Abstract
Context: Lichens produce specific secondary metabolites with different biological activity.
Objective: This study investigated the cytotoxic effects of physodic acid, in addition to the total phenolic content and cytotoxic and antioxidant activity of acetone extract from Hypogymnia physodes (L.) Nyl. (Parmeliaceae).
Materials and methods: Cytotoxicity of physodic acid (0.1–100 μM) was assessed in MDA-MB-231, MCF-7 and T-47D breast cancer cell lines and a nontumorigenic MCF-10A cell line using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, neutral red uptake and crystal violet assays during 72 h of incubation. An MTT assay was also used to assess the cytotoxic effects of the acetone extract (0.1–100 μg/mL) in the MDA-MB-231, MCF-7, T-47D breast cancer cell lines after 72 h. The total phenolic content of the acetone extract, expressed as the gallic acid equivalent, was investigated using Folin-Ciocalteu reagent. The antioxidant activity of the extract was assessed by 2,2-diphenyl-1-picrylhydrazyl and ferric-reducing antioxidant power assays.
Results: The cytotoxic activity of physodic acid appeared to be strong in the tumorigenic cell lines (IC50 46.0–93.9 μM). The compound was inactive against the nontumorigenic MCF-10A cell line (IC50 >100 μM). The acetone extract showed cytotoxicity in the breast cancer cell lines (IC50 46.2–110.4 μg/mL). The acetone extract was characterized by a high content of polyphenols, and it had significant antioxidant activity.
Discussion and conclusion: Physodic acid and acetone extract from H. physodes displayed cytotoxic effects in the breast cancer cell lines. Furthermore, acetone extract from H. physodes possessed significant antioxidant properties.
Introduction
Lichens (or lichenized fungi) consist of a fungus and a photosynthetic symbiont, which can be an alga or a cyanobacterium (Zambare & Christopher Citation2012). These organisms produce unprecedented secondary metabolites, which are extremely rare in the world of plants and nonlichenized fungi. These secondary metabolites include depsides, depsidones, quinones and xanthones, as well as derivatives of dibenzofuran, diketopiperazine and pulvinic acid (Huneck Citation1999; Müller Citation2001). Approximately 1050 secondary lichen metabolites have been identified to date (Molnár & Farkas Citation2010). Lichen-derived secondary metabolites have been shown to exert diverse biological activity, such as antiproliferative, antitumor, antioxidant, anti-inflammatory, antipyretic, analgesic, antiviral, antiprotozoal and photoprotective (Huneck Citation1999; Müller Citation2001).
Hypogymnia physodes (L.) Nyl. (Parmeliaceae) is a foliose lichen, which is widely distributed in the boreal and temperate forests of the Northern Hemisphere. H. physodes produces secondary metabolites with various chemical structures. Previous research has demonstrated the presence of depsides (atranorin and chloratranorin) and depsidones (protocetraric, physodic, isophysodic, hydroxyphysodic, methylphysodic and physodalic acids) in H. physodes (Molnár & Farkas Citation2010; Pavlović et al. Citation2013). Knowledge of the biological activity of physodic acid and its derivatives is limited. The antimicrobial activity of physodic acid, 3-hydroxyphysodic acid and extracts from H. physodes (Gollapudi et al. Citation1994; Türk et al. Citation2003, Citation2006; Yilmaz et al. Citation2005; Ranković et al. Citation2007), as well as the antimutagenic activity of physodic and physodalic acids (Shibamoto & Wei Citation1984; Osawa et al. Citation1991), has been reported. Studies also demonstrated that physodic (Neamati et al. Citation1997; Müller Citation2001) and hydroxyphysodic acids inhibited different enzymes (Proksa et al. Citation1994).
In the present study, physodic acid was isolated from H. physodes, and its cytotoxic effects were investigated in MCF-7, T47D and MDA-MB-231 breast cancer cell lines, as well as their normal counterparts, nontumorigenic epithelial cell line, MCF-10A. Furthermore, the cytotoxic and antioxidant activity of the acetone extract and its polyphenol content was evaluated.
