Abstract
Objectives. To compare valve-related morbidity among patients aged <70 and ≥70 years, receiving either a mechanical or a biological prosthesis in a population-based setting. Design. In total, 3279 patients (21 644 patient-years) were followed up through the Swedish National In-Patients Register, which registers all hospital admissions. Death, thromboembolism, bleeding, endocarditis, valve thrombosis and reoperations were all captured. Results. Survival was lower among patients aged <70 years with a bioprosthesis compared to a mechanical prosthesis (p < 0.0001), but equal among older patients. A mechanical prosthesis indicated a lower risk (p < 0.001) of thromboembolism (1.5% per patient year, ppy), than bioprosthesis (2.6% ppy), irrespective of age. Bleeding was increased (p = 0.002) with a mechanical prosthesis (1.7% ppy) compared to a bioprosthesis (1.1% ppy); the risk of bleeding increased early (<5 years) whereas thromboembolism increased late (>5 years). Event-free survival was higher in younger patients with a mechanical prosthesis compared to bioprosthesis recipients (p < 0.001), but equal among older patients. Conclusions. Survival was comparable in older patients irrespective of prosthesis type. Bleeding was increased with a mechanical prosthesis, especially in the elderly. The risk of thromboembolism was higher in patients with a bioprosthesis.
One major issue in attempts to establish as good a prognosis as possible after heart valve replacement is the choice of the optimal type of valve prosthesis for each individual patient. In this process, the patient's age at implantation is the major driving force. A mechanical valve is often chosen in younger patients due to its superior durability, although it implies a life-long risk of anticoagulant-related complications (Citation1–6). In older patients, the use of a bioprosthesis has been advocated for two major reasons: firstly, it is considered beneficial to be able to avoid oral anticoagulation in this group because of the increased risk of bleeding in the elderly (Citation5–10); and secondly, there is the advantage of a low risk of structural valve deterioration and thereby reoperation in older patients (Citation4,Citation11–13).
Today there is also a trend towards implanting bioprostheses in patients aged <70 years and thereby exposing them to more patient-years and also to ageing with their prosthesis (Citation14,Citation15).
Few studies have focused on valve-related complications with special attention to age and ageing and to the type of prosthesis. No study has investigated these complications at a population-based level over a very long time-period.
In the present study, the Swedish National In-Patients Register, in which every admission to hospital nation-wide is recorded, was used to study valve-related complications after heart valve surgery. Valve-related morbidity was compared among patients aged <70 years and ≥70 years receiving either a bioprosthesis or a mechanical prosthesis.
Material and methods
Patients
From 1 January 1985 to 31 December 2003, 3498 adult patients underwent primary heart valve replacement at Uppsala University Hospital. Of these patients, 219 died before hospital discharge (191 patients died within 30 days from surgery and 28 patients died in hospital after more than 30 days without ever being discharged). The 3279 patients discharged alive from their hospital stay constituted the basis of the present study.
Of these 3279 patients, 2176 received a mechanical prosthesis and 1103 a bioprosthesis. There were 1973 men (60%) and 1306 women (40%) with a mean age of 66 years, and 44% of the patients were aged ≥70 years. Concomitant coronary artery bypass grafting (CABG) was performed in 1289 (39%) of the patients. The majority of the patients were in New York Heart Association (NYHA) classes IIIA and IIIB (83%).
Selection of the type of prosthesis was mainly based on surgeon preference, heart rhythm and patient request. Mechanical prosthesis recipients were recommended life-long treatment with anticoagulants, while patients with a bioprosthesis were recommended three months treatment unless there were other indications for anticoagulation. Patients were advised to maintain an International Normalized Ratio (INR) within 2.0–3.0.
Data collection
All clinical data were collected at a preoperative interview and stored in a computer.
Follow-up and outcome
All patients were assigned a date of death or identified as being alive by computerized linkage of two national registers: the Swedish Cause of Death Register and a continuously updated population register. The last follow-up date with respect to survival was 31 December 31 2003. The total follow-up amounted to 21 644 years; in patients with a mechanical prosthesis 16 284 patient-years (mean 7.5), and in bioprosthesis recipients 5360 patient-years (mean 4.9).
