Abstract
Objectives. The management of sternal defects arisen after deep sternal wound infection is challenging and often requires extensive interdisciplinary teamwork between plastic and thoracic surgeons. In this study, the published literature on methods used to reconstruct sternal defects arisen as a result of deep sternal wound infection after open-heart surgery will be reviewed. Design. The Cochrane, Embase, PubMed, and SveMed + databases were searched in December 2011. Only papers regarding treatment of deep sternal wound infection after open-heart surgery in adults were included. Results. The literature search identified 224 original papers that met the inclusion criteria. The majority dealt with surgical techniques. None of the studies regarding reconstructive options were designed as randomized controlled trials, and the levels of evidence are generally low. Conclusion. The treatment of deep sternal wound infection has evolved considerably, but there is still little consensus regarding optimal surgical management and a general lack of a standard treatment protocol. The use of muscle flap transposition is well documented. Recent studies recommend the use of topical negative pressure therapy as an adjunct to surgical reconstruction.
Introduction
In 1957, Julian et al. performed the first median sternotomy, which is today a standard procedure in open-heart surgery. The procedure provides free access and a good overview of the thoracic organs, but with the introduction of a new procedure followed a new set of complications. One of the most severe being deep sternal wound infection (DSWI) with necrosis of the sternum. Today, DSWI post open-heart surgery (OHS) is a relatively rare complication with a reported global incidence in the range of 0.25–2.8% (Citation1–3) and a Nordic incidence in the range of 0.7–2.5%. However, due to a 30-day mortality in the range of 0–28% (Citation4,Citation5) and a significantly reduced long term survival compared to patients undergoing uncomplicated open heart surgery (Citation2,Citation3), DSWI is indeed a feared condition.
The sternal necrosis leaves a defect in the thoracic wall and thus exposes the thoracic organs, wherefore there is a need for reconstruction. The treatment of these patients is handled on a day-to-day basis by the thoracic surgery departments, but as the reconstruction often involves flap surgery, the reconstruction itself is often handled as an interdisciplinary teamwork between plastic and thoracic surgeons.
Since 1957, the treatment strategies for DSWI have undergone major advancements. Today, the treatment covers clearing of the sternal infection by debridement, at times treatment with topical negative pressure therapy and subsequently reconstruction with final closure of the sternal defect. Early treatment strategies primarily consisted of open wound care. In the ensuing period, the strategy changed to closed, retrosternal, and catheter based antibiotic irrigation. This treatment reduced mortality and morbidity, and is still, although in an updated version, a part of today's treatment. In 1976, Lee et al. described the use of transposed omentum to close sternal defects, and in 1980, Jurkiewicz et al. described the use of muscle flaps to reconstruct sternal defects. Since then, a variety of muscle flaps have been used to close mediastinum after sternal resection. The use of muscle flaps lowered the mortality even further and is today a well-integrated part of the treatment (Citation6).
Before reconstruction of the sternal defect, the wound must be debrided of necrotic tissue. This is achieved by a combination of removal of surgical wires and sutures, surgical debridement until the appearance of fresh bleeding tissue, open catheter irrigation, systemic, and local antibiotics (Citation6,Citation7). Debridement can be performed either as an independent procedure before the reconstruction, called a two-stage procedure, or as a single stage procedure where the wound is debrided and flaps are applied in the same operation. The single stage approach is recommended in recent studies as it yields shorter hospital stay and shorter postoperative periods of intensive care (Citation6). Furthermore, recent studies recommend the use of topical negative pressure (TNP) as an adjunct to surgical reconstruction as it promotes healing of the wound (Citation1,Citation4,Citation8–11). The size of the sternal defect determines how much tissue is needed for the reconstruction and thus directly influences the choice of flap. If the sternal defect is large, a supplementary skin graft may be necessary in order to avoid unnecessary wound tension.
