 7.5 mg CSE/kg/50 μl PBS; BAVCSE 1:50: BAV + 10 mg CSE/kg/50 μl PBS. The results are presented as the mean ± SEM for five animals. Differences between BAVCSE groups and BAV group were analyzed by one-way analysis of variance (ANOVA), followed by Tukey–Kramer test. Differences with an associated probability (p values) of less than 5% (p < 0.05) were considered significant. *p < 0.05; **p < 0.01.
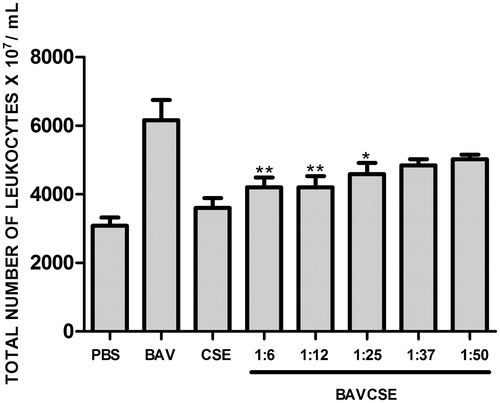
Figure 3. CSE effect on B. atrox venom coagulant activity. BAV: 20 μg/50 μl PBS. BAVCSE 1:1: BAV + 26 μg CSE/50 μl PBS; BAVCSE 1:2: BAV + 52 μg CSE/50 μl PBS; BAVCSE 1:5: BAV + 104 μg CSE/50 μl PBS; BAVCSE 1:10: BAV + 208 μg CSE/50 μl PBS; BAVCSE 1:20: BAV + 416 μg CSE/50 μl PBS. Each experiment was carried out in triplicate. Differences between BAVCSE groups and BAV group were analyzed by one-way analysis of variance (ANOVA), followed by Tukey–Kramer test. Results did not vary significantly as compared with BAV (p > 0.05).
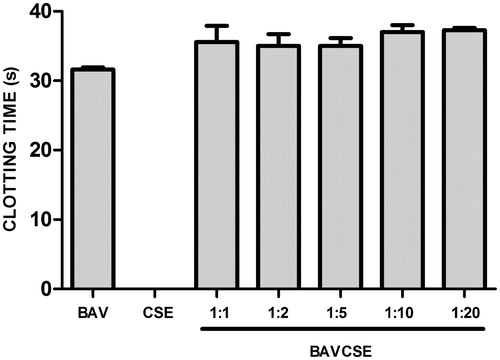
Figure 4. Effect of CSE on haemorrhage induced by B. atrox venom. BAV: 0.40 mg/50 μl PBS. BAVCSE 1:16: BAV + 3.2 mg CSE/kg/50 μl PBS; BAVCSE 1:32: BAV + 6.4 mg CSE/kg/50 μl PBS; BAVCSE 1:64: BAV + 12.8 mg CSE/kg/50 μl PBS; BAVCSE 1:96: BAV + 19.2 mg CSE/kg/50 μl PBS; BAVCSE 1:128: BAV + 25.6 mg CSE/kg/50 μl PBS. The results are presented as the mean ± SEM of five animals. Differences between BAVCSE groups and BAV group were analyzed by one-way analysis of variance (ANOVA), followed by Tukey–Kramer test. Results in BAVCSE experiments did not vary significantly as compared with BAV (p > 0.05).
