Abstract
Context: The African genus Aframomum (Zingiberaceae) is a group of diverse tropical plants frequently collected yet largely neglected taxonomically. The current and unprecedented loss of species due to man-made habitat destruction and climate change adds a desperate urgency not only to understand the phylogenetics, chemotaxonomy and biology, but also to preserve the quickly disappearing species.
Objectives: The present systematic review reports on the research progress in phytochemistry, pharmacology and toxicology of Aframomum species.
Methodology: Scientific databases such as MedSci, PubMed, Scopus, Google Scholar and Web of Knowledge were used to retrieve publications (from the year 1990 to 2014) related to Aframomum plants, isolated compounds and their bioactivity, phytochemistry and toxicology. The keywords combinations for the search were: Aframomum; chemotaxonomy, phylogenetics, pharmacology and bioactive metabolites and toxicology. A total of 71 research articles that report on the biological activity of extracts and chemical constituents were recovered and presented in this review.
Results: Most published data related to the potential of Aframomum melegueta, a medicinal plant from West and Central Africa. The potential of phenols and terpenoids isolated from Aframomum plants were generally much better documented than that of arylalkanoids.
Conclusion: Aframomum genus represents an enormous resource for novel compounds with a range of medicinal properties. However, these plants are under-researched and their conservation is poor. To unravel their full potential, efforts should be strengthened throughout the continent to establish the taxonomy, preserve the genus and explore novel medicinal properties.
Introduction
The Zingiberaceae constitute a family of terrestrial rhizomal herbs with over 1400 species distributed in over 50 genera. The genus Aframomum Schumann (1904) is the largest genus of Zingiberaceae comprising of 80 species of giant herbs peculiar to West and Central Africa. The genus was described by K. Schumann (1904) to accommodate the African species of Amomum (Cheek et al. Citation1996). Aframomum species range from Senegal in the west to Ethiopia in the north and Angola to Madagascar in the south. It is also found in the Gulf of Guinea Islands, Sao Tome and Principe. Some species, however, are ecologically specialized; for example, Aframomum longilgulatum is known only from forests in Cameroon, while the flooded forest in the Congo River basin is dominated by Aframomum pseudostipulare. Aframomum alboviolaceum is only found in the Savanna (Larsen et al. Citation1998; Tane et al. Citation2005). Aframomum species are also found in light gaps and forest margins and are common along roads and in old fields. They are perennials and aromatics when any part of the plant is crushed. They also possess highly coloured flowers and the peduncles are covered with sterile overlapping bracts (Fankam et al. Citation2011). These varieties of species are mostly used as laxatives, anthelmintics, toothache, antidiarrhoea, fever management, stomach ache, inflammatory conditions, postpartum haemorrhage and a tonic for sexual stimulation (Duke Citation2002). Additionally, they are reported to have antiulcer, antimicrobial, antinociceptive, antiplasmodial, hepatoprotective and anticancer activities (El-Halawany et al. Citation2014).
Various compounds have been isolated from the Aframomum plants since the 1970s and their chemistry and pharmacology have been reported (Adegoke & Skura Citation1994; Escoubas et al. Citation1995). Plant selection based on prior ethnomedicinal use has been fairly predictive of the possibility of identifying active leads (Kuete Citation2010). For example, ethnopharmacological uses as immune and skin disorders, and inflammatory ailments, should be considered when selecting plants to treat cancer symptoms (Kuete & Efferth Citation2010). The classes of compounds generally found in Aframomum species include diterpenoids, sesquiterpenoids, arylalkanoids and flavonoids. In this brief review, we summarize the recent progress in phytochemical and biological research of some of the most exploited Aframomum plants. Studies on the chemotaxonomy and molecular phylogeny are still under investigation to facilitate further conservation and exploitation of Aframomum pharmaceutical and food resources.
Molecular taxonomy, phylogeny and genomics
The taxonomy of Aframomum is extremely difficult and the genus is currently undergoing revision (Lock & Hall Citation1975). According to Harris et al. (Citation2000), Aframomum is monophyletic and is thought to have diversified during the late Oligocene to the Miocene. Presently, no criteria for the recognition of these species at the generic level has been established but studies using molecular sequence data (ITS-nrDNA and trnl-f-cpDNA, matK) have been utilized to generate hypotheses on the phylogenetic relationships among the genera of the Zingiberaceae, to evaluate past classifications of the family and to identify morphological features that characterize the various clades (Babu et al. Citation2011, Citation2012). The chloroplast region, psbA-trnH intergenic spacer has been proposed as a useful marker for DNA barcoding (Shaw et al. Citation2007). However, the sequencing has been reported to be hard, time-consuming and many of the psbA-trnH sequences are of poor quality. It was later observed that adding internally transcribed spacer region (ITS) and trnl-F databases improved the resolution of the phylogenetic tree. Hence, it is concluded that PsbA-trnH intergenic spacer alone might not be the most suitable for distinguishing taxa at the species level. Based on the DNA sequences of ITS, a system of classification was proposed. The genera of Zingiberaceae were grouped as: Alpinia, Aframomum, Amomum and Elettariopsis (which is near to Elettaria), Renealmia, Plagiostachys, Vanoverberghia, Hornstedtia and Etlingera in a single clade ‘Alpineae’, whereas Hedychium, Zingiber and Curcuma formed another clade ‘Zingibereae’ (Kress, Liu, et al. Citation2005; Kress, Wurdack, et al. Citation2005). This significant discrepancy in the chloroplast phylogenies might be due to intraspecific polymorphism and introgression. More studies are needed at population or species level to ensure a better characterization of Aframomum species.
