Abstract
Objectives. Statins have multiple pleiotropic effects that are independent of their cholesterol-lowering properties including rapid improvement of endothelial function in vitro. Hypertension is characterized by endothelial dysfunction and we hypothesized that a single-dose of atorvastatin may have an acute effect on vascular function. Design. Endothelium-dependent vasodilation (EDV) and endothelium-independent vasodilation were assessed with venous occlusion plethysmography during intra-arterial infusion of acetylcholine (ACH) and sodium nitroprusside (SNP), respectively, in 13 hypertensive men after wash-out from antihypertensive medication. Vasoconstrictive responses were evaluated in response to angiotensin II (Ang II) infusion. The protocol was repeated 1 h after 80 mg oral atorvastatin (ATV; Lipitor®). Results. ATV treatment significantly increased baseline forearm blood flow from 3.38 (0.27) to 4.31 (0.35) ml/min/100 ml tissue (p < 0.05). ATV did not affect ACH-induced EDV. Forearm vascular resistance in response to SNP was significantly lowered by ATV (p < 0.05). Vasoconstriction in response to Ang II was significantly inhibited by ATV treatment (p = 0.005). Conclusions. The observed acute statin effects in hypertension appear to be endothelium-independent and related to vascular smooth muscle cell function. These actions may in part contribute to the beneficial pleiotropic effects of statin therapy even in the acute in vivo setting.
Introduction
Hypertension and hypercholesterolemia are major cardiovascular risk factors. Treatment with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) is widely used in primary and secondary prevention of cardiovascular disease (Citation1–3). The endothelium is pivotal for blood pressure regulation, in part by releasing nitric oxide (NO), which relaxes the vascular smooth muscle cells (VSMCs). Hypertension is associated with endothelial dysfunction, which is a marker of future cardiovascular events (Citation4,Citation5). Antihypertensive drugs, including angiotensin converting enzyme inhibitors and angiotensin receptor blockers, can improve endothelial function (Citation6). In hypercholesterolemia, a normalization of impaired endothelial function has been demonstrated by long-term use of statins (Citation7,Citation8).
Statins have multiple effects independent of their cholesterol lowering actions, generally named pleiotropic effects. Statins improve endothelial function, reduce oxidative stress, and they have anti-inflammatory and anti-thrombotic properties (Citation9). All these pleiotropic effects of statins are counteracted by an elevated blood pressure level. There are reports on the very rapid onset of actions generated by statins in vitro (Citation10,Citation11). Statins’ effect on reversing endothelial dysfunction may explain this rapid improvement of vascular function. However, it remains unknown whether such results can be translated to in vivo conditions. In fact, it might be argued that an immediate initiation of statin therapy, regardless of the actual cholesterol level, could be of high clinical relevance for the outcome of patients with an acute coronary event (Citation12,Citation13). Therefore, in this pilot study we tested the hypothesis if a single-dose of atorvastatin could induce acute changes in endothelial and vascular function by means of a forearm blood flow (FBF) model in otherwise healthy hypertensive men.
Methods
Subjects
Thirteen non-smoking male subjects with mild to moderate hypertension were included after recruitment from newspaper advertisements. Anthropometric data and baseline characteristics are shown in . All patients were on ongoing antihypertensive medication and the treatment was withdrawn at least 4 weeks prior to the study. All subjects were statin-naïve. The study protocol was approved by the Ethics Committee of the University of Gothenburg and conducted according to the Declaration of Helsinki. The nature, purpose, and potential risks of the study were carefully explained to each subject before informed consent was obtained.
Table I. Baseline characteristics of the study subjects.
Study protocol
A baseline physical examination was performed. Actual blood pressure levels were equal to or exceeded 140/90 mmHg prior to study entry. During one study-day endothelium-dependent vasodilation (EDV) and endothelium-independent vasodilation (EIV) were assessed with venous occlusion plethysmography during intra-arterial infusion of acetylcholine (ACH) and sodium nitroprusside (SNP), respectively. Vasoconstrictive responses were evaluated in response to angiotensin II (Ang II) infusion. Ischemia-induced vasodilation was investigated after arterial occlusion of the upper arm. The protocol was repeated 1 h after single-dose of 80 mg oral atorvastatin.