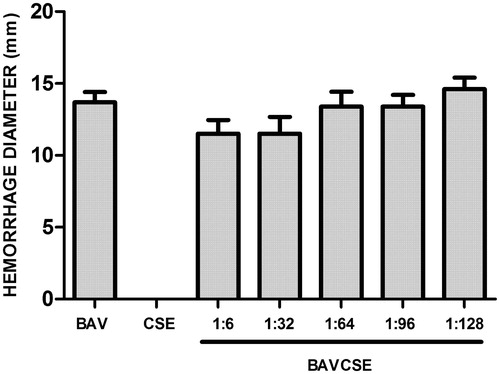
Figure 5. Effects of CSE on phospholipase A2 activity of B. atrox venom. BAV: 20 μg/50 μl PBS. BAVCSE 1:1: BAV + 26 μg CSE/50 μl PBS; BAVCSE 1:2: BAV + 52 μg CSE/50 μl PBS; BAVCSE 1:5: BAV + 104 μg CSE/50 μl PBS; BAVCSE 1:10: BAV + 208 μg BGE/50 μl PBS; BAVCSE 1:20: BAV + 416 μg CSE/50 μl PBS. The results are presented as the mean ± SEM of five animals. Differences between BAVCSE groups and BAV group were analyzed by one-way analysis of variance (ANOVA), followed by Tukey–Kramer test. Results in BAVCSE experiments did not vary significantly as compared with BAV (p > 0.05).
Discussion
The main clinical effects of Bothrops envenoming are local tissue damage (myonecrosis, haemorrhage and edema), life-threatening bleeding originating from blood coagulation disorders and shock (Gutiérrez Citation1995; Warrell Citation2004). In the present study, BAV was used to induce edema, peritonitis, nociception, coagulation, haemorrhage and indirect haemolytic activity, and we evaluated whether or not the aqueous extract obtained from the leaves of C. spicatus Swartz could quench the effects of the venom. Venom toxicity is due to its proteins content. Those are mainly metalloproteinases, lectins, serino-proteinases, bradykinin-potentiating peptides and PLA2 (Guércio et al. Citation2006; Neiva et al. Citation2009).
Quintans Júnior et al. (Citation2010) evaluated the antinociceptive and anti-inflammatory effects of the methanol extract obtained from the leaves of C. spicatus Swartz (MECs) on acetic acid-induced writhing, formalin, hot-plate and carrageenan-induced edema tests in rodents. Our studies in mice showed that BAV-induced paw edema immediately after administration and that the edema gradually decreased >4 h. This envenomation kinetics corroborates those from previous studies that demonstrated the ability of Bothrops venom to induce edema through the action of venom constituents on vascular endothelial cells and inflammatory mediators (Barbosa et al. Citation2008). Interestingly, we found that CSE diminished the edematogenic effect of BAV at all tested doses and that the effect lasted for 4 h. CSE also produced a significant inhibitory effect on the migration of inflammatory cells after the venom had been injected. The effect was stronger at the lowest concentrations tested. Many studies suggest that presence of glucan polysaccharides may act on the reticuloendothelial system, through phagocytosis stimulation, and can also induce vascular reactions, affecting the capillary permeability of the vessels (Whistler et al. Citation1976). Interestingly, glucans have been isolated from C. spicatus by da Silva and Parente (Citation2003). Quintans Júnior et al. (Citation2010) reported that the first phase of the edematous response was significantly lower in the presence of C. spicatus methanol extract (MECs) in rats, suggesting an inhibitory effect on the release of histamine and/or serotonin. MECs also inhibited significantly the second and third phases of the edema, suggesting inhibition of 5-lipoxygenase and/or cyclooxygenase, the enzymes involved in the formation of prostaglandins and leukotrienes.
The first experimental evidence that Bothrops venoms induce hyperalgesia (pain hypersensitivity) was provided by Teixeira et al. (Citation1994). In this study, the authors induced a long-lasting hyperalgesia mediated mainly by prostaglandin, leukotriens and PAF using B. jararaca venom. Chacur et al. (Citation2001) demonstrated that bradykin is involved in hyperalgesia induced by B. jararaca venom, which peaked 1 h after venom injection.
The pain phenomenon takes its origin from peripheral sensory nerve fibres through activation of nociceptors that relay noxious stimuli from the periphery to the central nervous system (Tortora & Grabowski Citation2002; Cummins et al. Citation2007). The results of present study showed that BAV produced a significant nociceptive effect both at 0–5 min and 20–30 min. The early phase may be due to direct effects on nociceptors, demonstrating that BAV causes an immediate sensation of pain and an increased responsiveness of neurons. However, more studies are needed to further investigate components and mechanism involved in the nociceptive action of the snake venoms. CSE exhibited antinociceptive activity in the first phase, probably by central action mechanism. Reports show that flavonoid-rich plant extracts can exhibit antinociceptive properties due to possible interactions of the flavonoids with the opioid system (Ghannadi et al. Citation2005; Maleki-Dizaji et al. Citation2007; Quintans Júnior et al. Citation2010).