Chemotaxonomy of Aframomum
Chemotaxonomy or chemosystematics is the attempt to classify and identify organisms (originally plants), according to demonstrable differences and similarities in their biochemical compositions (Smith Citation1976). Generally, it has been observed that plants of the same family usually synthesize compounds of similar classes due to the presence of similar classes of enzymes and hence similar biosynthetic pathways (Kuete & Efferth Citation2010). The Aframomum species as well as other Zingiberaceae are best known for the production of labdane diterpenoids and flavonoids. Other classes of compounds encountered in the genus include sesquiterpenoids and arylalkanoids. Out of the 12 most chemically studied Aframomum species, at least 11 contain diterpenoids (Tane et al. Citation2005). Within the Zingiberaceae family, labdane diterpenes and flavonoids may represent a chemotaxonomic marker of the genus Aframomum. However, Aframomum arundinaceum is one of the few species of Aframomum from which sesquiterpenoids are reported (Ayimele et al. Citation2004; Wabo et al. Citation2006).
Pharmacology of Aframomum extracts
Antioxidant and anti-inflammatory activity
The enzymatic sources of ROS/RNS include NADPH oxidase, xanthine oxidase, uncoupled endothelial nitric oxide synthase, arachidonic acid metabolizing enzymes such as cytochrome P450 enzymes, lipoxygenase (LOX) and cyclooxygenase (COX), and the mitochondrial respiratory chain. In a recent research conducted by El-Halawany et al. (Citation2014), it was found that methanol–chloroform extracts of A. melegueta significantly exhibited a protective role in CCl4-induced acute liver injury which might be due to elevated antioxidative defence potentials, suppressed inflammatory responses and apoptosis of liver tissue. Ethanolic extract of A. melegueta seeds also possessed anti-inflammatory activity, which was in part due to the inhibition of COX-2 enzyme activity and expression of pro-inflammatory genes (Ilic et al. Citation2014). Scavenging of free radicals is one of the major antioxidative mechanisms to inhibit the chain reaction of lipid peroxidation (LPO) and reduction of the deleterious effect of the cytotoxic products. Administration of aqueous extract of A. melegueta seeds to gamma radiation-induced liver damaged male Wistar rats at a dose of 200 or 400 mg/kg before and after irradiation was reported to have significantly decreased the elevated levels of LPO, restored glutathione (GSH) level near normal and enhanced catalase and glutathione peroxidase activities as well as significantly decreased the elevated levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities (Nwozo & Oyinloye Citation2011).
Inflammation is an important component of immune response to pathogens and damaged cell characterized by heat, redness, pains, swelling and sometimes loss of tissue functionality in chronic situation (Zhang & McNaughton Citation2006). The body’s reaction to this phenomenon may trigger inflammatory responses through the release of pro-inflammatory eicosanoids such as prostaglandins, prostacyclins and leukotrienes, and pro-inflammatory cytokines such as interleukins (IL-1B, IL-3, IL-6), interferons, tumour nuclear factor (TNF-α) and platelet-activating factor. COX and LOX oxidation of polyunsaturated fatty acid such arachidonic acid or linoleic acid forming bioactive eicosanoids are the major features of inflammatory response (Haeggström et al. Citation2010).
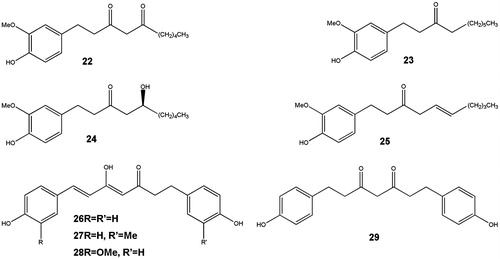
Phenolics, alkaloids and triterpenoids have been demonstrated to exhibit anti-inflammatory activities by exerting antioxidative properties in reducing O2– and malondialdehyde (MDA) production, plasma extravasations and cell migration mainly of leukocytes and potentiates the activity of superoxide dismutase in radical scavenging (Mosquera et al. Citation2007). Ethanolic extract of A. melegueta seeds demonstrated an anti-inflammatory activity in a rat paw oedema model and in pro-inflammatory gene expression assays. The whole extract reduced inflammation by 49% at 1000 mg/kg. The activity was in part attributed to the inhibition of COX-2 enzyme and expression of pro-inflammatory genes by [6]-paradol (23) and [6]-Shogaol (25) (Ilic et al. Citation2014).
Anticancer activity
Cancer cells rapidly acquire multidrug resistance (MDR), mainly due to the presence of two adenosine triphosphate-binding cassette transporters. MDR cancer cells reveal cross-resistance to a variety of chemically and functionally unrelated drugs. The structural diversity of plant’s secondary metabolites makes them an indispensable source for the discovery of new cytotoxic agents. Methanolic extracts of A. arundinaceum seeds demonstrated moderate, but selective, cytotoxicity towards leukaemia CEM/ADR5000 cells and glioblastoma U87MGΔEGFR compared to their respective sensitive counterparts CEM/CEM and U87MG cell lines (Kuete et al. Citation2014). One study noted that A. melegueta methanolic and chloroform seed extracts held cytotoxic potential against PANC-1 pancreatic cancer cells in vitro with IC50 values of 13.8 μg/mL and 47.8 μg/mL, respectively (Obike et al. Citation2014).