Thus, during the last 50 years, there have been considerable improvements in the survival and morbidity of the complication. This is attributable to the wide range of treatment options made available by advancements in medical and surgical science. However, there is a general lack of consensus regarding which treatment regimes and reconstructive techniques are best suited for the complication. Even though various attempts at proposing treatment algorithms have been made, none has reached a general acceptance. This justifies an extensive literature review of available reconstructive options, as each surgeon dealing with this type of defect must be aware of a variety of reconstructive options in order to make an individual assessment of the most suitable treatment protocol in each patient's case.
Aim
In this study, the published literature on methods used to reconstruct sternal defects arisen as a result of deep sternal wound infection after open-heart surgery will be reviewed.
Search strategy
In an initial electronic search, the Cochrane library, Embase, PubMed, and SveMed + databases were searched. The search were conducted using combinations of the following search terms: “deep sternal wound infection,” “flap,” “mediastinitis,” “open heart surgery,” “reconstructive surgical procedures,” “sternum,” and “surgical flap.” Furthermore, a series of more specific searches were conducted in PubMed with the following search terms: “latissimus dorsi sternal wound infection,” “omental transposition sternal wound infection,” “pectoralis major sternal wound infection,” “rectus abdominis sternal wound infection,” “VAC sternal wound infection,” and “TNP sternal wound infection.” All searches were performed on the 8th of December, 2011.
Inclusion and exclusion criteria
Only studies on human populations regarding the treatment and reconstruction of sternal defects arisen after deep sternal wound infection post open-heart surgery were included. If the studied population included children, it was excluded. Papers written in languages other than English or the Nordic languages were also excluded. The reference lists from the included articles were examined for additional relevant papers. After reviewing the reference lists, review articles were excluded. All included articles were classified according to the type of study by the primary author. The evidence level of each article was subsequently classified according to the Oxford Centre for Evidence-Based Medicine Levels of Evidence scale. Each article received a grade according to its medical evidence level, ranging from level I (highest level of evidence, i.e., randomized controlled trials) to level V (lowest level of evidence, i.e., expert opinions).
Results
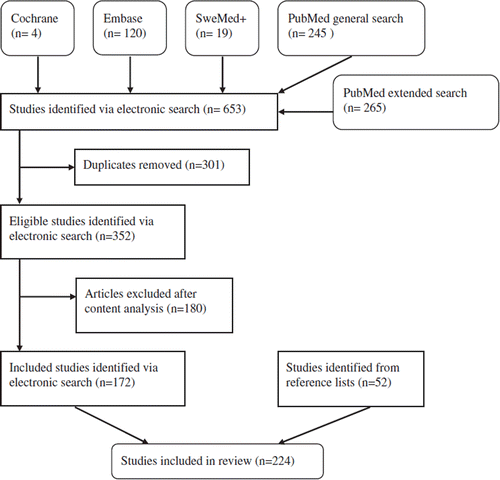
In the initial search, 103 original papers met the inclusion criteria. The searches aimed at the specific reconstructive surgical procedures identified 128 original papers, where 69 papers were not found in the initial search. An additional 52 articles were identified from reference list. Thus, a total of 224 original papers were included in the literature review. depicts a flow diagram of the search process. shows the distribution of levels of evidence in the included articles. No randomized controlled trials regarding flap surgery were identified and almost all identified studies regarding surgical flaps were either observational or retrospective studies. shows the included papers organized into groups according to their main outcome measure as well as the range of medical evidence level in the groups. As can be seen in , few studies directly compare the outcome of different flap types. Furthermore, only one identified study directly compared TNP therapy with conventional muscle flap surgery.
Table I. The level of evidence of included studies.*
Table II. Included articles and their main outcome measure.
Due to publication practice, the number of references in the printed version of this article has been limited. For a complete Supplementary Reference list please see the online version with the following direct link to the article http://informahealthcare.com/doi/abs/10.3109/14017431.2012.674549.