The date of hospital discharge was used as the starting point of the study. Every death or hospital readmission due to a valve-related event (defined as an embolic event, bleeding, prosthetic valve endocarditis, valve thrombosis, or reoperation, according to the Guidelines for Reporting Morbidity and Mortality after Cardiac Valvular Operations (Citation16), was identified by computerized linkage to the National In-Patients Register. The National In-Patient Register (Center of Epidemiology, National Board of Health and Welfare) registers all admissions to all Swedish hospitals, and a primary diagnosis, according to the International Classification of Diseases ICD-8: 1968–1986; ICD-9: 1987–1996; and ICD-10: 1997–, is assigned to every admission at discharge. Times to death or hospital readmission due to the ICD diagnostic codes corresponding to an embolic event, bleeding, prosthetic valve endocarditis, valve thrombosis, or reoperation were analyzed.
Statistical analyses
Patients’ survival and valve-related complications were determined by Kaplan-Meier actuarial analysis and expressed as percentage of patients who were event-free. Outcomes in the different groups were compared by using the log-rank method. Incidence rates of complications were also calculated in linearized fashion, expressed as percentage per patient year (ppy), and compared by the likelihood ratio test.
Standard Cox proportional hazards regression was used to determine independent predictors of all time-related events, i.e. death (from any cause), first embolic event, first hemorrhagic event, or any first valve-related complication or death (event-free survival). The relative hazard (RH) [i.e. exp(β1)] with 95% confidence intervals, was used as a measure of the risk of death in different categories, where β1 is the basic parameter in the Cox model.
Continuous variables were used in a logarithmic form (age) or with a set of dummy variables representing ranges, defined by commonly used or standard cut-off points (attained age at an event). The RH ratios and their 95% confidence intervals are in general given for the variable in both the optimal continuous form and the dichotomized form, as this way of presenting the results was considered most informative. A set of dummy variables was used for analysis of categorized variables.
As the basic models used imply the assumption that the RHs are constant over time, separate models were estimated for follow-up at <5–, 5–10, and ≥10 years after surgery. Further, stratified regression models were used for analysis of the interaction between type of prosthesis and age. Interaction was then tested by introduction of an interaction term.
All risk factor analyses were stratified for ages <70 and ≥70 years at the time of surgery.
Results
Mortality
The total 30-day mortality in all patients who underwent primary heart valve replacement during the study period was 5.7% (201/3498). In total there were 961 deaths during the follow-up period; of these deaths were cardiac, 56% (non-prosthesis–related). Valve-related deaths accounted for 10% of late deaths and 34% were non-cardiac.
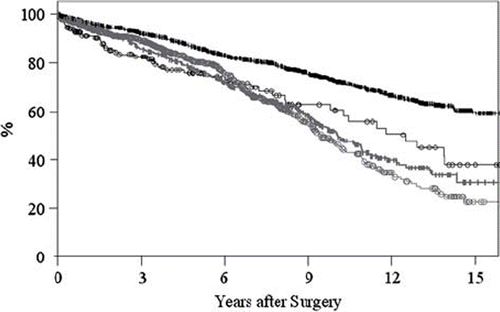
Among younger patients, those with a bioprosthesis had lower survival than recipients of a mechanical prosthesis, whereas there was no difference in survival in older patients between the two prosthesis groups (p < 0.0001; ).
Valve-related complications
Thromboembolism. The incidence of thromboembolism was increased (p < 0.0001) in patients a bioprosthesis (142 events, 2.6% ppy) compared to a mechanical prosthesis (248 events, 1.5% ppy; ). The incidence rate of thromboembolism was not related to age in either of the two types of prosthesis.
Table I. Linearized rates (per patient year) of prosthesis-related complications and number of patients with repeated events (n = 3279).
More than one event occurred in 59 patients. In 11 patients the thromboembolism was the cause of death without associated hospitalization. Mortality in relation to an embolic event (within 30 days) was 14% (53 events).