Antimicrobial activity
Many of the plant extracts and fractions used have good activities, especially the non-polar fractions of hexane and dichloromethane against the pathogens tested, and this may explain the traditional use of these medicinal plants. Plants of the genus Aframomum are known for their antibacterial activities, and this has been assigned to the presence of terpenoids such as aframodial (Ayafor et al. Citation1994a). Recently, great attention is being paid to exploitation of Aframomum plants as biodegradable fungicides in the control of most plant pathogenic fungi. Non-polar hexane seed extract of Aframomum sceptrum has been found to show the highest percentage inhibition of 60.26% against Hypocrea lixii (IMI 501885) while the polar ethanolic extracts with a percentage inhibition of 52.73% against Fusarium f. sp. elaeidis. Non-polar acetone seed extract and methanol extract showed the lowest percentage inhibitions of 42.45% against Fusarium oxysporum f. sp. elaeidis and 42.31% against H. lixii, respectively (Okogbenin et al. Citation2014). Aramomum melegueta has been reported to inhibit Bacillus cereus (a pathogen obtained from vegetable salad) with a lowest minimum inhibitory concentration of 31.25 mg/mL. This spice has henceforth been recommended to help in ensuring food safety (Uzeh & Oguntosin Citation2013). The difference in antimicrobial properties of a plant extract might be attributable to the age of the plant used, freshness of plant materials, physical factors (temperature, light and water), contamination by field microbes, adulteration and substitution of plants, incorrect preparation and dosage (Okigbo & Igwe Citation2007). It has also been reported that inactivity of plant extracts may be due to the age of the plant, extraction solvent, method of extraction and time of harvest of the plant materials (Alo et al. Citation2012).
Antidiabetic and antiobesity activity
Aqueous leaf extract of A. melegueta showed hypoglycemic activity on alloxan-induced diabetic and non-diabetic rats. The antihyperglycemic activity of the aqueous leaf extract was associated with an increase in plasma insulin level suggesting an insulinogenic activity, stimulating insulin secretion from the remnant β-cells or from regenerated β-cells (Adefegha & Oboh Citation2012b). Ethanolic extract of A. melegueta seeds [10 mg of 15.2% 6-gingerol (24), 12.5% 6-paradol (23), 1.7% 6-shogaol (25) and 4.0% 6-gingeredione] was able to increase the metabolic rate by increasing the energy expenditure through the activation of brown adipose tissue, a site of sympathetically mediated metabolic thermogenesis. Persons with no response to cold exposure (without substantial brown fat adipose tissue) failed to show a response (Sugita et al. Citation2013).
Antihypertensive activity
Lawal et al. (Citation2007) reported that, ingestion of A. melegueta seeds resulted in the lowering of cardiovascular indices such as systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure and mean arterial pressure in normotensives and hypertensives, respectively. All were found to be significantly different from control values (p < 0.01). Percentage reductions, though similar between normotensives and hypertensives, were however greater for SBP averaging (15–16%) than DBP (9–10%). A central effect was suggested as the cause but peripheral vasodilatation effect was not equally ruled out probably via the nitric oxide-cGMP pathway. The degree of reduction was found to be within safety limits, indicating its potential usefulness in managing hypertension in young and elderly hypertensive patients.
Male sex organs
The administration of 115 mg/kg of aqueous seeds extract of A. melegueta daily for 8 days resulted in an increase in the penile erection index, frequency of genital grooming and genital sniffing, and an increase in mounting frequency by 54%. Intromission latency was reduced by 32%, while ejaculation latency was increased (delayed) by 60% (with no significant influence on postejaculatory interval), and while all these effects were greater than control they were simultaneously lesser than the other herb tested, Piper guineense (although not by a statistically greater degree) (Kamtchouing et al. Citation2002). The same was also observed to increase the secretions of epididymis and seminal vesicle, which are accessory sex organs (Mbongue et al. Citation2012).
Antiestrogenic activity
El-Halawany and Hattori (Citation2012) reported that methanolic seeds extracts of A. melegueta inhibit 56.7 ± 3.4% of estrogenic activity in a yeast assay at the concentration of 100 μg/mL; this was decreased with naringinase pretreatment. Despite A. melegueta outperforming all other herbs in this study, it underperformed relative to the active control of tamoxifen (78% inhibition at 10 μM).
Antiageing activity
An assay based on image processing analysis demonstrated the antiwrinkle activity of a formulation containing Aframomum angustifolium seed extract. The data obtained in the two centre study suggests that the cosmeceutical containing A. angustifolium seed extract produces a global rejuvenation effect in terms of redness, pigmentation and fine lines similar to that noted utilizing an intense pulse light source. More research is currently under investigation (Andre et al. Citation2008)
Spasmolytic activity
The spasmolytic properties of the aqueous seed extracts of A. melegueta and Citrus aurantifolia (Christm and Panzer) (AMCA) mixture were tested on isolated rat trachea. Inhibition of the contraction was observed in the presence of the AMCA (EC50 1.80 ± 0.48 mg/mL) after a precontraction of the trachea by acetylcholine (10−5 M). The extract did not involve muscarinic receptor but likely inhibited cellular calcium. It also involved in part, β-adrenergic receptors (Ahounou et al. Citation2012).