Study design
The subjects attended the laboratory in the morning, and the study was conducted in a quiet, air- conditioned room with the subjects in a supine position. An arterial cannula was inserted into the brachial artery in the non-dominant arm for drug infusion. Intra-arterial blood pressure was recorded by electrical transducer (Statham P-23, Siemens-Elema) connected to a digital monitor. Electrocardiography monitoring was performed during the whole experiment. FBF was assessed by venous-occlusion plethysmography with a mercury-in-silastic strain gauge using the MAPPC® software (Elektromedicin AB, Kungsbacka, Sweden). The strain gauge, connected to the calibrated plethysmograph, was placed around the broadest part of the forearm resting at the level of the heart. Venous occlusion was achieved by rapidly inflating a blood pressure cuff on the upper arm to 50 mmHg. Hand circulation was excluded by inflating a pediatric wrist cuff to suprasystolic pressure 5 s prior to each measurement. Means of 3–5 recordings were expressed in millilitres per minute and 100 ml of tissue.
After cannulation of the artery, 30 min was allowed before starting the experiment with a baseline recording, followed by drug infusions. ACH 7.5 μg/ml (1, 2, and 3 ml/min), SNP 0.8 μg/ml (1, 2, and 3 ml/min), and Ang II 0.5 μg/ml (0.25, 0.5, 1, and 2 ml/min) were infused in the given order at increasing rates. Drug infusions were given at a constant rate over a period of 5 min for each dose, with 5-min wash-out period between the different drugs. FBF was measured at baseline and during the last minute of each dose-step for each infusion. The infusions were continued during FBF measurements. Immediately after the readings, the infusion was stopped and the intra-arterial systolic, diastolic, and mean arterial blood pressures were obtained. Before ischemia-induced vasodilation there was a 10-min wash-out period. Ischemia-induced vasodilation was assessed after 5 min of arterial occlusion of the upper arm. FBF and intra-arterial blood pressure were recorded immediately after deflating the cuff from suprasystolic pressure down to 50 mm Hg.
After the measurements, the subjects were treated with two Lipitor® 40 mg tablets (atorvastatin). One hour later, the infusion procedure was repeated. The subjects were not allowed to eat or drink anything else than water in between the experiments. The time window from intake of atorvastatin (ATV) to the repeated experiment was approximately 1 h and 15 min.
Baseline venous blood samples for lipid status, hemoglobin, creatinine, and fasting glucose levels were collected in the morning before the experiments. Analyses were performed by standard methods at the Department of Clinical Chemistry, Sahlgrenska University Hospital, Gothenburg, Sweden.
Calculations and statistical analysis
Forearm vascular resistance (FVR) was calculated as the ratio of mean arterial pressure (MAP) to FBF and expressed in arbitrary resistance units. Values were calculated at baseline and at each step of the different infusion protocols.
All statistical analysis were performed using the SPSS 18.0 software (SPSS Inc, Chicago, USA). Unless otherwise stated, values are presented as mean and standard error of the mean (SEM). Responses to vasoactive substances were analysed using two-way (treatment/no treatment and dose) ANOVA. A one-way ANOVA was used for the analysis of repeated measurements. Paired-Samples T-test was used when appropriate. Findings were considered significant at a significance level of p < 0.05.
Results
ATV treatment significantly increased baseline FBF from 3.38 (0.27) to 4.31 (0.35) ml/min/100 ml tissue (p < 0.05, ANOVA) whereas other hemodynamic parameters like blood pressure and heart rate were unchanged, .
Table II. Baseline hemodynamic variables.
Endothelium-dependent vasodilation
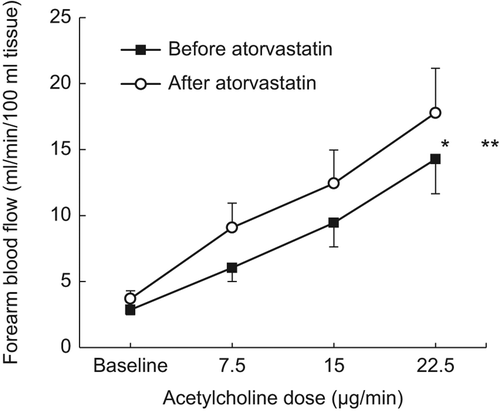
Intra-brachial ACH infusion increased FBF in a dose-dependent manner during both experiments (p < 0.001, ANOVA). ATV induced an upward adjustment of the dose-response curve (p < 0.05, ANOVA) but did not affect the EDV per se (, p = ns, two-way ANOVA). ACH infusion resulted in a dose-dependent decrease in FVR (p = 0.0002, ANOVA), from 4.7 (0.6) to 1.4 (0.4) arbitrary units (AU), and from 4.1 (0.5) to 1.2 (0.3) AU, before and after ATV treatment, respectively (p = ns, two-way ANOVA).