Discussion
The lack of randomized controlled trial studies comparing different types of reconstructive surgery may be explained by ethical considerations, the complexity of the condition, and the generally small study populations. Almost all identified studies regarding surgical flap methods are retrospective/observational, which results in limitations in the interpretation of their results. The retrospective/observational aspect makes it difficult to control for confounding variables and bias, which may lead to incorrect conclusions. The small study populations increase the risk of statistical type II errors, where one fails to observe a difference when in truth there is one, thus indicating poor sensitivity. Furthermore, few articles directly compare the outcome of various reconstructive techniques. The method used for classification of deep sternal wound infection is differing among the studies. Studies often use either the guidelines specified in the US Centers for Disease Control and Prevention, the classification proposed by Pairolero and Arnold, the classification proposed by El Oakley and Wright or a variation of these. However, a great part of the identified studies do not apply a standard classification method, and quite a few articles fail to mention how they define deep sternal wound infection. Moreover, the studies often cover large periods of time, and one may presume that the advances made in operative techniques, anesthesiology, and postoperative care during the duration of the studies might influence the results. Furthermore, the surgeon in charge may favor certain flaps due to personal preferences, and the choice of flaps could thus be dependent on the surgeon, which would be reflected in their published literature.
Risk factors
Generally, patients developing DSWI have higher comorbidity when compared to patients undergoing uncomplicated OHS (Citation8). The most accepted risk factors for developing DSWI and risk factors associated with increased mortality for patients who have developed DSWI is directly associated with the patient's habitual condition. Diabetes, chronic obstructive pulmonary disease, body mass index (BMI) over 30, old age, long term use of corticosteroids, non-elective OHS, re-operation due to bleeding complications, kidney failure, and smoking are all accepted as independent risk factors for developing poststernotomy DSWI (Citation2,Citation3,Citation8). Additionally, the length of the operation, how long the patient is under cardiopulmonary bypass, longer times in respirator, re-thoracotomy, and high postoperative blood glucose are also accepted as independent risk factors (Citation8). A few studies have investigated various suture techniques as risk factors, but results remain sparse. Furthermore, macromastia has been proposed as a risk factor and is addressed in two identified studies that describe a technique for combining sternal reconstruction with reduction mammaplasty. Whether the use of internal mammary artery (IMA)/bilateral internal mammary artery (BIMA) for grafting purposes is a risk factor is still debated (Citation12). It seems that the use of IMA/BIMA grafts on high-risk patients should, if possible, be avoided. If the IMA/BIMA grafts are used, the side arteries from IMA should be ligated as close as possible to IMA's main trunk as this tends to preserve most possible of the collateral blood supply to the sternum. Furthermore, for diabetics, it is recommended to give pre-, peri-, and postoperative i.v. insulin infusion with a tight regulation of the blood glucose level in order to lower the risk of developing DSWI. Several studies find a significant relationship between the European System for Cardiac Operative Risk Evaluation (EuroSCORE) and the New York Heart Association (NYHA) classification and the risk of developing DSWI (Citation8).
Musculus pectoralis flap
Jurkiewicz et al. first described the use of m. pectoralis major flaps to close sternal defects in 1980. Since then, the use of either unilateral or bilateral m. pectoralis major flaps to close sternal defects arisen after DSWI has become an established practice and is frequently described as the preferred method (Citation6,Citation13). The muscles are close to the wound and easy to dissect, thus, the reconstructive procedure yields only a small additional trauma with no risk of hernia formation. If the pectoralis flap is lifted as an advancement flap based on the thoracoacromial artery and vein, it is independent of the blood perfusion from IMA and can thus be used on patients where the IMA is unavailable (Citation14). When using m. pectoralis major flaps, skin is mobilized and the wound can often be closed without tension (Citation13). Whether use of the pectoralis flap can be done without postoperative functional disability is debated. Several studies conclude that using m. pectoralis major flaps provide good chest case stability, and that the postoperative mobility of arm and shoulder is not significantly decreased. Netscher et al. concludes, however, that maximum and average pectoral strength, supination, and adduction of the shoulder joint are all significantly decreased when the pectoral flaps is used compared to patients that have undergone uncomplicated sternotomy. Similarly, Eriksson et al. finds a significant long-term shoulder and arm disability in one third of the patients reconstructed with pectoralis flaps (Citation15). Habitual lung function, measured as forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and FEV1/FVC, is rapidly re-attained after the operation whereby the patients may be extubated quickly, thus lowering the risk of re-infection (Citation13). In addition, reconstruction with pectoralis major flaps provides an acceptable aesthetic result. However, if the sternal defect is localized in the most caudal third of the sternum, the m. pectoralis flap may provide insufficient coverage if it cannot be elevated as a turnover flap based on perforators from the ipsilateral IMA (Citation13,Citation14,Citation16).