Chemical components of the genus Aframomum and their biological activities
Terpenoids: diterpenoids and sesquiterpenoids saponins
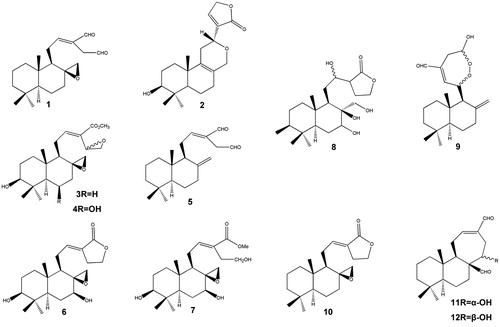
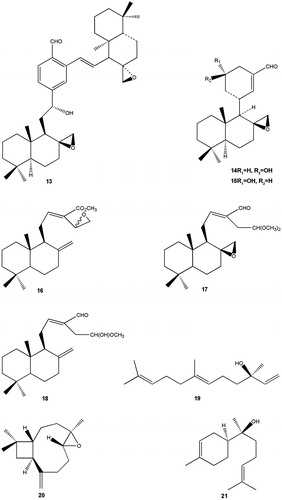
Diterpenoids (C20) and sesquiterpenoids (C15) are secondary metabolites of terpenoids. Their general chemical structures contain isoprene (C10H16). In medicinal plants, these compounds are also called quinta essentia, or essential oil fraction. Other terpenes include triterpenes, tetraterpenes and hemiterpenes (C30, C40 and C5). Some diterpenoids, aulacocarpinolide (2), aulacocarpin A (3) and aulacocarpin B (4) have been isolated from Aframomum aulacocarpos. Sesquiterpenoids are rarely found in Aframomum species. Up to date only three sesquiterpenoids derivatives have been reported in these species, these are 6,7-epoxy-3(15)-caryophyllene (20) and (−)-α-bisabolol (21) found in A. arundinaceum, (+)-S-nerolidol (19) isolated from A. sceptrum and Aframomum escapum (Ayafor et al. Citation1994b; Tomla et al. Citation2002; Wabo et al. Citation2006).
Antiplasmodial and antimicrobial activity
Diterpenoids and sesquiterpenoids are active against bacteria, fungi, viruses and protozoa. Some of the active metabolites that have been isolated are: labdane 3-deoxyaulacocarpin A from Aframomum zambesiacum; sesquiterpenoids oplodiol, (5E,10(14)-germacradien-1β,4β-diol and 1(10)E,5E-germacradien-4α-ol) with respective IC50 values of 4.17, 1.63 and 1.54 μM (Tchuendem et al. Citation1999; Kenmogne et al. Citation2006). In all cases, antiplasmodial activity was measured by inhibition of the chloroquine resistant W2 P. falciparum strain with IC50 <5 μM. The mechanism of action of terpenes is not fully understood, but is speculated to involve membrane disruption by the lipophilic compounds. Also, terpenoid molecules could insert into cell membrane and modify the composition, influence membrane fluidity, and potentially affect signalling by ligands and cofactors (Hao et al. Citation2013). Aframodial (dialdehyde, 8β,17-epoxy-12E labdane-15,16-dial) (1) has been isolated from the seeds of Aframomum polyanthum, Aframomum masuianum, Aframomum kayserianum, Aframomum sulcatum, Aframomum longifolius, A. arundinaceum and Aframomum latifolium (Ayafor et al. Citation1994a). Aframodial has been reported to actively inhibit growth of Salmonella enteriditis, Pseudomonas fragi, P. flourescens, Proteus vulgaris, Staphylococcus aureus, Aspergillus flavus, Streptococcus pyogens, A. parasiticus, A. ochraceus and A. niger in the bioassays of the extracts of Aframomum danielli. Their zones of inhibition in mm were 20, 22.33, 22.27, 23.29, 28.09, 26.54, 23.90, 33.95, 32.0 and 28.78, respectively (Adegoke & Skura Citation1994). It has also been reported that the extract of A. polyanthum is active against Enterobacter aerogenes EA294 with the lowest recorded minimal inhibitory concentration of 32 μg/mL (Djeussi et al. Citation2013). Other plant extracts that have also been found to possess antimicrobial activity include Aframomum giganteum (Agnaniet et al. Citation2004), and A. danielli (Fasoyiro & Adegoke Citation2007).
Anticancer activity
Recent studies on the cytotoxicity of four Aframomum species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines reported cytotoxic potential of Aframomum species and highlights the good activity of A. arundinaceum on sensitive and drug-resistant cancer cell lines. This must have been contributed by galanals A (11) and B (12), naringenin and kaempferol-3,7,4′-trimethylether (30). However, galanal A (11) and galanal B (12) were generally less active towards resistant cancer cells, with galanal B showing collateral sensitivity towards resistant breast adenocarcinoma MDA-MB-231/BCRP cells (Kuete et al. Citation2014). A. melegueta is reported to have inhibited the proliferation of the leukaemia ADR5000 cell lines with a reported IC50 value of 7.80 μg/mL (Kuete et al. Citation2011). Naringenin has also shown cytotoxicity in various human cancer cell lines and induced apoptosis via a transient induction of caspase-3/CPP32 activity, in the human promyeloleukaemia cell line HL-60 (Kanno et al. Citation2003; Li et al. Citation2009). The moderate cytotoxicity of galanals A (11) (IC50 of 18 μM or 5.62 μg/mL) and B (12) (IC50 of 32 μM or 12.21 μg/mL) towards human T lymphoma Jurkat cells has also been reported (Miyoshi et al. Citation2003) ( and )
Polyphenols, flavonoids and phenolic glycosides
The relationship between total phenol contents and antioxidant activity has been widely studied in different foodstuffs (Ghasemi et al. Citation2009). Antioxidant activity of foodstuff significantly increases with the presence of high concentration of total phenol and flavonoid contents. Phenols are broadly distributed in plants and are the most abundant secondary metabolites. Plant phenols have drawn increasing attention due to their significant effects in the prevention of multiple oxidative stress associated diseases such as cancer. Flavonoids (hydroxylated phenolic substances) have shown great potential in management of coronary heart disease (Rice-Evans et al. Citation1997) and effective against a wide array of microorganisms such as polio type 1 and Coxsackie B4 viruses (Özçelik et al. Citation2011). Flavonoids are also known to act on the inflammatory response via many routes and block molecules like COX, iNOS, cytokines, nuclear factor-кB and matrix metalloproteinases.