Materials and methods
Chemicals
Acetone, anhydrous citric acid, ethyl acetate, formic acid, hexane, hydrochloric acid and toluene were purchased from POCH (Gliwice, Poland). Amphotericin B, ascorbic acid, 3-tert-butyl-4-hydroxyanisole (BHA), crystal violet (CV), Dulbecco’s modified Eagle’s medium (DMEM), dimethylsulphoxide (DMSO), 2,2-diphenyl-1-picrylhydrazyl (DPPH), epidermal growth factor (EGF), foetal bovine serum (FBS), gallic acid, glutamine, horse serum, hydrocortisone, insulin, iron(III) chloride hexahydrate (FeCl3·6H2O), TPTZ (2,4,6-tripyridyl-s-triazine), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 3-amino-7-dimethylamino-2-methylphenazine hydrochloride (NR), phosphate buffered saline (PBS) containing calcium, magnesium, penicillin and streptomycin, sodium dodecyl sulphate (SDS) and trypsin-EDTA solution, were obtained from Sigma-Aldrich Co. (St Louis, MO).
Silica gel (Sigma-Aldrich) 15–40 μm, with an average pore diameter of 60 Å, was used for column chromatography. Silica gel 60 F254 plates (Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC) to monitor the obtained fractions, and the resulting spots were visualized under UV light and also in VIS after spraying the plates with anisaldehyde-H2SO4 reagent and heating at 105 °C/10 min. Extraction was done in an ultrasonic bath (Elmasonic S 180H, Elma, Germany). 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively, using a Bruker AVANCE 400 NMR spectrometer (Rheinstetten, Germany). For the MS analysis, a Bruker microTOF-q (Bremer, Germany) mass spectrometer was used. For UV spectra registration, the UV/VIS Lambda 35 PerkinElmer (Waltham, MA) spectrophotometer was employed. For cell viability assays, the UV absorbance was measured using a spectrophotometric plate reader ELx-800 (BioTek, San Francisco, CA).
Plant material
The lichen H. physodes was collected in October 2008 in the Notec Primeval Forest, Poland and authenticated by Dr. Daria Zarabska-Bożejewicz (The Institute for Agricultural and Forest Environment of the Polish Academy of Sciences in Poznan). A voucher specimen (ES 2008.1001) has been deposited in the herbarium of the Department of Pharmacognosy, Poznan University of Medical Sciences.
Preparation of the acetone extract
Dried, cleaned and fragmented thalli of H. physodes (4.0 g) were sonicated at 35 °C for 4 × 30 min with acetone (100 mL × 4) in an ultrasonic bath. The extracts were filtered using Whatman filter paper No. 1 and concentrated by evaporation using a rotary evaporator under vacuum at 35–40 °C to afford a solid residue (439.2 mg). The yield of the acetone extract was 10.98%.
Isolation of physodic acid
Dried, cleaned and fragmented thalli of H. physodes (2.3 g) were successively extracted for 5 × 1 h with hexane (200 mL × 5) and then for 3 × 1 h with acetone (160 mL × 3) by shaking at room temperature. These extracts were filtered using Whatman filter paper. No. 1 and concentrated under reduced pressure at 35 °C, yielding 50.0 mg (yield 2.17%) and 798.0 mg (yield 34.70%) of solid residue of hexane and acetone extract, respectively. Subsequently, the acetone extract (350 mg) was chromatographed on a glass column of silica gel (2.5 × 50 cm) with the gradient of a solvent system of hexane–AcOEt (75:25), toluene–hexane (75:35, 90:30) and toluene–AcOEt (75:30) to yield 107 fractions. Fractions 24–29 were crystallized as physodic acid.
Determination of the total phenolic content
The total phenolic content in the lichen acetone extract was determined spectrophotometrically using Dóka and Bicanic’s (Citation2002) modified method, with Folin-Ciocalteu reagent. Briefly, 0.1 mL of the acetone extract dissolved in DMSO (2 mg/mL) was mixed with 7.4 mL of distilled water and 0.5 mL of the Folin-Ciocalteu reagent. The mixture was shaken vigorously, and a 20% (w/v) sodium dicarbonate solution (2 mL) was added after 1 min. Next, the samples were incubated for 30 min at room temperature. The absorbance was measured at 760 nm. The total polyphenol concentration was calculated using gallic acid (0.02–0.08 mg/mL) as a standard from a calibration curve (y = 0.1016x − 0.0029, R2 = 0.9993). The concentration of the total phenolic compounds in the extract was expressed as mg of gallic acid equivalent per gram of the dry extract. The values are expressed as the mean of 12 replications ± SD.
