Abstract
Objective. To investigate whether the effect of 48-month usage of coenzyme Q10 and selenium on cardiac function was different for participants with different levels of cardiac wall tension as measured by plasma levels of N-terminal natriuretic peptide (NT-proBNP) at baseline. Methods. A 48-month randomized double-blind controlled trial in a cohort of community-dwelling elderly (mean age 78 years) was carried out. A total of 443 participants were given coenzyme Q10 combined with selenium, or a placebo. NT-proBNP measured at baseline and 48 months was used to evaluate the cardiac wall tension. Results. After 48 months, supplementation of coenzyme Q10 and selenium had varying impacts depending on the severity of impairment of cardiac function. Analyses of the responses in the different quintiles of baseline NT-proBNP showed that those with active supplementation, and a plasma level of NT-proBNP in the second to fourth quintiles demonstrated significantly reduced NT-proBNP levels (p = 0.022) as well as cardiovascular mortality after 48 months (p = 0.006). Conclusion. Long-term supplementation of coenzyme Q10/selenium reduces NT-proBNP levels and cardiovascular mortality in those with baseline NT-proBNP in the second to fourth quintiles indicating those who gain from supplementation are patients with mild to moderate impaired cardiac function.
Key words::
Introduction
Coenzyme Q10 (ubiquinone) is present in all cells of the body and is an essential factor in the mitochondrial production of energy. Optimal level of coenzyme Q10 is therefore especially important in the cardiac muscle cells where the energy requirements are high. Decreases in the endogenous myocardial synthesis of coenzyme Q10 may lead to mitochondrial dysfunction, energy starvation, and oxidative stress of the cardiac muscle (Citation1–2). Increasing age results in a decreased synthesis of coenzyme Q10 (Citation3) and also increased elements of fibrosis of the cardiac cells can be seen (Citation4). Furthermore the incidence and prevalence of heart failure also increase with age (Citation5), and significantly lower plasma levels of coenzyme Q10 have been found in patients with cardiomyopathy compared with healthy controls (Citation6). Molyneux et al. (Citation7) reported that plasma coenzyme Q10 above the cut-point (0.73 μmol/L) was an independent predictor of survival in patients with heart failure. Supplementation of coenzyme Q10 in heart failure patients has in a recent meta-analyses (Citation8–9) been shown to lead to a small but significant improvement of cardiac systolic function. However, patients with the most severe heart failure seemed not to benefit from this intervention. This suggests that the effect of supplementation of coenzyme Q10 may vary depending on the severity of heart failure.
Selenium is another nutrient essential for vital processes within the body such as antioxidant defence, oxidative metabolism, and immune surveillance. A relationship between selenium and cardiovascular disease was discovered with Keshan disease, an endemic cardiomyopathy that was found to be more prevalent in areas with a deficiency of selenium (Citation10). In patients with acute coronary syndromes, Lubos et al. (Citation11) reported that low levels of selenium increased the risk of cardiovascular death. The suggestion has therefore been made that selenium-dependent antioxidant enzymes such as glutathione peroxidase (Gpx), thioredoxin reductase (TrxR), and selenoprotein P may prevent the development of cardiovascular disease. However, the efficacy of selenium supplementation on cardiovascular disease has not been well studied (Citation10). It is important to note that a complex relationship between coenzyme Q10 and selenium exists, where selenium is needed in order to activate coenzyme Q10 (Citation12). Also, the antioxidant effect of coenzyme Q10 requires continuous reduction of ubiquinone and regeneration to the active ubiquinol form that requires selenium (Citation13). Moreover, in an earlier study of heart failure patients, which was performed by members of this research group, a relative down-regulation of the Trx-regenerating protein— TrxR1—was demonstrated, which indicates a selenium deficiency (Citation14).
We have recently shown that dietary supplementation of coenzyme Q10 combined with selenium given to 443 community-dwelling elderly for 48 months decreased both deterioration in cardiac function and cardiovascular mortality (Citation15). However, the effect of supplementation of coenzyme Q10 seems to vary depending on different levels of cardiac function. Moreover, it has been discussed that heart failure patients with low levels of myocardial wall tension, as measured by N-terminal natriuretic peptide (NT-proBNP), are unlikely to benefit from treatment of heart failure, whereas those with high levels will die despite therapy (Citation16). One may therefore assume that participants with the lowest or highest cardiac wall tension might not have the same cardiac benefit from supplementation of Q10 and selenium. This therefore justified a sub-group analysis of our data to explore treatment effects of Q10 and selenium in participants with expected (moderate cardiac wall tension) versus unexpected (lower or higher cardiac wall tension) treatment effect. The aim of the present study was to investigate if the effect of 48 months intervention of coenzyme Q10 and selenium on cardiac function and cardiovascular mortality was different for participants with different levels of cardiac wall tension at baseline.