Rectus abdominis flap
Jurkiewics et al. first described the use of the rectus abdominis (RA) flap to close sternal defects after DSWI in 1980. Even though the RA flap is not a first choice flap today, it has proven to be useful for sternal reconstruction after DSWI, especially if the pectoralis flap is unavailable or does not provide sufficient coverage. One of the RA flap's advantages is its ability to cover defects in the most caudal third of the sternum where other flaps may have insufficient reach (Citation16). Furthermore, the RA flap provides ample volume to fill the dead-space, is located close to the sternal defect, and can be lifted as a musculocutaneous flap (Citation16). Harvesting the RA flap requires extensive dissection with a risk of ventral hernia formation and uneven contour of the abdominal wall. If the RA flap is based on a. and v. epigastrica superior, it may be unreliable if the ipsilateral IMA is not available (Citation13). However, several authors have successfully used an inter-costal artery based RA flap when the ipsilateral IMA is unavailable. Two studies describe a method where the RA flap is based on arteria epigastrica superior in spite of an unavailable IMA, although this requires the flap to be raised as a pure muscle flap. In a study published in 1997 by Cohen et al., FVC, FEV1, and FEV1/FVC were measured preoperatively and on average 10.6 months post operatively on patients who had undergone sternal reconstruction with, respectively, RA flap and m. pectoralis major flap. The study concluded that use of the RA flap provided a significantly worse postoperative lung function as compared to the pectoralis flap.
Omental transposition
In 1976, Lee et al. described the use of transposed omentum to cover mediastinal defects. Since then the use of omental transposition to cover sternal defects has been used less frequently as a result of the entry of muscular flaps (Citation6). With its rich blood supply and well-developed lymphatic system, several studies conclude that transposed omtentum still retains an important place in the reconstruction of sternum post DSWI, either alone or in combination with muscle flaps (Citation7,Citation17). The omentum has a high concentration of vascular endothelial growth factor and thus promotes neo angiogenesis in the area where it is placed. An omental flap can reach more or less every part of a sternal defect, is highly pliable, and can thus fill irregular defects sufficient. However, in order to transpose the omentum, one must enter into a visceral cavity with the risk of spreading infection and a risk of ventral hernia formation (Citation7,Citation13,Citation17). Several studies have reported donor-site hernias when transposing the omentum (Citation18). Only two identified studies directly compares the outcome of omentum transposition and muscle flaps to reconstruct sternal defects post DSWI. In the first study, Lopez-Monjardin et al. found that the sepsis related mortality was significantly decreased for omentum transposition as compared with pectoralis major flaps in patients undergoing reconstruction after DSWI post OHS. In spite of this, no differences were observed in total mortality between the two groups (Citation7). In the second study, Milano et al. found that the hospital stay for the omentum transposition group was significantly shorter as compared with a group of pectoralis flap recipients (Citation17).
Latissimus dorsi flap
The free latissimus dorsi (LD) flap is rarely used for sternal reconstruction after DSWI (Citation6,Citation13,Citation19). This is reflected in the literature search where few studies using the method were identified. The LD flap is independent of blood supply from the IMA and may be elevated as a musculocutaneous flap (Citation20). Additionally, LD flap usage is not associated with a risk of hernia formation. Use of the LD flap does not result in a significantly decreased postoperative FEV1 or a significant postoperative impairment of the shoulders strength or passive/active shoulder joint mobility (Citation20). However, the pedicled LD flap cannot sufficiently cover a sternal defect in the caudal third of the sternum. Furthermore, the patient has to be repositioned during surgery. Donor–site seroma is reported as a frequent complication in studies where the LD flap is used for breast reconstruction. Only a couple of studies have reported donor-site seroma when using the LD flap for sternal reconstruction after DSWI.