Cells and culture conditions
Human breast cancer cell lines (MCF-7, T47D and MDA-MB-231), and a nontumorigenic epithelial cell line (MCF-10A), were purchased from the European Type Culture Collection (Sigma-Aldrich Co.). The cells were grown at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. The cancer cell lines were maintained in phenol red-free DMEM medium, supplemented with 10% FBS, penicillin (100 U/mL), streptomycin (0.1 mg/mL) and 2 mM glutamine. The MCF-10A cells were cultured in DMEM, supplemented with 0.1% insulin solution, 0.02% EGF solution, 0.05% hydrocortisone solution, 5% horse serum and 1% antibiotics solution (104 U penicillin, 10 mg streptomycin, 25 μg amphotericin B). All the cell lines were routinely maintained in T75 flasks in a 37 °C humidified environment of 5% CO2/95% air, and they were passaged twice a week using 0.05% trypsin/0.02% EDTA.
Determination of cell viability
The cell viability was measured using assays: MTT (Berridge et al. Citation2005), neutral red uptake (NRU) (Fotakis & Timbrell Citation2006) and CV (Chiba et al. Citation1998). Briefly, the cells were trypsinized, seeded into 96-well microtiter plates at a density of 20,000 cells/well, and allowed to attach overnight. Stock solutions of physodic acid and acetone extract were prepared in DMSO, diluted with a medium to the desired concentrations (100–0.1 μM or 100–0.1 μg/mL, respectively), and transferred to the cells. The final concentrations of DMSO never exceed 0.1%. The control cells were cultured with a medium containing 0.1% DMSO. The cells were incubated for 72 h.
MTT assay
The MTT assay is commonly used in the screening of anticancer compounds. The tetrazolium ring of MTT is cleaved by dehydrogenases in mitochondria of living cells to produce a purple formazan. The MTT test was carried out according to the protocol described by Berridge et al. (Citation2005). Briefly, after 72 h of incubation with the tested substances, the medium was aspirated, and 170 μL of the mixture of the medium and the MTT solution (7.5:1) were added to the wells. The cells were incubated for 4 h. Excessive MTT was then aspirated, and the formazan that formed was solubilized by the addition of 150 μL SDS. The plates were shaken (3 min, 650 rpm), and the formazan that formed was solubilized. The mixture of the medium and the MTT solution (7.5:1) was used as a blank. The absorbance was read at 570 nm using a microplate reader. IC50 values were calculated from curves constructed by plotting the cell survival versus the drug concentration. The values are expressed as the mean of six replications ± SD.
NRU assay
The NRU assay, which is based on the uptake and subsequent lysosomal accumulation of the supravital dye neutral red, was used to quantify the number of viable cells after their exposure to the cytotoxic substances. After incubation with NR the dye was extracted from the tested cells and measured spectrophotometrically. The absorbance of the dye extracted from the cells is linear to the number of living cells in a tested sample. The NRU test was carried out following the protocol described by Fotakis and Timbrell (Citation2006). In short, after 72 h of incubation, the medium was aspirated. The medium was then supplemented with neutral red (150 μL) and incubated for 2 h. Subsequently, the neutral red-containing medium was aspirated, and the cells were washed twice with PBS (100 μL). A mixture of acetic acid (1%) and ethanol (50%) was then poured into each well. The plate was shaken (10 min, 100 rpm) to extract the dye. The absorbance, as a measure of the viable cell number, was read at 540 nm by a scanning multiwell spectrophotometer (a microplate reader). The IC50 values for cell viability were obtained by plotting the percentage of cell viability versus the concentration of the test substance on a graph. The values are expressed as the mean of six replications ± SD.
CV assay
The CV assay is based on the reduction in the growth rate reflected by the colorimetric determination of stained cells what allows to assess the number of viable cell after incubation with tested substances. The CV stains DNA upon solubilization in acetic acid, and the amount of the dye bound to cellular DNA is then quantitated using a plate reader. However, in the CV assay, the absorbance of the stained cells does not represent the amount of viable cells when the cells are cultured after reaching confluence. The results of a CV assay often vary when cells grow uniformly on the bottom of each well. In some cases, all adherent cells are dead cells, but still attached to the bottom of the well cells, are considered viable cells because CV nonspecifically stains them. This also occurs frequently when using other protein assays.