Methods
The study
Eligible subjects in this study were elderly persons who lived in a rural community of 10 300 inhabitants in the southeast of Sweden. The design of the study has been previously described (Citation15). Briefly, all inhabitants aged between 69 and 87 years were invited to participate in a study primarily designed to investigate the prevalence of impaired cardiac function and heart failure in an elderly population. Of 675 individuals invited to the present study, 443 agreed to dietary supplementation with coenzyme Q10 and selenium, or a placebo, and could thus be included. At baseline, all study participants were examined by a cardiologist, who took a new patient history and performed a clinical examination. Blood pressure was measured with the participant resting in a supine position, and blood samples were drawn after fasting overnight. All participants were evaluated every sixth month during the study period of 48 months. Diabetes mellitus was defined as an ongoing treatment for diabetes, or a blood glucose level above 7 mmol/L. Hypertension was defined as a previous diagnosis, or a resting blood pressure of more than 140/90 mm. Ischemic heart disease was defined as a history of angina pectoris, and/or myocardial infarction or changes on ECG indicating ischemic heart disease. A respiratory disease was established if the participant was diagnosed with such, or was undergoing treatment for obstructive lung disease. In the present study the analyses of the change in NT-proBNP levels were performed on the 211 participants (active n = 117 and placebo n = 94) who completed the 48-month study period and who had blood samples at both baseline and 48 months collected. Analyses of the systolic cardiac function through echocardiography were performed where the systolic function was expressed as ejection fraction and cardiovascular mortality was also analyzed on all participants included in the study (n = 443).
Measurement of cardiac function
NT-proBNP was used to measure the cardiac wall tension; this peptide provides information about myocardial wall tension and is sensitive to subtle alterations in cardiac filling pressures. Analysis of this peptide is frequently used as a diagnostic and prognostic instrument in the handling of heart failure patients (Citation5,Citation17). In the present study, we used plasma values of NT-proBNP collected at baseline, and at the end of the study (i.e., 48 months). Blood samples of NT-proBNP were drawn with the patient in a sitting position after overnight fasting and resting for 30 min. EDTA vials were used. The vials were chilled on ice before centrifugation at 3000 g, 4°C, and were then frozen at − 70°C. No sample was thawed more than twice. NT-proBNP was analyzed on the Elecsys 2010 platform (Roche Diagnostics, Mannheim, Germany). The total coefficient of variation was 4.8% at 220 ng/L and 2.1% at 4254 ng/L (n = 70) at our laboratory.
Doppler echocardiographic examinations (Accuson XP-128c) were performed with participants in the left lateral position. Normal systolic function was defined as ejection fraction (EF) ≥ 50%, and severely impaired systolic function was defined as EF less than 30%.
Study intervention
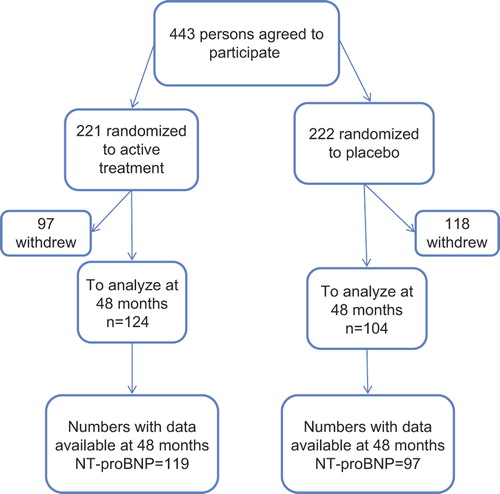
A flow chart of the study is given in . After informed consent, all participants were randomized in a double-blind manner and given either a combination of 200 mg/day of coenzyme Q10 (Bio-Quinon 100 mg B.I.D, Pharma Nord, Vejle, Denmark) and 200 μg/day of organic selenium (SelenoPrecise 200 μg, Pharma Nord), or a placebo. The selenium source was patented selenium yeast, SelenoPrecise®. The coenzyme Q10 tablets were identical to medicinal quality tablets registered for heart failure in a European Union trial (Myoqinon®¸ authorization no. OGYI 11494-2010). A total of 221 participants received active substances, whereas 222 received a placebo. All participants were followed for 48 months and were re-examined every 6 months. The study tablets were taken in addition to regular medication. All study tablets (active drug and placebo) not consumed were returned and counted. The study was approved by the Regional Ethical Committee. The Medical Product Agency declined to review the study protocol since the study was not considered a trial of a medication for a certain disease but rather one of the food supplement commodities that are commercially available. The study was registered at clinical trial.gov NCT01443780.