In the late phase, BAV nociceptive effect can be due to an inflammatory response. Inflammation induced by Bothrops venom is characterized by a complex network of chemical mediators and the cellular components responsible for such effects. Besides the role of stored and newly generated inflammatory mediators, enzymes present in the venom, mainly PLA2 and metalloproteinases, appear to play a role in inflammation and hyperalgesia induced by B. asper venom (Teixeira et al. Citation2009). Our experiments showed that CSE exhibited antinociceptive activity also in the second phase as well. da Silva et al. (Citation2000) isolated flavonol diglycosides tamarixetin 3-O-neohesperidoside, kaempferide 3-O-neohesperidoside and the known quercetin 3-O-neohesperidoside together with six known flavonoids from C. spicatus leaves. Tamarixetin 3-O-neohesperidoside and kaempferide 3-O-neohesperidoside showed slight inhibitory activity on nitric oxide production by activated macrophages. This information suggests that CES may act on inflammatory disorders, probably by a peripheral mechanism. These findings corroborate the traditional analgesic and anti-inflammatory indication of the leaf infusion. On the other hand, our work demonstrated that CSE was not effective to inhibit coagulant, haemorrhagic and indirect haemolytic activities induced by BAV.
Conclusion
This study demonstrates that C. spicatus leaf aqueous extract has a significant inhibitory effect on edema and migration of inflammatory cells, mainly at the lowest concentrations tested. Moreover, CSE significantly reduced BAV-induced nociception in the first and second phases. CSE presumably acts through a central inhibitory mechanism while inhibiting prostaglandin synthesis as well. The present study contributes to validate the traditional use of C. spicatus leaf infusion against inflammatory disorders including those caused by snake envenomation and accentuates the importance of popular knowledge in the search for alternative treatments to snakebite.
Funding information
The authors wish to thank the Amapa State Research Foundation (FAPEAP) and the National Council for Scientific and Technological Development (CNPq) for financial support. This work has also benefited from an ‘Investissement d’Avenir’ grant managed by the Agence Nationale de la Recherche (CEBA, ref ANR-10-LABX-0025).
Acknowledgements
The authors are grateful to the Instituto Evandro Chagas, the Central de Armazenamento e Distribuição de Imunobiológicos do Amapá, the Instituto de Pesquisas Científicas e Tecnológicas do Amapá, the Neotrópica Tecnologia Ambiental LTDA, Dra. Camila Moreira Barreto Gomes, and Dra. Wegliane Campelo da Silva Aparício for their contributions.
Disclosure statement
The authors report no declarations of interest.
References
- Araújo VT, Domingos CD, Monteiro APS, et al. 1999. Evaluation of the antiurolithiatic activity of the extract of Costus spiralis Roscoe in rats. J Ethnopharmacol. 66:193–198.
- Ávila-Agüero ML, París MM, Hu S, et al. 2001. Systemic cytokine response in children bitten by snakes in Costa Rica. Pediatr Emerg Care. 17:425–429.
- Barbosa AM, Villaverde AB, Guimarães-Souza L, et al. 2008. Effect of low-level laser therapy in the inflammatory response induced by Bothrops jararacussu snake venom. Toxicon. 51:1236–1244.
- Bittencourt JAH, Oliveira NKS, Cabral MS, et al. 2014. Antiophidian activity of Brosimum guianense (Aubl) Huber. Am J Pharmacol Toxicol. 9:148–156.
- Borges CC, Sadahiro M, Dos-Santos MC. 1999. Aspectos epidemiológicos e clínicos dos acidentes ocorridos nos municípios do Estado do Amazonas. Rev Soc Bras Med Trop. 32:637–646.