Multivariate analyses showed that a mechanical prosthesis decreased the risk of at least one thromboembolic event (RH = 0.5) in both age-groups ().
Table II. Number of first events and relative hazards associated with a mechanical prosthesis versus bioprosthesis; multivariate analysis* based on the study cohort (n = 3279).
Analyses that allowed the RH to change over time were made separately (). During the first five-year period the risk for thromboembolism was comparable for mechanical prosthesis and bioprosthesis; whereas after five years, patients with a bioprosthesis had increased risk compared to patients with a mechanical one.
Table III. Comparison of relative hazards at <5 years, 5–10 years, and >10 years after heart valve surgery, in patients with a mechanical prosthesis. Reference is patients with bioprosthesis in the corresponding time interval.
Bleeding. Bleeding was more common in patients with a mechanical prosthesis; 271 bleeding episodes (1.7% ppy) compared to 57 (1.1% ppy) in bioprosthesis recipients (p = 0.002; ). In eight patients the hemorrhage was the cause of death without associated hospitalization. Of the hemorrhages, 14% (46 events) were fatal.
Bleeding was equally common in younger patients among the two types of prosthesis (p = 0.3). Older patients with a mechanical prosthesis had a higher incidence of bleeding (2.1% ppy) as compared to younger ones (1.5% ppy, p < 0.001; ). Among patients with a bioprosthesis, patients age was not related to the incidence of bleeding.
In patients aged ≥70 years a mechanical prosthesis increased the risk of bleeding (RH = 1.9; ).
Compared to a bioprosthesis, a mechanical prosthesis was associated with an increased risk of bleeding in analyses of RH over time. After five years, the risk of a bleeding event was comparable between the two types of prosthesis ().
Other valve-related complications. In total, bacterial endocarditis occurred on 144 occasions in 88 patients, and more than once in 12 patients. Prosthetic-valve endocarditis was more common (p = 0.005) in patients with a bioprosthesis (44 events, 0.8% ppy) than in those with a mechanical prosthesis (79 events, 0.5% ppy; ). Younger bioprosthesis recipients had the highest incidence of endocarditits (1.4% ppy).
Mechanical complications occurred in 30 patients with a mechanical prosthesis and in two patients with a bioprosthesis. There were 38 reoperations for valve-related complications, 23 in patients (0.15% ppy) with a mechanical prosthesis and 15 (0.3% ppy) with a bioprosthesis.
Event-free survival (i.e. freedom from any valve-related complication and death)
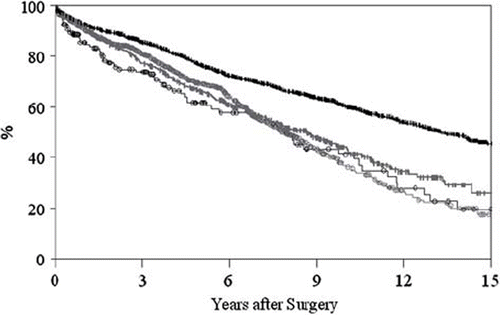
Younger patients with a mechanical prosthesis had superior event-free survival compared to bioprosthesis recipients in the same age-group (p < 0.0001; ). In the older group, there was no significant difference in event-free survival between patients with the two types of prosthesis.
Figure 2. Event-free survival in relation to age and type of prosthesis in 3279 patients who underwent heart valve surgery during 1985–2003. Vertical black bars = Mechanical <70 years. Black circles = Biological <70 years. Vertical grey bars = Mechanical ≥70 years. Grey circles = Biological≥70 year.
Discussion
The principal findings in the present study were:
Among older patients, there was no difference in survival between the two prosthesis groups, whereas among younger patients those with a bioprosthesis had lower survival than patients with a mechanical prosthesis.
Thromboembolism was increased with a bioprosthesis compared to a mechanical prosthesis irrespective of age.
A mechanical prosthesis was associated with an increased risk of bleeding especially in older patients.