Figure 1. Endothelium-dependent vasodilation. FBF during baseline and in response to intra-arterial infusion of ACH, before atorvastatin (■) and after atorvastatin (○) treatment. *p < 0.05 (ANOVA for treatment), **p = ns (2-way ANOVA, treatment × dose). Mean and SEM.
Ischemia-induced reactive hyperemia resulted in a substantial increase in the FBF, from 2.8 (0.5) to 31.9 (1.2) ml/min/100 ml tissue, and from 3.5 (0.6) to 32.6 (2.6) ml/min/100 ml tissue, before and after ATV treatment, respectively (p = ns, T-test).
Endothelium-independent vasodilation
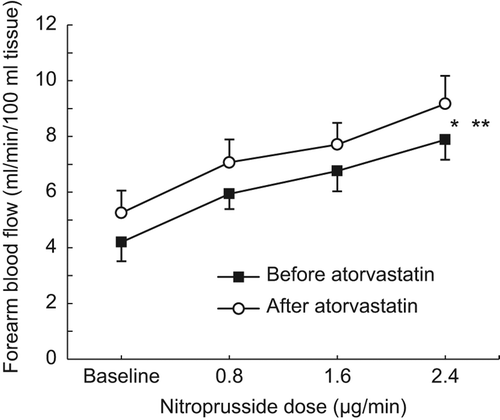
Intra-brachial SNP infusion resulted in a significant and dose-dependent increase in FBF (p < 0.01, ANOVA), and ATV treatment induced an upward shift in the dose-response curve (, p < 0.05, ANOVA). In parallel, FVR decreased (p < 0.0001, ANOVA), from 3.7 (0.6) to 1.6 (0.1) AU, and from 2.7 (0.3) to 1.5 (0.2) AU, before and after ATV treatment, respectively (p < 0.05, two-way ANOVA).
Angiotensin II mediated vasoconstriction
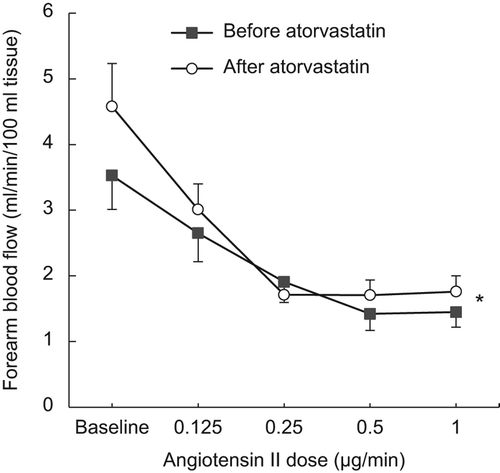
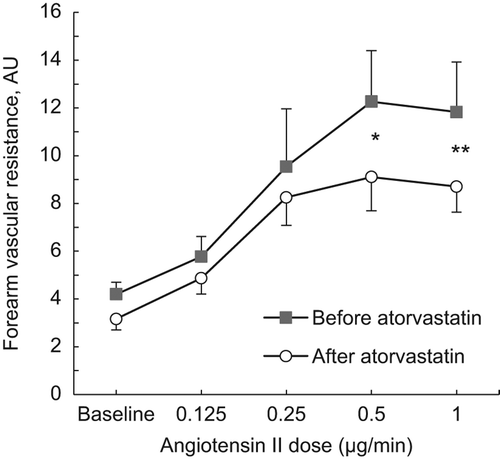
Intra-brachial infusion of Ang II induced a dose- dependent decrease in FBF (p < 0.001, ANOVA). This vasoconstrictor response during Ang II was inhibited by ATV treatment (, p = 0.005, two-way ANOVA). FVR increased during Ang II infusion and the response was inhibited by ATV ().
Discussion
In this study, we report the acute effects of a single-dose of atorvastatin (ATV) on basal FBF, peripheral vascular resistance, and vascular responses to vasoactive substances in hypertensive men. Basal blood flow, EIV, and Ang II-induced vasoconstriction were all significantly modified by ATV whereas endothelium-dependent vasodilator responses were unchanged.