- Calixto JB, Beirith A, Ferreira J, et al. 2000. Naturally occurring antinociceptive substances from plants. Phytother Res. 14:401–418.
- Calvete JJ, Sanz L, Angulo Y, et al. 2009. Venoms, venomics, antivenomics. FEBS Lett. 583:1736–1743.
- Campbell JA, Lamar WW. 2004. The venomous reptiles of the western hemisphere. Ithaca (NY): Comstock Publishing Associates. Cornell University Press.
- Carneiro AS, Ribeiro OG, De Franco M, et al. 2002. Local inflammatory reaction induced by Bothrops jararaca venom differs in mice selected for acute inflammatory response. Toxicon. 40:1571–1579.
- Carriconde C, Morais D, Von Fritschen M, et al. (1996). Plantas medicinais e alimentícias. Olinda, Brazil: Centro Nordestino de Medicina Popular. Universidade Federal Rural de Pernambuco.
- Chacur M, Picolo G, Gutiérrez JM, et al. 2001. Pharmacological modulation of hyperalgesia induced by Bothrops asper (terciopelo) snake venom. Toxicon. 39:1173–1181.
- Cummins TR, Sheets PL, Waxman SG. 2007. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain 131:243–257.
- da Silva BP, Bernardo RR, Parente JP. 2000. Flavonol glycosides from Costus spicatus. Phytochemistry 53:87–92.
- da Silva BP, Parente JP. 2003. Bioactive polysaccharides from Costus spicatus. Carbohydr Polym. 51:239–242.
- da Silva JO, Coppede JS, Fernandes VC, et al. 2005. Antihemorrhagic, anti-nucleolytic and other antiophidian properties of the aqueous extract from Pentaclethra macroloba. J Ethnopharmacol. 100:145–152.
- De Sousa EA, Bittencourt JAM, De Oliveira NKS, et al. 2012. Influence of a low-level semiconductor gallium arsenate laser in experimental envenomation induced by Bothrops atrox snake venom. Am J Pharmacol Toxicol. 7:141–148.
- Farsky SH, Walber J, Costa-Cruz M, Cury Y, et al. 1997. Leukocyte response induced by Bothrops jararaca crude venom: in vivo and in vitro studies. Toxicon. 35:185–193.
- Ghannadi A, Hajhashemi V, Jafarabadi H. 2005. An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food. 8:488–493.
- Guércio RA, Shevchenko A, Shevchenko A, et al. 2006. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. 4:11.
- Gutiérrez JM, Avila C, Rojas E, Cerdas L. 1988. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 26:411–413.
- Gutiérrez JM, Lomonte B. 1989. Local tissue damage induced by Bothrops snake venoms. A review. Mem Inst Butantan. 51:211–223.
- Gutiérrez JM. 1995. Clinical toxicology of snake bite in Central America. In: Meier J, White J, editors. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton (FL): CRC Press. p. 645–665.
- Gutiérrez JM. 2002. Comprendiendo los venenos de serpientes: 50 anos de investigaciones em América Latina – understanding snake venoms: 50 years of research in Latin America. Rev Biol Trop. 50:377–394.
- Havsteen B. 1983. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 32:1141–1148.
- Hunskaar S, Hole K. 1987. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30:103–104.
- Kondo H, Kondo S, Ikezawa H, Murata R, Ohsaka A. 1960. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol. 13:43–51.
- Laing GD, Clissa PB, Theakston RDG, Moura-da-Silva AM, Taylor MJ. 2003. Inflammatory pathogenesis of snake venom metalloproteinase induced skin necrosis. Eur J Immunol. 33:3458–3463.
- Lomonte B, León G, Angulo Y, Rucavado A, Núñez V. 2009. Neutralization of Bothrops asper venom by antibodies, natural products and synthetic drugs: contributions to understanding snakebite envenomings and their treatment. Toxicon. 54:1012–1028.
- Maleki-Dizaji N, Fathiazad F, Garjani A. 2007. Antinociceptive properties of extracts and two flavonoids isolated from leaves of Danae racemosa. Arch Pharm Res. 30:1536–1542.