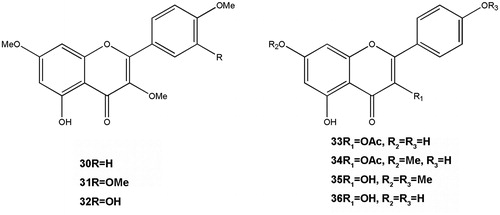
Amarowicz and Shahidi (Citation1997) attributed the pharmacological activities (antiulcer, anti-inflammatory, antiallergic, antiviral, antibacterial, antiosteoporotic, and antihepatotoxic actions) of flavonoids to their potent antioxidant activities. In addition, flavonoids are good free radical scavengers that donate hydrogen, inhibit LPO and are metal ion chelators. However, the antioxidant power of flavonoids depends on some important structural prerequisites such as the number and the arrangement of hydroxyl groups, the extent of structural conjugation and the presence of electron-donating and electron-accepting substituents on the ring structure (Middleton et al. Citation2000). Eight flavonoids reported in five different species of the genus are summarized in .
Antioxidant activity
In general, ortho-substitution with an electron donor, such as the methoxy groups of curcumin, increases the antioxidant activity of phenols by enhancing the stability of the phenoxyl radical by an inductive effect (El-Halawany et al. Citation2014). RP-HPLC analysis showed abundance of quercetin and kaempferol in phenolic characterization on seeds of A. melegueta and chlorogenic acid in A. danielli. These phenolic and non-phenolic constituents contributed to the inhibitory effect of these extracts on acetylcholinsterase activity and their antioxidant property (Adefegha & Oboh Citation2012a). Among all the Aframomum species, A. melegueta has captured attention to possess functional ingredients to prevent inflammation and ROS-related diseases. Quercetin and kaempferol isolated from A. giganteum has been shown to possess antibacterial activities. They also display potent antiviral and anti-inflammatory activities. In addition, quercetin and kaempferol inhibit the release of rat mast cell histamine, and they are also efficient radical scavengers (Vidari et al. Citation1971; Kim et al. Citation2004; Park et al. Citation2008). Quercetin and its methylated derivatives (31 and 32) have been reported to have strong activity against polio type 1 and Coxsackie B4 viruses both in vitro and in vivo (Tane et al. Citation2005).
Arylalkanoids
The diarylheptanoids (22–25) are groups of compounds which bears the 1,7-diphenylheptane skeletons as a special character. They were isolated for the first time from the seeds of Aframomum letestuianum. Haining and She (Citation2012) grouped them into linear and cyclic diarylheptanoids. They further classified linear diarylheptanoids into four groups: oxy bridges structures, flavonoid moiety, dimeric linear diarylheptanoids and those having unusual structures. Cyclic diarylheptaoids were grouped into 2-metaparacyclophanes and 10-metaparacyclophanes. Diarylheptanoids are known to exhibit a broad range of potent biological activities as anti-inflammatory, antioxidant, estrogenic, antihepatoxic, leishmanicidal, melanogenesis, neuroprotective, antibacterial and antitumour. This group of compounds has also recently been shown to have an inhibitory activity against nitric oxide production in activated murine macrophages (Kim et al. Citation2005). Currently, ongoing investigations are being carried out on gingerdione (22), [6]-paradol (23), [6]-gingerol (24) and [6]-shogaol (25). In a recent report, chloroform fraction of A. melegueta seeds yielded one new diarylheptanoid named 3-(S)-acetyl-1-(49-hydroxy-39,59-dimethoxyphenyl)-7-(30,40,50-trihydroxyphenyl)heptane (El-Halawany et al. Citation2014), in addition to four other diarylheptanoids previously isolated from A. letestuianum, namely (4Z,6E)-5-hydroxy-1,7-bis(4-hydroxyphenyl)hepta-4,6-dien-3-one (26), letestuianin A (27), B (28) and C (29) (Tane et al. Citation2005). [6]-Gingerol (24) and [6]-shogaol (25), also isolated from A. melegueta, have been reported to exhibit significant anti-feedant activity (Ukeh et al. Citation2009) (). A summary of the isolated compounds from reviewed species and their biological activities are outlined in .
Table 1. Summary of Aframomum species, isolated compounds and their biological activities.
Toxicology
With the current emphasis on research and development of medicinal plants worldwide, it is important to have some information regarding the toxicity potentials and efficacies of plants utilized ethnobotanically to treat ailments. In ethnopharmacological studies of medicinal plants, a search in scientific literature should be made for known toxic properties of plants of interest before embarking on biological activity studies. However, where toxic effects are unavailable, the inclusion of cytotoxicity and other toxicity protocols in the study are useful in detecting potential toxicity. This strategy is applicable when screening plant extracts or isolated natural products for some other biological activities such as anti-infectious, anti-inflammatory, antioxidant, anti-diarrhoea and antiparasitic property (Shahid Citation2012).