Potential explanations for our findings include different vasoprotective effects of statins which are exerted independent of their cholesterol-lowering actions (Citation10,Citation14). These pleiotropic effects include improvement of endothelial function via reduction of oxidative stress (Citation15) and rapid activation of endothelial nitric oxide synthase (Citation11,Citation16). Furthermore, statins may induce acute vasodilation through both endothelium-dependent and endothelium- independent mechanisms in vitro, where the latter depends on vascular smooth muscle cell hyperpolarizations (Citation17).
Endothelial dysfunction, specifically the finding of a decreased capacity for EDV, is a common denominator of different cardiovascular risk factors. Rapid improvement of endothelial function has previously been shown in non-invasive studies within 24 h after initiation of atorvastatin treatment in healthy subjects and in smokers (Citation18,Citation19). However, we were not able to demonstrate any effect of a single-dose of ATV on EDV or ischemia-induced vasodilation in hypertensive subjects. Whether the lack of effect could be related to the hypertensive state of the subjects or to other methodological differences of the studies remains unknown. In a recent study by Schneider et al., investigating vascular effects of fluvastatin in patients with hypertension and normal cholesterol levels, no effect was found on either EDV or EIV after 2 weeks of statin treatment. Schneider et al. conclude that the lack of effect on EDV is likely due to an inability of fluvastatin to reduce oxidative stress in the vasculature (Citation20) suggesting that the observed effects of ATV are probably drug- and not class specific.
Statins are able to induce acute vasorelaxing endothelium-independent effects on aortic rings from spontaneously hypertensive rats. It is discussed that the effects on VSMC involve blocking of extracellular calcium entry (Citation21). In coronary circulation, acute vasodilating effects of a single dose of ATV have been demonstrated within 1 h in man (Citation22). In that study, the diastolic blood flow velocity of the left anterior descending artery was assessed at baseline and during hyperemic conditions, before and after ATV treatment. They showed that baseline diastolic blood flow velocity increased 32% after ATV. This is in line with our observation of 28% increase in baseline FBF after acute ATV treatment, demonstrating a vasorelaxing statin effect that might be independent of the endothelium.
The primary vascular target for Ang II is the VSMC and we found that ATV diminished Ang II-induced vasoconstriction. The G protein-coupled Ang II Type 1 receptors on VSMC regulate vascular tone via multiple mechanisms leading to increased intracellular free calcium concentration, which leads to muscle contraction. Ang II also induces contraction through a RhoA/Rho-kinase (ROCK) pathway. By affecting Rho-kinase, statins can acutely inhibit vasoconstrictive responses in vitro (Citation21). Statins also inhibit Rho activation caused by Ang II both in cultured VSMCs and in vivo (Citation23). Recently, it has been shown that statins reduce ROCK activity in man (Citation24). Our present results indicate that ATV has a direct attenuating effect on Ang II-induced vasoconstriction also in vivo, suggesting an acute statin effect on VSMCs.
Rapid initiation of statin therapy might be of clinical significance in patients with impairment of blood flow and tissue perfusion. Recent findings from clinical trials and meta-analysis on patients with acute coronary syndrome (ACS) have suggested that very early initiation of statin treatment after the diagnosis of ACS (on the first day of hospitalization), may decrease 1-month mortality (Citation12,Citation13). Vice versa, acute statin withdrawal during ACS increased short-term mortality and this adverse outcome was independent of the effect on cholesterol level (Citation25). Altogether, these results demonstrate rapid effects of statins in humans, and the mechanisms are thought to involve beneficial pleiotropic properties of statins.
The study used forearm plethysmography, the state of the art method for in vivo vascular function assessment. In addition, individuals were carefully evaluated regarding blood pressure, lipid status, and other risk factors for endothelial dysfunction. One limitation of the study is that it was not placebo-controlled. We cannot exclude that the observed beneficial effects may be attributed to factors other than ATV treatment. However, we carefully controlled the conditions of the repeated measurements and attempted to exclude external factors, which may have affected the FBF measurements. Another limitation is the short time window between ATV treatment and reinvestigation. However, in vitro effects have been observed within minutes. It cannot be ruled out that a longer time window between test and re-test could lead to positive results regarding improvement of the EDV. Furthermore, the subjects in our study showed normal low-density lipoprotein (LDL) levels, but relatively high levels of high- density lipoprotein (HDL). As HDL carries several protective effects on the endothelial function (Citation26), we cannot exclude that subjects with low HDL might respond differently to acute statin treatment. We are considering a follow-up study with atorvastatin in patients with adverse cholesterol levels.