Other methods
A few identified studies describe the successful use of transverse or horizontal fixation of the sternum with titanium plates, either alone or in combination with other treatment modalities such as flap surgery or TNP therapy. The fixation of the sternum appears to provide a better thorax stability than flap surgery alone, which in turn allows for extubation to be performed faster (Citation21,Citation22). However, use of transverse or horizontal fixation of the sternum with plates of synthetic material does not seem to have gained general acceptance in the literature and results/reports are sparse. Furthermore, a few studies describe their use of advanced antibiotic treatment regimes, CO2 laser sterilization, electrolyzed strong acid in aqueous solution, and activated macrophage suspension. In addition, a single case report of a successful sternal reconstruction with the use of an osteocutaneous flap from the scapula was identified.
Topical negative pressure
TNP therapy is based on the application of a fixed subatmospheric pressure in the wound. The negative pressure increases local blood flow, decreases edema and increases the formation of granulation tissue. These factors are known to promote wound healing (Citation23). Furthermore, the negative pressure in the sternal defect keeps the sternal edges relatively closer together (Citation24). The approximation of the sternal edges provides extra stability to the thorax, thereby allowing for a faster extubation (Citation25). Furthermore, the use of TNP therapy rapidly allows the patients to regain mobility, resulting in a significant impact on nursing care requirement and a lowered risk for deep vein thrombosis (Citation1). The use of TNP as a supplement in the treatment of DSWI has proven a very effective method to control infection, promote wound healing and lower the mortality for patients suffering from post sternotomy DSWI (Citation1,Citation4,Citation8–11). In addition, progression of the wound healing can be easily monitored as TNP therapy allows for immediate wound inspection.
Recently, several studies have compared TNP therapy with conventional wound care strategies and concluded that use of TNP therapy provides lower risk of treatment failure, a significantly shorter hospital stay, faster control over the infection, and a significantly lowered short and long term mortality (Citation18,Citation26). The increased formation of granulation tissue presumably will lead to smaller defects, which in turn facilitates the use of smaller flaps. Thus, TNP therapy may influence which reconstructive method is used. Furthermore, a couple of studies have had success with TNP therapy as the sole treatment for DSWI and thus advocates a more restrictive use of flaps (Citation26). The use of TNP therapy might very well play a crucial part in lowering the mortality for DSWI even further, and thus the technique should be implemented as standard procedure prior to an eventual flap reconstruction.
TNP therapy is generally regarded as a very safe treatment method with few complications. However, a couple of cases of fatal right ventricular rupture during TNP therapy for DSWI have been reported (Citation27,Citation28). The complication is proposed to be a pure mechanical problem, and Malmsjö et al. have shown that inserting a rigid barrier over the heart may prevent it. Bapat et al. continues to conclude that use of TNP treatment for more than 3 weeks appears to be associated with recurrent problems of the sternal wound. Gdalevitch et al. have attempted to unveil predictors of TNP treatment failure and concludes that TNP treatment failure is significantly associated with a positive blood culture, a high degree of bony exposure and sternal instability as well as a wound depth of 4 cm or above (Citation22).