Liver toxicity
Aframomum melegueta seed oil has the potential of ameliorating benign prostatic hyperplasia and cardiac dysfunction as indicated by testosterone, PSA, lipid profile and troponin levels. The LD50 of 273.86 mg/kg body weight indicated mild toxicity with lower than normal Hb and RBC confirming the possibility of toxicity (Akpanabiatu et al. Citation2013). An ethanolic extract of A. melegueta seeds at an oral dose of 450–1500 mg/kg for 28 days in rats was noted to cause dose-dependent increase in liver weight with a mild increase in LDH and non-significant increases in AST. However, toxicity was not detected by histological examination (no detectable necrosis, steatohepatitis or cirrhosis) (Ilic et al. Citation2010). Methanolic extract of A. melegueta seeds was found to be potentially hepatotoxic (p < 0.05) at 300 mg/kg under prolonged ingestion at high dose. This was supported by the remarkable decrease in the mean body weight of all extract-treated rats, with elevations in serum AST, ALT, ALP and total bilirubin. There was also a decrease in the mean relative liver weight of test rats compared to the control as well as histopathological lesions on treated rat liver. However, low and high doses of aqueous extract of A. melegueta seeds have shown no toxicity in the histological observation of the adrenal glandular cells (Chinaka et al. Citation2014).
Pregnancy
A study in rats that used 50 mg of A. melegueta seeds mixed in 20 g rat chow (1% of feed; the food lasted for 4 days and then normal chow was fed to the rats) noted that the control group had an average litter size of seven. The experimental group fed with the seeds of A. melegueta failed to deliver pups although no other side-effects appeared apparent. This dose equated to 286–345 mg/kg in rats, and lower doses (0.5–2 mg injections) have since failed to adversely affect litter size. High doses have also been implicated in antifertility actions in females (Inegbenebor et al. Citation2009).
Conclusion
The genus Aframomum is a group of diverse tropical plants that has been used as an ethnomedicine since ancestral times. We have reported a number of studies that have been carried out on the uses and applications of Aframomum extracts and chemical constituents. It was observed that A. giganteum and A. melegueta are the most studied species. The whole plant of A. giganteum yielded mostly flavonoids, while hydroxyphenyl alkanoids have been reported from A. melegueta (Charles & Simon Citation1990). The information presented in this review on the phytochemistry and various pharmacological properties of the different extracts, essential oils and the constituents might provide incentive for proper evaluation of the use of the species in medicine. Test conducted in animal models for anti-inflammatory, analgesic, antinociceptive, reproductive capability, anticancer, gastroprotective and other biological activities have shown promising results without any adverse effect within normal stated doses. The major constituents (diterpenoids and sesquiterpenoids) of the essential oils have been shown to produce precocious effects in insect control as well as in bacterial and fungal infection. Further conservation of the species at the genomic level, epigenomic level, transcriptional and post-transcriptional levels is essential for the sustainable development and utilization of Aframomum medicinal and food resources. The integration of biological systems and ‘omics’ techniques could dramatically enhance Aframomum research and development.
Disclosure statement
We declare no competing interest.
References
- Adefegha SA, Oboh G. 2012a. Acetylcholinesterase (AChE) inhibitory activity, antioxidant properties and phenolic composition of two Aframomum species. J Basic Clin Physiol Pharmacol. 23:153–161.
- Adefegha SA, Oboh G. 2012b. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spices. Pharm Biol. 50:857–865.
- Adegoke G, Skura B. 1994. Nutritional profile and antimicrobial spectrum of the spice Aframomum danielli K. Schum. Plant Foods Hum Nutr. 45:175–182.
- Agnaniet H, Menut C, Bessière JM. 2004. Aromatic plants of tropical central Africa. Part XLIX: chemical composition of essential oils of the leaf and rhizome of Aframomum giganteum K. Schum from Gabon. Flavour Frag J. 19:205–209.
- Ahounou JF, Ouedraogo GG, Gbenou JD, Ouedraogo S, Agbodjogbe WK, Dansou PH, Moudachirou M. 2012. Spasmolytic effects of aqueous extract of mixture from Aframomumum melegueta (K. Schum) – Citrus aurantifolia (Christm and Panzer) on isolated trachea from rat. Afr J Tradit Complement Altern Med. 9:228–232.
- Akpanabiatu MI, Ekpo ND, Ufot UF, Udoh NM, Akpan EJ, Etuk EU. 2013. Acute toxicity, biochemical and haematological study of Aframomum melegueta seed oil in male Wistar albino rats. J Ethnopharmacol. 150:590–594.
- Alo M, Anyim C, Igwe J, Elom M, Uchenna D. 2012. Antibacterial activity of water, ethanol and methanol extracts of Ocimum gratissimum, Vernonia amygdalina and Aframomum melegueta. Adv Appl Sci Res. 3:844–848.
- Amarowicz R, Shahidi F. 1997. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem. 58:355–359.
- Andre P, Renimel I, Sauvan N, Razafimambimby H. 2008. Cosmetic composition in particular with anti-ageing activity comprising an extract of Aframomum angustifolium or longoza plant. [cited 2016 Apr 9]. Available from: http://www.google.com/patents/US7381436.
- Ayafor J, Tchuendem M, Nyasse B, Tillequin F, Anke H. 1994a. Aframodial and other bioactive diterpenoids from Aframomum species. Pure Appl Chem. 66:2327–2330.
- Ayafor JF, Tchuendem MH, Nyasse B, Tillequin F, Anke H. 1994b. Novel bioactive diterpenoids from Aframomum aulacocarpos. J Nat Prod. 57:917–923.
- Ayimele GA, Tane P, Connolly JD. 2004. Aulacocarpin A and B, nerolidol and β-sitosterol glucoside from Aframomum escapum. Biochem Syst Ecol. 32:1205–1207.