The CV assay was carried out following the protocol described by Chiba et al. (Citation1998), with some slight modification. Briefly, after 72 h of incubation with the tested substances, the medium was aspirated, and the cells were washed twice with PBS containing calcium and magnesium. The CV solution (0.1% CV in 10% ethanol) was then added to each well for at least 10 min. The stain was removed, and the plates were rinsed using the calcium and magnesium-containing PBS. Glacial acetic acid (50%) was added to all the wells and shaken (10 min; 650 rpm). The absorbance was read at 570 nm using a microplate reader. The IC50 values for cell viability were calculated by plotting the percentage of cell viability versus the concentration of the test substance on a graph. The values are expressed as the mean of six replications ± SD.
Radical scavenging activity (DPPH) assay
DPPH is a stable free radical, which is commonly used to evaluate the ability of compounds to act as free radical scavengers or hydrogen donors and to measure the antioxidant activity of tissue extracts. The reaction of an antioxidant or a reducing compound with DPPH results in the production of hydrazine DPPH2. This reaction may be tracked spectrophotometrically, as the colour of the DPPH solution changes from purple (the absorbance at 517 nm) to yellow. The free radical scavenging capacity of the acetone extract from H. physodes was determined using the DPPH method described by Annegowda et al. (Citation2010). Briefly, 0.1 mL of the acetone extract dissolved in DMSO (6.0–0.094 mg/mL) was added to 2.9 mL of the DPPH solution (0.1 mM in methanol; the final assay concentrations were 200.0–3.125 μg/mL). The reaction mixture was shaken and incubated in the dark at room temperature for 30 min. The absorbance was measured at 517 nm against a blank (2.9 mL of methanol + 0.1 mL of DMSO). The negative control was a mixture of 2.9 mL of DPPH solution and 0.1 mL of DMSO. Vitamin C and BHA were used as positive controls. The following equation was used to calculate the concentration of the DPPH radicals:
where A0 is the absorbance of the negative control and A1 is the absorbance of the reaction mixture or standards. The IC50 values of the antioxidant were calculated as the concentration required to inhibit the activity of the DPPH radicals by 50%. The activity of the H. physodes extract at a concentration of 50 μg/mL was expressed as the vitamin C equivalent (VCE) (μg VCE/mL tested sample). The values are expressed as the mean of 12 replications ± SD.
Reducing power (FRAP assay)
The FRAP (ferric-reducing antioxidant power) assay was carried out as described previously (Arnous et al. Citation2002), with some modification. Briefly, 50 μL of the acetone extract was dissolved in acetone–DMSO (4:1) (1.5 mg/mL) and mixed with 0.95 mL of freshly prepared FRAP reagent (0.9 mL of TPTZ in 0.05 M HCl and 0.05 mL of 3 mM FeCl3 in 5 mM citric acid), followed by incubation for 30 min in a water bath at 37 °C. The final assay concentration was 50.0 μg/mL. The absorbance was measured at 620 nm against a blank containing 0.05 mL of the acetone–DMSO (4:1) mixture and 0.95 mL of the FRAP reagent. The results were expressed as VCE (μg VCE/mL tested sample). The values are expressed as the mean of 12 replications ± SD.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) and Microsoft Excel 2010 software (Microsoft, Redmond, WA). The median effect concentrations (IC50 values) were determined using a concentration–response curve.
Results
Phytochemical analysis
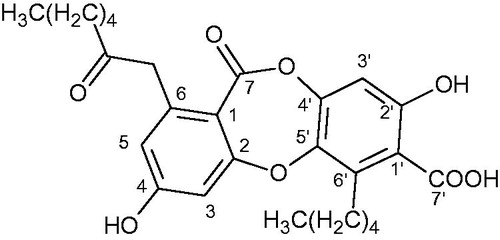
As a result of the phytochemical investigation, the acetone extract and physodic acid were obtained from the thallus of H. physodes. Physodic acid (15 mg) () was isolated from the acetone extract using column chromatography. The identity of this compound, which has already been detected in this species (Culberson Citation1979; Purvis et al. Citation1992), was confirmed using TLC and by UV, 1H, 13C NMR and MS analyses.
Physodic acid: Rf = 0.53 (toluene–AcOEt–formic acid, 139:83:8); white crystals; UV (MeOH) λmax 209, 267, 314 nm; ESIMS (positive mode) m/z 471 [M + H]+; 1H NMR (acetone-d6, 400 MHz) and 13C NMR (acetone-d6, 100 MHz). The data were in full agreement with findings reported in the literature (Huneck & Yoshimnura Citation1996).
Total phenolic content
The total amount of phenolic compounds in the acetone extract was determined as the gallic acid equivalent. The results indicated that the extract contained 299.40 ± 4.94 mg of gallic acid equivalent/g of the dry extract.