Conclusion
The results of this study indicate that a single oral dose of atorvastatin affects peripheral vascular reactivity in hypertensive subjects. Basal blood flow increased while the EDV was unaffected by atorvastatin. Ang II effect on vasoconstriction was inhibited by atorvastatin. The results suggest acute statin effects in hypertension that are independent of the endothelium, indicating that VSMCs are affected more rapidly than the endothelial cells. These actions may in part contribute to the beneficial pleiotropic effects of statins.
Acknowledgements
We want to thank laboratory technicians Ann Nolby and Marie Söderblom for excellent assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by grants from the Swedish Research Council, the Swedish Heart-Lung Foundation, and the Swedish Hypertension Society.
References
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–9.
- Waters DD. What the statin trials have taught us. Am J Cardiol. 2006;98:129–34.
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–58.
- Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7.
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001; 104:191–6.
- Ghiadoni L, Taddei S, Virdis A. Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol. 2012;10:42–60.
- John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia: potential mechanisms and differences. J Hypertens. 2000;18:363–74.
- O’Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997;95:1126–31.
- Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F.
- Zhou Q, Liao JK. Pleiotropic effects of statins. - Basic research and clinical perspectives. Circ J. 2010;74:818–26.
- Datar R, Kaesemeyer WH, Chandra S, Fulton DJ, Caldwell RW. Acute activation of eNOS by statins involves scavenger receptor-B1, G protein subunit Gi, phospholipase C and calcium influx. Br J Pharmacol. 2010;160:1765–72.
- Angeli F, Reboldi G, Garofoli M, Ramundo E, Verdecchia P. Very early initiation of statin therapy and mortality in patients with acute coronary syndrome. Acute Card Care. 2012; 14:34–9.
- Angeli F, Reboldi G, Mazzotta G, Garofoli M, Cerasa MF, Verdecchia P. Statins in acute coronary syndrome: very early initiation and benefits. Ther Adv Cardiovasc Dis. 2012;6: 163–74.
- Prinz V, Endres M. The acute (cerebro)vascular effects of statins. Anesth Analg. 2009;109:572–84.
- Wassmann S, Laufs U, Baumer AT, Muller K, Ahlbory K, Linz W, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–7.
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35.
- Mukai Y, Shimokawa H, Matoba T, Hiroki J, Kunihiro I, Fujiki T, et al. Acute vasodilator effects of HMG-CoA reductase inhibitors: involvement of PI3-kinase/Akt pathway and Kv channels. J Cardiovasc Pharmacol. 2003; 42:118–24.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol. 2001;88:1306–7.
- Iida K, Goland S, Akima T, Luo H, Birnbaum Y, Siegel RJ. Effect of a single 20-mg tablet of Atorvastatin on brachial artery blood flow in normolipidemic male smokers versus nonsmokers. Am J Cardiol. 2007;100:881–4.
- Schneider MP, Schmidt BM, John S, Schmieder RE. Effects of statin treatment on endothelial function, oxidative stress and inflammation in patients with arterial hypertension and normal cholesterol levels. J Hypertens. 2011;29:1757–64.
- Perez-Guerrero C, Marquez-Martin A, Herrera MD, Marhuenda E, Alvarez de Sotomayor M. Regulation of vascular tone from spontaneously hypertensive rats by the HMG-CoA reductase inhibitor, simvastatin. Pharmacology. 2005;74:209–15.
- Hinoi T, Matsuo S, Tadehara F, Tsujiyama S, Yamakido M. Acute effect of atorvastatin on coronary circulation measured by transthoracic Doppler echocardiography in patients without coronary artery disease by angiography. Am J Cardiol. 2005;96:89–91.
- Ruperez M, Rodrigues-Diez R, Blanco-Colio LM, Sanchez-Lopez E, Rodriguez-Vita J, Esteban V, et al. HMG-CoA reductase inhibitors decrease angiotensin II- induced vascular fibrosis: role of RhoA/ROCK and MAPK pathways. Hypertension. 2007;50:377–83.
- Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119: 131–8.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002; 105:1446–52.
- Tran-Dinh A, Diallo D, Delbosc S, Varela-Perez LM, Dang Q, Lapergue B, et al. HDL and endothelial protection. Br J Pharmacol. 2013;169:493–511.