- Babu K, Jayakumar V, Minoo D, Venugopal M, Sudarshan M, Radhakrishnan V, Backiyirani S, Narayanaswami M, Peter K, Parthasarathy V. 2012. Genetic diversity and phylogenetic relationships among small cardamom (Elettaria cardamomum Maton.) cultivars and related genera using DNA markers. Int J Innovat Horticult. 1:47–57.
- Babu KN. 2011. Biotechnology in conservation and development of spices. Souvenir Abstr. SYMSAC VI:79–93.
- Charles DJ, Simon JE. 1990. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J Am Soc Hortic Sci. 115:458–462.
- Cheek M, Cable S, Hepper F, Ndam N, Watts J. 1996. Mapping plant biodiversity on Mount Cameroon. In: Van der Maesen LJG, Van der Burgt XM, Van Medenbach de Rooy JM editors. Mapping plant biodiversity on Mount Cameroon. Berlin (Germany): Kluwer Academic Publishers. p. 110–120.
- Chinaka N, Eban L, Ode J, Ejiofor C, Igile G. 2014. Hepatotoxicity of methanol seed extract of Aframomum melegueta [Roscoe] K. Schum. in Sprague-Dawley Rats. Am J Biomed Sci. 2:61–66.
- Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, Nkuete AH, Kuete V. 2013. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement Altern Med. 13:164.
- Duke JA. 2002. CRC handbook of medicinal spices. Boca Raton (FL): CRC Press.
- El-Halawany AM, Dine RSE, El Sayed NS, Hattori M. 2014. Protective effect of Aframomum melegueta phenolics against CCl4-induced rat hepatocytes damage; role of apoptosis and pro-inflammatory cytokines inhibition. Sci Rep. 4:5880.
- El-Halawany AM, Hattori M. 2012. Anti-oestrogenic diarylheptanoids from Aframomum melegueta with in silico oestrogen receptor alpha binding conformation similar to enterodiol and enterolactone. Food Chem. 134:219–226.
- Escoubas P, Lajide L, Mizutani J. 1995. Termite antifeedant activity in Aframomum melegueta. Phytochemistry. 40:1097–1099.
- Fankam A, Kuete V, Voukeng I, Kuiate J, Pages JM. 2011. Antibacterial activities of selected Cameroonian spices and their synergistic effects with antibiotics against multidrug-resistant phenotypes. BMC Complement Altern Med. 11:104.
- Fasoyiro S, Adegoke G. 2007. Phytochemical characterization and the antimicrobial property of Aframomum danielli extract. Afr J Agric Res. 2:76–79.
- Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. 2009. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 22:277–281.
- Haeggström JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. 2010. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun.396:135–139.
- Haining L, She G. 2012. Naturally occurring diarylheptanoids – a supplementary version. Rec Natl Prod. 6:321–333.
- Hao D, Gu X, Xiao P, Liang Z, Xu L, Peng Y. 2013. Research progress in the phytochemistry and biology of Ilex pharmaceutical resources. Acta Pharm Sin B. 3:8–19.
- Harris D, Poulsen A, Frimodt-Møller C, Preston J, Cronk QB. 2000. Rapid radiation in Aframomum (Zingiberaceae): evidence from nuclear ribosomal DNA internal transcribed spacer (ITS) sequences. Edinb J Bot. 57:377–395.
- Ilic N, Schmidt BM, Poulev A, Raskin I. 2010. Toxicological evaluation of grains of paradise (Aframomum melegueta) [Roscoe] K. Schum. J Ethnopharmacol. 127:352–356.
- Ilic NM, Dey M, Poulev AA, Logendra S, Kuhn PE, Raskin I. 2014. Anti-inflammatory activity of grains of paradise (Aframomum melegueta Schum) extract. J Agric Food Chem. 62:10452–10457.
- Inegbenebor U, Ebomoyi MI, Onyia KA, Amadi K, Aigbiremolen AE. 2009. Effect of aqueous extract of alligator pepper (Zingiberaceae Aframomum melegueta) on gestational weight gain. Niger J Physiol Sci. 24:165–169.
- Kamtchouing P, Mbongue G, Dimo T, Watcho P, Jatsa H, Sokeng S. 2002. Effects of Aframomum melegueta and Piper guineense on sexual behaviour of male rats. Behav Pharmacol. 13:243–247.
- Kanno S-I, Shouji A, Asou K, Ishikawa M. 2003. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. J Pharmacol Sci. 92:166–170.
- Kenmogne M, Prost E, Harakat D, Jacquier M-J, Frédérich M, Sondengam LB, Zeches M, Waffo-Téguo P. 2006. Five labdane diterpenoids from the seeds of Aframomum zambesiacum. Phytochemistry. 67:433–438.
- Kim H-J, Yeom S-H, Kim M-K, Shim J-G, Paek I-N, Lee M-W. 2005. Nitric oxide and prostaglandin E2 synthesis inhibitory activities of diarylheptanoids from the barks of Alnus japonica Steudel. Arch Pharmacal Res. 28:177–179.
- Kim HP, Son KH, Chang HW, Kang SS. 2004. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 96:229–245.
- Kress WJ, Liu A-Z, Newman M, Li Q-J. 2005. The molecular phylogeny of Alpinia (Zingiberaceae): a complex and polyphyletic genus of gingers. Am J Bot. 92:167–178.
- Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. 2005. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci USA. 102:8369–8374.
- Kuete V. 2010. Potential of Cameroonian plants and derived products against microbial infections: a review. Planta Med. 76:1479–1491.
- Kuete V, Ango PY, Yeboah SO, Mbaveng AT, Mapitse R, Kapche GD, Ngadjui BT, Efferth T. 2014. Cytotoxicity of four Aframomum species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines. BMC Complement Altern Med. 14:340.