Cytotoxic activity
The results of the MTT assay showed that the cytotoxic effects of the tested lichen substances differed in the three types of cancer cell lines (oestrogen receptor (ER)-negative MDA-MB-231; ER-positive MCF-7, T47D) (). Physodic acid was potently active and caused the death of 100% of cells at the concentration used. The cytotoxicity of the acetone extract was lower than that of the physodic acid. Based on these data, the tested cell lines were exposed to increasing concentrations of acetone extract and physodic acid. Subsequent MTT assays revealed that the acetone extract from H. physodes exerted cytotoxic effects at concentrations from 0 to 100 μg/mL. The IC50 values are presented in . In decreasing order of toxicity, the values were as follows: MDA-MB-231 > T47D > MCF-7. presents the results of the MTT, NR and CV assays of physodic acid at a final concentration range of 0–100 μM and the cytotoxic properties of this compound in the human nontumorigenic immortalized breast epithelial MCF-10A cell line. In all the tests, physodic acid exerted the most pronounced cytotoxic activity against the MCF-7 cells, followed by the T47D and MDA-MB-231 cells (). Physodic acid at the tested concentrations (IC50 >100 μM) did not affect the viability of the MCF-10A cells.
Table 1. Cytotoxic effect of physodic acid and acetone extract obtained from H. physodes against selected human cancer cell lines.
Table 2. In vitro cytotoxic activities of acetone extract from H. physodes.
Table 3. In vitro cytotoxic activities of physodic acid.
Antioxidant activity
The results of the DPPH assay showed that the acetone extract of H. physodes had pronounced antioxidant activity. However, it was weaker than that of the referenced substances (BHA and vitamin C) (). The potency of the extract of H. physodes was about five times lower than that of compounds regarded as potent antioxidants. As shown by the results of the FRAP assay, at a concentration of 50 μg/mL, the antioxidant activity of the acetone extract was six times lower than that of vitamin C. This result confirmed the strong antioxidant properties of the tested extract revealed by the DPPH assay (50 μg/mL showed the antioxidant activity 5.39 ± 0.23 μg/mL of VEC).
Table 4. Antioxidant activity of acetone extract from H. physodes.
Discussion
Polyphenols are secondary metabolites, which have been shown to exert various biological activities, such as antioxidant, cytotoxic, antibacterial and anti-inflammatory (Quideau et al. Citation2011). Several authors suggested that the biological properties of plant and lichen extracts were associated with their polyphenol content (Lu & Foo Citation2002; Kosanić & Ranković Citation2011). Research has also shown that lichens synthesized polyphenolic compounds, such as depsides, depsidones and dibenzofuranes (Gulluce et al. Citation2006). The results of the present study revealed a high content of polyphenols in the acetone extract from H. physodes. The content was higher than that reported in a previous study (141.59 mg GA/g of the dry extract) (Mitrović et al. Citation2011).
Lichens have been used in folk medicine since ancient times (Pieroni Citation2000; Agelet & Valles Citation2003). However, their biological activity has not been extensively studied, including that of H. physodes, physodic acid and its derivatives. To the best of our knowledge, only the cytotoxic effects of methanolic extract from H. physodes has been studied in MCF-7 and MDA-MB-231 cell lines (IC50 = 50 and 44 μg/mL, respectively) (Ari et al. Citation2014). The cytotoxic activity of physodic acid isolated from H. physodes in FemX (IC50 = 19.52 μg/mL) and LS174 (IC50 = 17.89 μg/mL) has also been studied (Ranković et al. Citation2014). Pavlović et al. (Citation2013) reported the biological activity of physodic, physodalic, 3-hydroxyphysodic and isophysodic acids isolated from H. physodes in rat thymocytes. Among the studied acids, physodalic acid demonstrated cytotoxic activity at the lowest concentrations (1 and 10 μg/well). Physodic acid and 3-hydroxyphysodic acid exhibited weaker activity (10 μg/well), whereas isophysodic acid did not affect the viability of the thymocytes.