Statistics
Both descriptive and inferential statistics were used to analyze the data in the present study. Group comparisons of categorical variables were tested with Chi-square tests while continuous variables were analyzed with the Student's t-test. To study participants with different levels of cardiac function they were split into five equally sized groups (quintiles) based on their baseline plasma levels of NT-proBNP. Quintile 1 was considered having a low cardiac wall tension, whereas quintiles 2–4 were considered to have moderate cardiac wall stress and finally quintile 5 was considered to have a high cardiac wall tension. Since no, or minor effect of the treatment was expected in those with the highest or lowest cardiac wall tension, a three-way ANOVA with quintile (Q1 and Q5, Q2–4), group (active and placebo) and follow-up (baseline and 48 months) was used to analyze subgroup treatment effect interactions. To control for skewed distributions, NT-proBNP was subjected to a log 10 transformation. Kaplan–Meier curve analysis and Log-rank comparisons in the different groups (i.e., Q1, Q2–4, and Q5 of NT-proBNP) were performed to explore if there was a difference in cardiovascular mortality between active substance and placebo. In the Kaplan–Meier analyses censored participants were those still living at the end of the study period, or who had died of reasons other than cardiovascular disease. Completed participants were those who had died due to cardiovascular disease.
A p value less than 0.05 was considered statistically significant. SPSS version 20.0 (IBM SPSS Inc, Chicago, IL, USA) was used for statistical computing.
Results
Study population and drop-out analyses
For information regarding the basic characteristics of the study population see reference (Citation15). The mean age of the participants who completed the 48-month study period (n = 211) was 77 years, among which 47% were males (). Hypertension was the most commonly occurring disease and was found in 70% of the included participants. The two groups were equal with respect to all study variables, with the exception of respiratory disease found in 22% and 9% (p = 0.006) of those randomized to placebo or active substance respectively. EF less than 40% was found in 7% of the baseline population ().
Table I. Background characteristics of the studied population who had plasma values of NT-proBNP collected at both baseline and at the end of the study (i.e., 48 months) and in relation to active substance or placebo.
Table II. Description of participants (n = 211) with plasma values of NT-proBNP collected at both baseline and 48 months according to baseline quintiles of NT-proBNP.
Table III. Ejection fraction distributed into the different quintiles of NT-proBNP in the total study population (n = 443).
During the 48-month study period a total of 215 participants out of the 443 primarily included withdrew (). Out of these 215, 129 discontinued based on own decision whereas 86 died, thus a drop-out rate of 29%. A more detailed description of reasons for drop-out is given in a previous publication (Citation15). However, the major argument to discontinue the study from the participants themselves was that they had to take too many tablets per day as well as their regular medication (n = 58, 13%). The only adverse effect reported was by the 16 participants (3%) who dropped out due to gastrointestinal symptoms/diarrhoea.
describes the characteristics of the participants in the five different quintiles of the baseline plasma value of NT-proBNP. Ischemic heart disease was present in 5% of those in quintile 1, and 23% and 19% respectively were on beta-blockers or diuretics. Of those in quintile 2–4, an average of 23%, had ischemic heart disease and an average 43% were on beta-blockers or diuretics. In quintile 5, however, 38% had ischemic heart disease and approximately half of them were on beta-blockers and diuretics. Examining the study population with Doppler-echocardiography showed a distribution of systolic cardiac function as presented in the different quintiles of NT-proBNP as presented in . It could be seen that there is a good relation between the echocardiographic evaluation of the systolic cardiac function and the plasma concentration of NT-proBNP.