- Kuete V, Efferth T. 2010. Cameroonian medicinal plants: pharmacology and derived natural products. Front Pharmacol. 1:123–127.
- Kuete V, Krusche B, Youns M, Voukeng I, Fankam AG, Tankeo S, Lacmata S, Efferth T. 2011. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J Ethnopharmacol. 134:803–812.
- Larsen K, Lock J, Maas H, Maas P. 1998. Zingiberaceae. New York (NY): Springer. p. 474–495.
- Lawal B, Aderibigbe A, Essiet G, Essien A. 2007. Hypotensive and antihypertensive effects of Aframomum melegueta seeds in humans. Int J Pharmacol. 3:311–318.
- Li Y, Yang Z-Y, Wang M-F. 2009. Synthesis, characterization, DNA binding properties and antioxidant activity of Ln(III) complexes with hesperetin-4-one-(benzoyl) hydrazone . Eur J Med Chem. 44:4585–4595.
- Lock J, Hall J. 1975. Taxonomic studies in the genus Aframomum (Zingiberaceae). Boissiera. 24a:225–231.
- Mbongue G, Kamtchouing P, Dimo T. 2012. Effects of the aqueous extract of dry seeds of Aframomum melegueta on some parameters of the reproductive function of mature male rats. Andrologia. 44:53–58.
- Middleton E, Kandaswami C, Theoharides TC. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 52:673–751.
- Miyoshi N, Nakamura Y, Ueda Y, Abe M, Ozawa Y, Uchida K, Osawa T. 2003. Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in Human T lymphoma Jurkat cells. Cancer Lett. 199:113–119.
- Mosquera OM, Correa YM, Buitrago DC, Niño J. 2007. Antioxidant activity of twenty five plants from Colombian biodiversity. Mem Inst Oswaldo Cruz. 102:631–634.
- Nwozo SO, Oyinloye BE. 2011. Hepatoprotective effect of aqueous extract of Aframomum melegueta on ethanol-induced toxicity in rats. Acta Biochim Pol. 58:355–358.
- Obike H, Ezejindu D, Akingboye A. 2014. The effects of Aframomum melegueta aqeous extract on the adrenal gland of adult Wistar rats. Int J Med Health Sci. 3:1–6.
- Okigbo R, Igwe D. 2007. Antimicrobial effects of Piper guineense ‘Uziza’ and Phyllantus amarus ‘Ebe-benizo’ on Candida albicans and Streptococcus faecalis . Acta Microbiol Immunol Hung. 54:353–366.
- Okogbenin O, Emoghene A, Okogbenin E, Airede C. 2014. Antifungal effect of polar and non polar extracts of Aframomum Sceptrum on two isolates of oil palm. J Appl Sci Environ Manag. 18:173–183.
- Özçelik B, Kartal M, Orhan I. 2011. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm Biol. 49:396–402.
- Park H-H, Lee S, Son H-Y, Park S-B, Kim M-S, Choi E-J, Singh TS, Ha J-H, Lee M-G, Kim J-E. 2008. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharmacal Res. 31:1303–1311.
- Rice-Evans C, Miller N, Paganga G. 1997. Antioxidant properties of phenolic compounds. Trends Plant Sci. 2:152–159.
- Shahid AA. 2012. Biological activities of extracts and isolated compounds from Bauhinia galpinii (Fabaceae) and Combretum vendae (Combretaceae) as potential antidiarrhoeal agents. Pretoria (South Africa): University of Pretoria.
- Shaw J, Lickey EB, Schilling EE, Small RL. 2007. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot. 94:275–288.
- Smith PM. 1976. The chemotaxonomy of plants. London: Edward Arnold.
- Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, Iwanaga T, Kameya T, Kawai Y, Saito M. 2013. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br J Nutr. 110:733–738.
- Tane P, Tatsimo SD, Ayimele GA, Conolly JD. 2005. Bioactive metabolites from Aframomum species. 11th NAPRECA symposium book of proceedings, Antananarivo, Madagascar. p. 214–223.
- Tchuendem MH, Mbah JA, Tsopmo A, Ayafor JF, Sterner O, Okunjic CC, Iwu MM, Schuster BM. 1999. Anti-plasmodial sesquiterpenoids from the African Reneilmia cincinnata. Phytochemistry. 52:1095–1099.
- Tomla C, Kamnaing P, Ayimele GA, Tanifum EA, Tsopmo A, Tane P, Ayafor JF, Connolly JD. 2002. Three labdane diterpenoids from Aframomum sceptrum (Zingiberaceae). Phytochemistry. 60:197–200.
- Ukeh DA, Birkett MA, Pickett JA, Bowman AS, Mordue AJ. 2009. Repellent activity of alligator pepper, Aframomum melegueta, and ginger, Zingiber officinale, against the maize weevil, Sitophilus zeamais. Phytochemistry. 70:751–758.
- Uzeh RE, Oguntosin DO. 2013. Efficacy of essential oils from some African spices against two strains of Bacillus cereus isolated from vegetable salad. J Food Res. 2:48–54.
- Vidari G, Finzi PV, de Bernardi M. 1971. Flavonols and quinones in stems of Aframomum giganteum. Phytochemistry. 10:3335–3339.
- Wabo HK, Tane P, Connolly JD. 2006. Diterpenoids and sesquiterpenoids from Aframomum arundinaceum. Biochem Syst Ecol. 34:603–605.
- Zhang X, McNaughton PA. 2006. Why pain gets worse: the mechanism of heat hyperalgesia. J Gen Physiol. 128:491–493.