This is the first study to demonstrate the cytotoxic activity of acetone extract from H. physodes and isolated physodic acid against breast cancer cells (ER-negative MDA-MB-231 and ER-positive MCF-7, T47D). As shown in and , the intensity of the cytotoxic effect depended on the type of cell line. Furthermore, depsidone affected the tumorigenic breast cancer cells, whereas the nontumorigenic cells (MCF-10A) were resistant to physodic acid at the applied doses. The cytotoxic activity of physodic acid was confirmed using three different methods. The results suggest that physodic acid is an interesting anticancer candidate and worthy of future research to determine the potential usefulness of this natural substance. The results of the experiments point to a possible interaction of physodic acid with cellular factors, receptors, organelles or signalling pathways involved in the survival of cancer cells. The lowest IC50 values for the cancer cells incubated with physodic acid were obtained in the NRU and CV assays (), pointing to the interaction of physodic acid with DNA or lysosomes in cancer cells. However, several different mechanisms of action are possible. According to a recent study, a broad spectrum of mechanisms may be responsible for the resistance of MCF-10A cells to anticancer compounds. These include differences in the subcellular localization of Bik (Trejo-Vargas et al. Citation2015) and the KLF8/EGFR signalling pathway (Li et al. Citation2015), differences in stearoyl-CoA desaturase-1 activity (Belkaid et al. Citation2015) and combined inhibition by glycolysis and AMP-activated protein kinase (Wu et al. Citation2015), and differences in mitochodrial-mediated apoptosis (Taha et al. Citation2015). Therefore, the exact mechanism responsible for the selective activity of physodic acid in breast cancer should be further investigated.
In the present study, the potency of the acetone extract and physodic acid differed, with the latter exerting stronger cytotoxic activity. In our opinion, this might be the result of the synergistic or antagonistic effect of several compounds, including the physodic acid in the extract.
The biological activity of antioxidants is related to the ability of these antioxidants to inhibit the oxidation of macromolecules, such as proteins and DNA, which prevents mutagenesis or carcinogenesis. A high concentration of reactive oxygen species can give rise to numerous diseases. Consequently, antioxidants have an important role in the treatment of medical and cosmetology problems (Christaki et al. Citation2012; Herman & Herman Citation2013). The present study is used DPPH and FRAP assays to establish the antioxidant properties of acetone extract from H. physodes. DPPH assays have frequently been used to study the scavenging capacities of plants (Dudonné et al. Citation2009). The FRAP method was proposed for the first time to evaluate the antioxidant properties of lichens. A few studies have investigated the antioxidant properties of extracts obtained from H. physodes. For example, Mitrović et al. (Citation2011) and Stojanović et al. (Citation2010) reported the IC50 values for methanolic extracts (45.57 μg/mL and 79.70 μg/mL), and Kosanić et al. (Citation2011) reported the percentage of inhibition of DPPH radicals by methanolic (73.18%), acetone (60.18%) and aqueous (30.98%) extracts from H. physodes. Climatic conditions, such as high light intensity, high temperature and air pollution, have been shown to play important roles in the production of lichen metabolites and to influence their biological properties (Bartak et al. Citation2004; Weissman et al. Citation2006; Millot et al. Citation2007). Thus, the biological properties of extract from the thalli of H. physodes grown in Poland may differ from those of H. physodes in other countries, where the lichen may be exposed to other environmental factors. According to the results of the present study, the antioxidant activity of the acetone extract from H. physodes was two to three times higher than that reported for methanolic extract in previous studies (Stojanović et al. Citation2010; Mitrović et al. Citation2011). The strong antioxidant activity may be attributed to its high content of polyphenols. Although the antioxidant properties of H. physodes were significant, those of the chemical standard BHA and vitamin C were stronger.
Conclusions
In summary, the results suggest that physodic acid and acetone extract obtained from H. physodes have strong anticancer activity in vitro. Previous studies examined the effects of physodic acid and acetone extract in other cancer cell lines (Pavlović et al. Citation2013; Ari et al. Citation2014; Ranković et al. Citation2014). This is the first study to investigate their cytotoxic activity in breast cancer cell lines (MCF-7, T47D and MDA-MB-231). It should be stressed that these substances showed pronounced activity against both oestrogen dependent (MCF-7 and T47D) and independent breast cancer cells (MDA-MB-231). Although few therapeutic strategies for the treatment of oestrogen dependent breast cancer focused on ERs were developed; however, they are very often connected with severe side effects. Moreover, the treatment of oestrogen independent breast cancer is still connected with very high percent of therapeutic failures, therefore presented in our paper compounds should be further tested. Interestingly, although physodic acid displayed cytotoxic effects in the breast cancer cell lines, it was nontoxic against the nontumorigenic MCF-10A cells at the tested concentrations. The acetone extract from H. physodes possessed significant antioxidant properties, possibly due to the high level of polyphenols in the tested extract. Since it was presented is several studies that polyphenolic antioxidants may dysregulate delicate redox balance in cancer cell the pronounced antioxidant properties of physodic acid and the acetone extract obtained from H. physodes could explain their cytotoxicity; however, this problem should be subject of further research.