Analyses of time trends of bioprothesis and thromboembolism showed an increased risk especially late after surgery (>5 years), whereas a mechanical prosthesis was associated with an increased risk of bleeding early (<5 years) after surgery.
Event-free survival was superior in younger patients with a mechanical prosthesis but was not influenced by valve type in older patients.
Our study was based on 3279 consecutive patients in a defined geographical area. With a mean follow-up of 6.6 years corresponding to 21 644 patient-years this analysis permits valid conclusions to be drawn on valve-related complications.
The follow-up was conducted through computerized linkage with the National In-Patients Register. This register has high validity (Citation17) and should provide reliable information. The use of this register only captured the adverse events which lead to hospital admission. Thus this study may have underestimated valve-related morbidity; however, all clinically important events should have been identified.
The incidence rates of valve-related complications in this study were similar to expectations (Citation1–4, Citation12,Citation15). Although this study also focused on age and type of prosthesis, this should not affect the data concerning hospital admission and diagnosis of a valve-related complication.
Only two randomized trials have compared patient outcomes in association with mechanical and bioprosthetic valves; one concluded that there were no difference in survival rate between the two groups, a higher reoperation rate in the bioprosthesis group and more hemorrhage in the mechanical group (Citation2). The other trial concluded that survival was higher with aortic mechanical than with aortic biological valves, that bleeding was increased with a mechanical valve, and that there was no difference in thromboembolism between patients with the two types of prosthesis (Citation3).
A contrasting finding in the present study is the increased incidence of thromboembolism associated with bioprostheses, especially late after surgery. We believe that other studies may have underestimated the incidence of embolism since they did not report from a National In-Patients Register. In our study, the risk for embolism increased over time and was highest very late (>10 years) after surgery. It is reasonable to assume that the risk-factor burden for thromboembolism, such as age, heart failure and atrial fibrillation accumulate over time and therefore anticoagulation treatment would serve protectively in this group of patients. We believe that some patients with a bioprosthesis are undertreated with regard to antithrombotic or anticoagulant agents. Approximately one-third to one half of all bioprosthesis require anticoagulation at some time during long-term follow-up, mainly because of atrial fibrillation (Citation1). In addition, one must consider the current trend with increasing use of bioprosthesis in patients aged 55–70 years in order to avoid anticoagulation treatment. In these borderline patients, the risk of the anticoagulation therapy must be balanced against the need for a reoperation. Geldorp et al. showed that the lifetime risk of reoperation with a bioprosthesis might be as high as 35% in a 55-year-old patient (Citation6). The present results show the increased risk of thromboembolism over time must also be considered when choosing a prosthesis.
Not surprisingly, bleeding was more frequent in patients with a mechanical prosthesis. In relation to age, there was no difference in bleeding between the two types of prosthesis in younger patients. On the contrary, older patients had more bleeding events than did younger ones. In the older age-group, the risk was increased during the entire period after surgery, but even more pronounced during the initial five years. Earlier studies showed that international normalized ratio (INR) variability, and thereby increased bleeding, were associated with both high age and advanced NYHA class (Citation18–21). Therefore, there should be special focus on increasing anticoagulation control in the elderly. This should include close long-term follow-up, patient education and self-management when possible.
The essential question is which prosthesis offers the better prognosis in each individual patient? Event-free survival provides a composite measure of most competing risks involved.
Survival was increased among younger patients with a mechanical prosthesis, however, one must consider that the patients considered for a bioprosthesis in younger ages often have a large burden of comorbidities, reducing survival per se.
In this study, an interaction was found between type of prosthesis and age, in that a bioprosthesis was associated with decreased event-free survival in patients aged <70 years. In addition, ageing with a mechanical prosthesis was associated with increasing risk of an adverse event; however, there was no such trend in patients ageing with a bioprosthesis.
The risk of reoperation for structural valve deterioration in patients receiving a bioprosthesis at an older age is low. In our study, the 10-year survival in patients aged ≥70 years was approximately 50%. Considering that the risk of structural valve deterioration in this age-group is <15% (Citation15) , reoperation will be necessary in very few of the older patients receiving a bioprosthesis.