Response to treatment in different biomarker groups
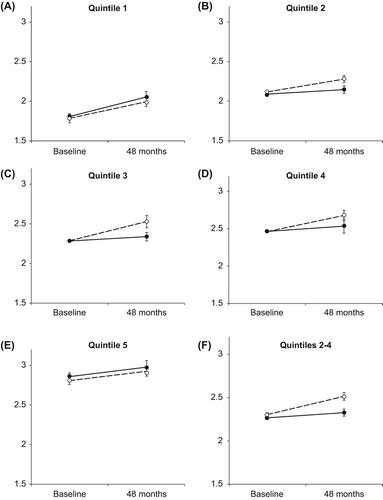
The three-way ANOVA with quintile (Q1–5), group (active and placebo), and follow-up (baseline and 48 months) showed a significant treatment effect (i.e., group*follow-up interaction), F(1, 201) = 4.02, p = 0.046, in favor for the treatment group. There was no significant treatment subgroup interaction, but change of quintiles (Citation1–5) to groups with and without expected treatment effect (Q1 and Q5, Q2–4) revealed such a significant three-way interaction, F(1, 207) = 5.36, p = 0.022, that is, different treatment effects in different subgroups. Follow-up analyses using two-way ANOVA with group (active and placebo) and follow-up (baseline and 48 months) for separate quintiles (Q1 and Q5, Q2–4) showed significant treatment effects for quintiles 2–4 (p = 0.006) (; separate analyses for Q1–5 showed treatment effects for Q3 and similar tendencies for Q2 and Q4).
Figure 2. (A) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months)and with NT-proBNP as outcome measure (logarithmic transformed) according to the quintile 1 of baseline NT-proBNP (NT-proBNP 15 ng/L-104 ng/L). Two-way ANOVA, F (1, 41) = 0.210, p = 0.65. (B) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months)and with NT-proBNP as outcome measure (logarithmic transformed) according to the quintile 2 of baseline NT-proBNP (NT-proBNP 104.01–149.80 ng/L). Two-way ANOVA, F (1, 39) = 2.23, p = 0.14. (C) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months)and with NT-proBNP as outcome measure (logarithmic transformed) according to the quintile 3 of baseline NT-proBNP (NT-proBNP 149.81–227 ng/L). Two-way ANOVA, F (1, 42) = 4.25, p = 0.045. (D) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months)and with NT-proBNP as outcome measure (logarithmic transformed) according to the quintile 4 of baseline NT-proBNP (NT-proBNP 227.01–395.40 ng/L). Two-way ANOVA, F (1, 39) = 1.60, p = 0.21. (E) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months)and with NT-proBNP as outcome measure (logarithmic transformed) according to the quintile 5 of baseline NT-proBNP (NT-proBNP 395.41–3083 ng/L). Two-way ANOVA, F (1, 40) = 0.00, p = 0.99. (F) Analysis of the interaction between group (active substance or placebo) and follow-up (baseline or 48 months) and with NT-proBNP as outcome measure (logarithmic transformed) according to the baseline quintiles 2–4 of NT-proBNP (NT-proBNP 104.01–395.40 ng/L). Two-way ANOVA, F (1, 124) = 7.95, p = 0.006.
We also evaluated the impact of the active substance on cardiovascular mortality according to the different quintiles of NT-proBNP. This analysis was performed on all study participants (n = 443) included at baseline (Supplementary Figures 1–3 are only available in the online version of the journal. Please find this material with the following direct link to the article: http://informahealthcare.com/doi/abs/10.3109/14017431.2013.820838.)
In quintile 1, 5.1% of those with active substance compared with 3.1% of those in placebo suffered cardiovascular mortality (p = 0.68). In the second to fourth quintile, the rate of cardiovascular mortality in those allocated to active substance and placebo was 1.8% and 10.6%, respectively, a rate that was significant different (p = 0.006). In quintile 5, the corresponding rate for cardiovascular mortality in those with active substance was 13.8%, whereas, it was 19.7% for those with placebo (p = 0.35).
Discussion
This prospective double-blind, randomized, controlled trial showed that the impact of dietary supplementation of coenzyme Q10 and selenium for 48 months varied in relation to the baseline concentration of NT-proBNP. An effect of the long-term intervention of coenzyme Q10 and selenium combined was found only in participants with signs of light to moderately increased cardiac wall tension, as reflected by the plasma levels of NT-proBNP and cardiovascular mortality. It is important to note that, as this was a post-hoc subgroup analysis of data collected from a randomized trial and the sample sizes were small, the results should be interpreted with caution.