Disclosure statement
The authors report no declarations of interest.
References
- Agelet A, Valles J. 2003. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part III. Medicinal uses of non-vascular plants. J Ethnopharmacol. 84:229–234.
- Annegowda HV, Anwar LN, Mordi MN, Ramanathan S, Mansor SM. 2010. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacognosy Res. 2:368–373.
- Ari F, Celikler S, Oran S, Balikci N, Ozturk S, Ozel MZ, Ozyurt D, Ulukaya E. 2014. Genotoxic, cytotoxic, and apoptotic effects of Hypogymnia physodes (L.) Nyl. on breast cancer cells. Environ Toxicol. 29:804–13.
- Arnous A, Makrisa DP, Panagiotis K. 2002. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J Food Compost Anal. 15:655–665.
- Bartak M, Hajek J, Vrablíkova H, Dubová J. 2004. High-light stress and photoprotection in Umbilicaria antarctica monitored by chlorophyll fluorescence imaging and changes in zeaxanthin and glutathione. Plant Biol. 3:331–341.
- Belkaid A, Duguay SR, Ouellette RJ, Surette ME. 2015. 17β-Estradiol induces stearoyl-CoA desaturase-1 expression in estrogen receptor-positive breast cancer cells. BMC Cancer. 15:440.
- Berridge MV, Herst PM, Tan AS. 2005. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 11:127–152.
- Chiba K, Kawakami K, Tohyama K. 1998. Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol in Vitro. 12:251–258.
- Christaki E, Bonos E, Giannenas I, Florou-Paneri P. 2012. Functional properties of carotenoids originating from algae. J Sci Food Agric. 15:5–11.
- Culberson CF. 1979. Chemical and botanical guide to lichen products. Koenigstein: Otto Koeltz Science Publishers.
- Dóka O, Bicanic D. 2002. Determination of total polyphenolic content in red wines by means of the combined He–Ne laser optothermal window and Folin–Ciocalteu colorimetry assay. Anal Chem. 74:2157–2161.
- Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon JM. 2009. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 57:1768–1774.
- Fotakis G, Timbrell JA. 2006. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 160:171–177.
- Gollapudi SR, Telikepalli H, Jampani HB, Mirhom YW, Drake SD, Bhattiprolu KR, Vander Velde D, Mitscher LA. 1994. Alectosarmentin, a new antimicrobial dibenzofuranoid lactol from the lichen, Alectoria sarmentosa. J Nat Prod. 57:934–938.
- Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, Agar G, Sokmen A. 2006. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine. 13:515–521.
- Herman A, Herman AP. 2013. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol Physiol. 26:8–14.
- Huneck S. 1999. The significance of lichens and their metabolites. Naturwissenschaften. 86:559–570.
- Huneck S, Yoshimnura I. 1996. Identification of lichen substances. Berlin: Springer-Verlag.
- Kosanić M, Ranković B. 2011. Lichens as possible sources of antioxidants. Pak J Pharm Sci. 24:165–170.
- Kosanić M, Ranković B, Vukojević J. 2011. Antioxidant properties of some lichen species. J Food Sci Technol. 48:584–590.
- Li T, Lu H, Mukherjee D, Lahiri SK, Shen C, Yu L, Zhao J. 2015. Identification of epidermal growth factor receptor and its inhibitory microRNA141 as novel targets of Krüppel-like factor 8 in breast cancer . Oncotarget. 6:21428–21442.
- Lu Y, Foo LY. 2002. Polyphenolics of Salvia – a review. Phytochemistry. 59:117–140.
- Millot M, Tomasi S, Articus K, Rouaud I, Bernard A, Boustie J. 2007. Metabolites from the lichen Ochrolechia parella growing under two different heliotropic conditions. J Nat Prod. 70:316–318.
- Mitrović T, Stamenkovic S, Cvetkovic V, Tošić S, Stanković M, Radojević I, Stefanović O, Čomić L, Đačić D, Ćurčić M, et al. 2011. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci. 12:5428–5448.
- Molnár K, Farkas E. 2010. Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C, J Biosci. 65:157–173.