- Neiva M, Arraes FB, de Souza JV, Rádis-Baptista G, Prieto da Silva ARB, Walter MEMT, Brigido MM, Yamane T, Lopez-Lozano JL, Astolfi-Filho S. 2009. Transcriptome analysis of the Amazonian viper Bothrops atrox venom gland using expressed sequence tags (ESTs). Toxicon. 53:427–436.
- Núñez V, Cid P, Sanz L, De La Torre P, Angulod Y, Lomonte B, Gutiérrez JM, Calvete JJ. 2009. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J Proteomics. 73:57–78.
- Quintans Júnior LJ, Santana MT, Melo MS, Sousa DP, Santos IS, Siqueira RS, Lima TC, Silveira GO, Antoniolli AR, Ribeiro LAA, Santos MRV. 2010. Antinociceptive and anti-inflammatory effects of Costus spicatus in experimental animals. Pharm Biol. 48:1097–1102.
- Rocha MMT, Furtado MFD. 2007. Análise das atividades biológicas dos venenos de Philodryas olfersii (Lichtenstein) e Philodryas patagoniensis (Girard) (Serpentes Colubridae). Rev Bras Zool. 24:410–418.
- Rothschild AM, Rothschild Z. 1979. Liberation of pharmacologically active substances by snake venoms. In: Lee CY, editor. Handbook of experimental pharmacology. Vol. 52. Berlin: Springer. p. 591–628.
- Silva RBL. 2002. A etnobotânica de plantas medicinais da comunidade quilombola de Curiaú, Macapá-AP, Brasil. Belém, Pará, Brasil: Universidade Federal Rural da Amazonia.
- Soares AM, Ticli FK, Marcussi S, Lourenço MV, Januário AH, Sampaio SV, Giglio JR, Lomonte B, Pereira PS. 2005. Medicinal plants with inhibitory properties against snake venoms. Curr Med Chem. 12:2625–2641.
- Soares CC, Marques TM, Rigolin GG, Neis E, Friaça AMV, Silva AS. 2009. Atividade analgésica do extrato da Pectis jangadensis (S. Moore). Braz J Pharmacogn. 19:77–81.
- Souza GE, Ferreira SH. 1985. Blockade by antimacrophage serum of the migration of PMN neutrophilis into the inflamed peritoneal cavity. Agents Actions. 17:97–103.
- Souza SMC. 2006. Efeito do extrato hidroalcóolico de Elipta prostata em modelo de inflamação in vivo, induzido pelo veneno de serpente Bothrops moojeni. São José dos Campos, SP, Brazil: Universidade do Vale do Paraíba.
- Teixeira C, Cury Y, Moreira V, Picolo G, Chaves F. 2009. Inflammation induced by Bothrops asper venom. Toxicon. 54:988–997.
- Teixeira CF, Cury Y, Oga S, Jancar S. 1994. Hyperalgesia induced by Bothrops jararaca venom in rats: role of eicosanoids and platelet activating factor (PAF). Toxicon. 32:419–426.
- Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. 2003. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 42:947–962.
- Theakston RD, Reid HA. 1983. Development of simple standard assay procedures for the characterization of snake venoms. Bull World Health Organ. 61:949–956.
- Tortora GJ, Grabowski SR. 2002. Principles of anatomy and physiology. 10th ed. Hoboken (NJ): John Wiley.
- Trebien HA, Calixto JB. 1989. Pharmacological evaluation of rat paw edema induced by Bothrops jararaca venom. Agents Actions. 26:293–299.
- Warrel DA. 2004. Snakebites in Central and South America: epidemiology, clinical features and clinical management. In: Campbell JA, Lamar WW, editors. The venomous reptiles of the Western hemisphere. Ithaca (NY): Comstock Publishing Associates.
- Whistler RL, Bushway AA, Singh PP. 1976. Noncytotoxic, antitumor polysaccharides. Adv Carbohydr Chem Biochem. 32:235–275.