TNP therapy has thus proven a valuable component in the treatment of DSWI and may facilitate the use of smaller flaps, but the need for skin grafting for final closure of the sternal defect will prevail. With the recent development seen in the area of TNP therapy, we face a new predicament as the DSWI patients possible will be at the thoracic surgery departments for longer times before the plastic surgeons become involved. Hence, one must determine: how long will one be willing to use TNP therapy before deciding on plastic surgery options? As long as standard treatment algorithms are not available, a heightened focus on early interdisciplinary teamwork between plastic and thoracic surgeons when deciding upon a treatment plan for the individual patient is necessary. Furthermore, one must investigate how the TNP therapy aided results compare to traditional early plastic surgery flap treatment, not only in survival and disability statistics, but also in terms of quality of life for the patient. How does the outcomes of a relatively quick but extensive flap reconstruction compare to a smaller reconstructive procedure after a couple of weeks use of TNP therapy? Future studies focusing on this aspect of DSWI treatment are awaited.
It is as always important to evaluate each patient in order to find the best procedure. The number of different flap procedures used suggests that the ideal reconstructive flap procedure does not exist. At times a combination of different flaps and techniques are required, and it is thus important that the surgeon is familiar with several flaps and techniques.
The low levels of medical evidence presented in the identified studies make it difficult to develop evidence-based guidelines. Guidelines may still be presented, but it is of great significance to understand the limitations of the evidence on which the guidelines are based. In order to gain more valid knowledge, it is necessary to perform larger, prospective, internationally coordinated studies as well as decide on an internationally accepted standard for the classification of DSWI. Berdajs et al. recently presented a protocol for a randomized controlled trial, testing delayed primary versus late secondary wound closure. The results are greatly anticipated, and hopefully this may inspire new attempts of designing randomized prospective studies in the field of sternal reconstruction post DSWI.
Conclusion
Sternal reconstruction after DSWI still poses a complex therapeutic challenge. The literature mainly consists of studies with low-level evidence, and randomized controlled trials regarding flap surgery do not exist. Although reconstruction with flaps is well described in the literature, there is little consensus regarding optimal reconstructive technique and a general lack of a standard treatment protocol.
http://www.informahealthcare.com/10.3109/14017431.2012.674549
Download PDF (153.7 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Baillot R, Cloutier D, Montalin L, Cote L, Lellouche F, Houde C, . Impact of deep sternal wound infection management with vacuum-assisted closure therapy followed by sternal osteosynthesis: A 15-year review of 23,499 sternotomies. Eur J Cardiothorac Surg. 2010;37:880–7.
- Braxton JH, Marrin CA, McGrath PD, Morton JR, Norotsky M, Charlesworth DC, . 10-year follow-up of patients with and without mediastinitis. Semin Thorac Cardiovasc Surg. 2004;16:70–6.
- Filsoufi F, Castillo JG, Rahmanian PB, Broumand SR, Silvay G, Carpentier A, . Epidemiology of deep sternal wound infection in cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:488–94.
- Eyileten Z, Akar AR, Eryilmaz S, Sirlak M, Yazicioglu L, Durdu S, . Vacuum-assisted closure and bilateral pectoralis muscle flaps for different stages of mediastinitis after cardiac surgery. Surg Today. 2009;39:947–54.
- Ascherman JA, Patel SM, Malhotra SM, Smith CR. Management of sternal wounds with bilateral pectoralis major myocutaneous advancement flaps in 114 consecutively treated patients: Refinements in technique and outcomes analysis. Plast Reconstr Surg. 2004;114:676–83.
- Jones G, Jurkiewicz MJ, Bostwick J, Wood R, Bried JT, Culbertson J, . Management of the infected median sternotomy wound with muscle flaps. The emory 20-year experience. Ann Surg. 1997;225:766–76; discussion 776–8.
- Lopez-Monjardin H, de-la-Pena-Salcedo A, Mendoza-Munoz M, Lopez-Yanez-de-la-Pena A, Palacio-Lopez E, Lopez-Garcia A. Omentum flap versus pectoralis major flap in the treatment of mediastinitis. Plast Reconstr Surg. 1998;101:1481–5.
- Sjogren J, Nilsson J, Gustafsson R, Malmsjo M, Ingemansson R. The impact of vacuum-assisted closure on long-term survival after post-sternotomy mediastinitis. Ann Thorac Surg. 2005;80:1270–5.