In conclusion, the present study showed equal survival in patients aged ≥70 irrespective of type of prosthesis. The incidence of bleeding was increased in older patients with a mechanical prosthesis especially in the first five years after surgery. Thromboembolism was higher with a bioprosthesis and even more pronounced very late after surgery (>10 years).
Acknowledgements
Financial support for this study was provided by the Swedish Heart Lung Foundation. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Oxenham H, Bloomfield P, Wheatley DJ, Lee RJ, Cunningham J, Prescott RJ, . Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89:715–21.
- Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152–8.
- Hanania G, Michel PL, Montely JM, Warembourg H, Nardi O, Leguerrier A. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve in patients between 60 and 70 years of age. J Am Coll Cardiol. 2002;39(Suppl A):423A.
- Peterseim D, Can Y-Y, Cheruvu S, Landolfo K, Bashore TM, Lowe JE. Longterm outcome after biological versus mechanical aortic valve replacement in 841 patients. J Thorac Cardiovasc Surg. 1999;335:587–9.
- Schelbert EB, Vaughan-Sarrazin MS, Welke KF, Rosenthal GE. Valve type and long-term outcomes after aortic valve replacement in older patients. Heart. 2008;94:1181–8.
- Van Geldorp M, Jamieson E, Kappetein P, Ye J, Fradet GJ, Eijkemans MJ. Patients outcome after aortic valve replacement with a mechanical or biological prosthesis: Weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881–6.
- Milano A, Guglielmi C, De Carlo M, Di Gregorio O, Borzoni G, Verunelli F, . Valve-related complications in elderly patients with biological and mechanical aortic valves. Ann Thorac Surg. 1998;66:S82–7.
- Hanania G. Which heart valve prosthesis for patients aged between 60 and 70 years? Heart. 2003;89:481–2.
- Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A. Bleeding complications of oral anticoagulant treatment: An inception-cohort, prospective collaborative study. Lancet. 1996;348:423–8.
- Birkmeyer N, Birkmeyer JD, Tosteson A, Grunkemeier GL, Marrin C, O'Connor GT. Prosthetic valve type for patients undergoing aortic valve replacement. Ann Thorac Surg. 2000;70:1946–52.
- Bloomfield P. Choice of heart valve prosthesis. Heart. 2002;87:583–9.
- Grunkemeier G, Naftel DC, Starr A, Rahimtoola SH. Long-term performance of heart valve prostheses. Curr Probl Cardiol. 2000;25:73–156.
- Yun KL, Miller DC, Moore KA, Mitchell RS, Oyer PE, Stinson EB. Durability of the Hancock MO bioprosthesis compared with the standard aortic valve bioprosthesis. Ann Thorac Surg. 1995;60:s221–8.
- Brown J, O'Brien S, Wu C, Sikora J, Griffith B, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years. Changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137: 82–90.
- Rahimtoola SH. Choice of prosthetic heart valve in adults. J Am Coll Cardiol. 2010;55:2413–26.
- Edmunds LH Jr, Clark RE, Cohn LH, Miller DC, Weisel RD. Guidelines for reporting morbidity and mortality after cardiac valvular operations. Ann Thorac Surg. 1996;62:932–5.
- Hammar N, Larsen FF, de Faire U. Are geographical differences and time trends in myocardial infarction incidence in Sweden real? Validity of hospital discharge diagnoses. J Clin Epidemiol. 1994;47:685–93.
- Butchart EG, Payne N, Hui-Hua L, Buchan K, Mandana K, Grunkemeier GL. Better anticoagulation control improves survival after valve replacement. J Thorac Cardiovasc Surg. 2002;123:715–23.
- Cannegeiter SC, Rosendaal F, Briet E. Tromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41.
- Holper K, Wottke M, Lewe T, Baumer L, Meisner H, Paek SU. Bioprosthetic and mechanical valves in the elderly: Benefit and risks. Ann Thorac Surg. 1995;60:S443–6.