- Müller K. 2001. Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol. 56:9–16.
- Neamati N, Hong H, Mazumder A, Wang S, Sunder S, Nicklaus MC, Milne GW, Proksa B, Pommier Y. 1997. Depsides and depsidones as inhibitors of HIV-1 integrase: discovery of novel inhibitors through 3D database searching. J Med Chem. 40:942–951.
- Osawa T, Kumon H, Reece CA, Shibamoto T. 1991. Inhibitory effect of lichen constituents on mutagenicity induced by heterocyclic amines. Environ Mol Mutagen. 18:35–40.
- Pavlović V, Stojanović I, Jadranin M, Vajs V, Djordjević I, Smelcerovic A, Stojanovic G. 2013. Effect of four lichen acids isolated from Hypogymnia physodes on viability of rat thymocytes. Food Chem Toxicol. 51:160–164.
- Pieroni A. 2000. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J Ethnopharmacol. 70:235–273.
- Proksa B, Adamcova J, Sturdikova M, Fuska J. 1994. Metabolites of Pseudevernia furfuracea (L.) Zopf. and their inhibition potential of proteolytic enzymes. Pharmazie. 49:282–283.
- Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM, editors. 1992. The lichen flora of Great Britain and Ireland. London: Natural History Publications.
- Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. 2011. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl. 50:586–621.
- Ranković B, Kosanić M, Manojlović N, Rančić A, Stanojković T. 2014. Chemical composition of Hypogymnia physodes lichen and biological activities of some its major metabolites. Med Chem Res. 23:408–416.
- Ranković B, Misić M, Sukdolak S. 2007. Antimicrobial activity of extracts of the lichens Cladonia furcata, Parmelia caperata, Parmelia pertusa, Hypogymnia physodes and Umbilicaria polyphylla. Br J Biomed Sci. 64:143–148.
- Shibamoto T, Wei CI. 1984. Mutagenicity of lichen constituents. Environ Mutagen. 6:757–762.
- Stojanović G, Stojanović I, Stankov-Jovanović V, Mitić V, Kostić D. 2010. Reducing power and radical scavenging activity of four Parmeliaceae species. Cent Eur J Biol. 5:808–813.
- Taha H, Looi CY. Arya, Wong WF, Yap LF, Hasanpourghadi M, Mohd MA, Paterson IC, Mohd Ali H. 2015. (6E,10E) Isopolycerasoidol and (6E,10E) isopolycerasoidol methyl ester, prenylated benzopyran derivatives from Pseuduvaria monticola induce mitochondrial-mediated apoptosis in human breast adenocarcinoma cells. PLoS One. 10:e0126126.
- Trejo-Vargas A, Hernandez-Mercado E, Ordonez-Razo RM, Lazzarini R, Arenas-Aranda DJ, Gutierrez-Ruiz MC, Konigsberg M, Luna-Lopez A. 2015. Bik subcellular localization in response to oxidative stress induced by chemotherapy, in two different breast cancer cell lines and a non-tumorigenic epithelial cell line. J Appl Toxicol. 35:1262–1270.
- Türk AO, Yilmaz M, Kivanç M, Türk H. 2003. The antimicrobial activity of extracts of the lichen Cetraria aculeata and its protolichesterinic acid constituent. Z Naturforsch C, J Biosci. 58:850–854.
- Türk H, Yilmaz M, Tay T, Türk AO, Kivanç M. 2006. Antimicrobial activity of extracts of chemical races of the lichen Pseudevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetoric acid constituents. Z Naturforsch C, J Biosci. 61:499–507.
- Weissman L, Fraiberg M, Shine L, Garty J, Hochman A. 2006. Responses of antioxidants in the lichen Ramalina lacera may serve as an early-warning bioindicator system for the detection of air pollution stress. FEMS Microbiol Ecol. 58:41–53.
- Wu Y, Sarkissyan M, Mcghee E, Lee S, Vadgama JV. 2015. Combined inhibition of glycolysis and AMPK induces synergistic breast cancer cell killing. Breast Cancer Res Treat. 151:529–539.
- Yilmaz M, Tay T, Kivanç M, Garty J, Hochman A. 2005. The antimicrobial activity of extracts of the lichen Hypogymnia tubulosa and its 3-hydroxyphysodic acid constituent. Z Naturforsch C, J Biosci. 60:35–38.
- Zambare VP, Christopher LP. 2012. Biopharmaceutical potential of lichens. Pharm Biol. 50:778–798.