- Domkowski PW, Smith ML, Gonyon DL Jr, Drye C, Wooten MK, Levin LS, . Evaluation of vacuum-assisted closure in the treatment of poststernotomy mediastinitis. J Thorac Cardiovasc Surg. 2003;126:386–90.
- Agarwal JP, Ogilvie M, Wu LC, Lohman RF, Gottlieb LJ, Franczyk M, . Vacuum-assisted closure for sternal wounds: A first-line therapeutic management approach. Plast Reconstr Surg. 2005;116:1035–40; discussion 1041–3.
- Chen Y, Almeida AA, Mitnovetski S, Goldstein J, Lowe C, Smith JA. Managing deep sternal wound infections with vacuum-assisted closure. ANZ J Surg. 2008;78:333–6.
- De Paulis R, de Notaris S, Scaffa R, Nardella S, Zeitani J, Del Giudice C, . The effect of bilateral internal thoracic artery harvesting on superficial and deep sternal infection: The role of skeletonization. J Thorac Cardiovasc Surg. 2005;129:536–43.
- Pairolero PC, Arnold PG, Harris JB. Long-term results of pectoralis major muscle transposition for infected sternotomy wounds. Ann Surg. 1991;213:583–9; discussion 589–90.
- Castello JR, Centella T, Garro L, Barros J, Oliva E, Sanchez-Olaso A, . Muscle flap reconstruction for the treatment of major sternal wound infections after cardiac surgery: A 10-year analysis. Scand J Plast Reconstr Surg Hand Surg. 1999;33:17–24.
- Eriksson J, Huljebrant I, Nettelblad H, Svedjeholm R. Functional impairment after treatment with pectoral muscle flaps because of deep sternal wound infection. Scand Cardiovasc J. 2011;45:174–80.
- Davison SP, Clemens MW, Armstrong D, Newton ED, Swartz W. Sternotomy wounds: Rectus flap versus modified pectoral reconstruction. Plast Reconstr Surg. 2007;120:929–34.
- Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg. 1999;67:377–80; discussion 380–1.
- Fuchs U, Zittermann A, Stuettgen B, Groening A, Minami K, Koerfer R. Clinical outcome of patients with deep sternal wound infection managed by vacuum-assisted closure compared to conventional therapy with open packing: A retrospective analysis. Ann Thorac Surg. 2005;79:526–31.
- Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg. 1994;93:1208–14; discussion 1215–6.
- Banic A, Ris HB, Erni D, Striffeler H. Free latissimus dorsi flap for chest wall repair after complete resection of infected sternum. Ann Thorac Surg. 1995;60:1028–32.
- Sansone F, Mossetti C, Bruna MC, Oliaro A, Zingarelli E, Flocco R, . Transomental titanium plates for sternal osteomyelitis in cardiac surgery. J Card Surg. 2011;26:600–3.
- Gdalevitch P, Afilalo J, Lee C. Predictors of vacuum-assisted closure failure of sternotomy wounds. J Plast Reconstr Aesthet Surg. 2010;63:180–3.
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 1997;38:563–76; discussion 577.
- Pettersson G, Larsson S, Sudow G, Holmstrom H. Use of muscle flaps in the treatment of infected sternotomy. Scand J Thorac Cardiovasc Surg. 1986;20:1–4.
- Calcaterra D, Garcia-Covarrubias L, Ricci M, Salerno TA. Treatment of mediastinitis with wound-vacuum without muscle flaps. J Card Surg. 2009;24:512–4.
- Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: Vacuum-assisted closure versus conventional treatment. Ann Thorac Surg. 2005;79:2049–55.
- Bapat V, El-Muttardi N, Young C, Venn G, Roxburgh J. Experience with vacuum-assisted closure of sternal wound infections following cardiac surgery and evaluation of chronic complications associated with its use. J Card Surg. 2008;23:227–33.
- Sartipy U, Lockowandt U, Gabel J, Jideus L, Dellgren G. Cardiac rupture during vacuum-assisted closure therapy. Ann Thorac Surg. 2006;82:1110–1.