The active substance slowed the increase in NT-proBNP and decreased cardiovascular mortality in those with baseline plasma values of NT-proBNP in the second to fourth quintiles, whereas no clear effects were seen in those in the first and fifth quintiles. Sander et al. (Citation8) also reported that the impact on cardiac function of coenzyme Q10 supplementation varied, and reported no benefit for those patients presenting with the most severe heart failure (i.e., NYHA class III). The same experience was reported in a recent meta-analysis of the effect of coenzyme Q10 supplementation on patients with heart failure. That study showed that the improvement in ejection fraction only was found for those with mild or moderate impairment of cardiac function (i.e., EF ≥ 30%) (Citation9). The exact mechanisms explaining this can only be speculated upon, but Sander et al. (Citation8) discussed that the number of damaged myocytes may limit the ability of the myocardium to respond to the effects of coenzyme Q10, which concurs with many other studies in cardiology where patients with advanced structural damage do not benefit from interventions of non-specific agents regarding cardiovascular deaths or cardiovascular events such as myocardial infarction or worsening heart failure (Citation18). In our study only 5% had ischemic heart disease in quintile 1 compared with 12–23% in quintiles 2–4 and 38% in quintile 5. According to the echocardiographic evaluation, it could be seen that in those with a substantial structural myocardial damage as seen as severely impaired systolic function, the part that had high plasma concentration of NT-proBNP was substantial, indicating that a high plasma concentration of NT-proBNP could indicate substantial myocardial damage, but also on the contrary, a low plasma concentration of NT-proBNP could indicate low probability of substantial myocardial damage, or a well-treated heart failure. Our result is to some extent compatible to the results from the studies of Sander et al. (Citation8) and Fotino et al. (Citation9), suggesting that supplementation of coenzyme Q10 can be expected to be beneficial in persons who are in the early stages of the development of cardiac dysfunction or in those who have less severe heart failure.
In the present study approximately 60% (i.e., Q2, Q3, and Q4) of the participants were in the groups in which supplementation of coenzyme Q10 and selenium had an impact on the cardiac wall stress and cardiovascular mortality. However, there might be a treatment effect even in the group with low or high plasma concentration of NT-proBNP, even if the small size of the groups in this study prevented us from showing this. The effect of coenzyme Q10 is dependent on presence of selenium (Citation12). Also, coenzyme Q10 plays an important part in the synthesis of selenocystein, which is required for the antioxidant effects of coenzyme Q10 (Citation19). We are not aware of any previous studies evaluating this combination in community-dwelling elderly. The major cardiovascular effect of coenzyme Q10 is speculated to be due to its antioxidant properties. Small randomized studies in patients with coronary heart disease have shown that supplementation of coenzyme Q10 reduces malondialdehyde (i.e., a marker of oxidative stress), increases catalase and super oxide dismutase (i.e., increased antioxidant activity) and reduces plasma levels of interleukin-6 (i.e., inflammation) (Citation20–21). Based on the obtained results, we suggest a positive effect of supplementation mainly in those patients with at least mild to moderate impaired cardiac function.
Limitations. The results in the present study have to be seen as hypothesis generating due to small sizes and because of being a post-hoc subgroup analysis. However, in our previous study we found that the intervention significantly improved cardiac function as measured by echocardiography as well as NT-proBNP (Citation15). The present evaluation of the change in NT-proBNP levels contains only those who participated during the whole study period of 48 months. Thus, the results may not be generalized to all included participants, such as those who died during the course of the study. Also, the evaluated study population was elderly, with a mean age of 78 years why it is not possible to extrapolate the results into other age groups. Only 6% of those in quintile 5 had an EF below 30%. This could be explained partly by the fact that some of the patients with severely impaired cardiac function were treated with heart failure medication that decreases the cardiac wall tension and thus decreases the stimulus for secretion of NT-proBNP. The drop-out rate of the initial study population, after exclusion for mortality, was 29%. We do not consider this rate as remarkable; however, it is higher compared with other studies also with long-follow periods, such as the Heart Protection Study (Citation22) or the Aspirin for Prevention of Cardiovascular Events study (Citation23). However, it is important to note that the design of these two studies differs compared with that of our study. Firstly, both studies offered a medical treatment to individuals in high risk to die because of cardiovascular disease (Citation22) or to individuals with a high risk to develop a vascular disease, as established by a low ankle brachial index, (Citation23). In our study, we offered nutritional supplement to community-dwelling elderly without any pre-selected criterions of having or not having a specific risk for a cardiovascular disease. One may assume that individuals at a risk to die because of or to develop a cardiovascular disease may be more motivated to complete a study that evaluates a medical treatment. Secondly, in the Heart Protection Study all individuals who were less likely to complete the 5-year study period was excluded (i.e., 26%). In our study no such exclusion was done however the percentages who were excluded in that study is compatible with the 29% of those who discontinued based on own decision in our study. Thirdly, in Heart Protection Study 28% were above 70 years (Citation22), whereas the mean age in the Aspirin for Prevention of Cardiovascular Events study (Citation23) was approximately 62 years. Hence, both studies included younger populations compared with the mean age of 78 years of the population in our study. Despite the limitations of the present study, we still argue that the results presented are of interest.
Conclusion
In conclusion, supplementation of coenzyme Q10 and selenium in a cohort of community-dwelling elderly reduced the progression of cardiac wall tension, as measured by the cardiac biomarker NT-proBNP, and cardiovascular mortality mainly in participants whose baseline plasma NT-proBNP ranged from the second to the fourth quintiles of the peptide. This could be interpreted to indicate that the therapeutic response may be more pronounced in participants who are in the early stages of development of cardiac dysfunction based on an intervention time of 48 months. This study could provide a basis to initiate larger randomized trials evaluating the effect of coenzyme Q10 and selenium on patients with heart failure. Inclusion of younger populations as well as patients at different severity levels in such studies may be of importance to unravel the effect of supplementation of coenzyme Q10 and selenium in different patients.
Supplementary Figures 1–3
Download PDF (207.7 KB)Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Grants or other support: Part of the analysis costs was supported by grants from The County Council of Linköping University and the Swedish Research Council. The selenium tablets and Q10 capsules were kindly donated by Pharma Nord Aps.
References
- Mortensen SA. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end-points of “Q-symbio”–a multinational trial. Biofactors. 2003;18:79–89.
- Kalen A, Norling B, Appelkvist EL, Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta. 1987;926:70–8.
- Kaikkonen J, Nyyssonen K, Tuomainen TP, Ristonmaa U, Salonen JT. Determinants of plasma coenzyme Q10 in humans. FEBS Lett. 1999;443:163–6.
- Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2:158–73.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10:933–89.
- Senes M, Erbay AR, Yilmaz FM, Topkaya BC, Zengi O, Dogan M, Yucel D. Coenzyme Q10 and high-sensitivity C-reactive protein in ischemic and idiopathic dilated cardiomyopathy. Clin Chem Lab Med. 2008;46:382–6.
- Molyneux SL, Florkowski CM, George PM, Pilbrow AP, Frampton CM, Lever M, Richards AM. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J Am Coll Cardiol. 2008;52:1435–41.
- Sander S, Coleman CI, Patel AA, Kluger J, White CM. The impact of coenzyme Q10 on systolic function in patients with chronic heart failure. J Card Fail. 2006;12: 464–72.
- Fotino AD, Thompson-Paul AM, Bazzano LA. Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. Am J Clin Nutr. 2013;97:268–75.
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, Hurst R. Selenium in human health and disease. Antioxid Redox Signal. 2011;14:1337–83.
- Lubos E, Sinning CR, Schnabel RB, Wild PS, Zeller T, Rupprecht HJ, et al. Serum selenium and prognosis in cardiovascular disease: results from the AtheroGene study. Atherosclerosis. 2010;209:271–7.
- Xia L, Nordman T, Olsson JM, Damdimopoulos A, Bjorkhem-Bergman L, Nalvarte I, et al. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J Biol Chem. 2003;278:2141–6.
- Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53:764–70.
- Jekell A, Hossain A, Alehagen U, Dahlstrom U, Rosen A. Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur J Heart Fail. 2004;6:883–90.
- Alehagen U, Johansson P, Bjornstedt M, Rosen A, Dahlstrom U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol. 2012. http://dx.doi.org/10.1016/j.ijcard.2012.04.156 (in press).
- Cleland JG, Taylor J, Freemantle N, Goode KM, Rigby AS, Tendera M. Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: a report from the PEP-CHF study. Eur J Heart Fail. 2012;14:487–94.
- Alehagen U, Lindstedt G, Eriksson H, Dahlstrom U. Utility of the amino-terminal fragment of pro-brain natriuretic peptide in plasma for the evaluation of cardiac dysfunction in elderly patients in primary health care. Clin Chem. 2003;49:1337–46.
- Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–61.
- Moosmann B, Behl C. Selenoproteins, cholesterol-lowering drugs, and the consequences: revisiting of the mevalonate pathway. Trends Cardiovasc Med. 2004;14:273–81.
- Lee BJ, Huang YC, Chen SJ, Lin PT. Effects of coenzyme Q10 supplementation on inflammatory markers (high- sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28:767–72.
- Lee BJ, Huang YC, Chen SJ, Lin PT. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012;28:250–5.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
- Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303: 841–8.