Abstract
Background. It has been debated whether or not heparin infusion before or after non-heart-beating donors are declared dead improves the quality of pulmonary grafts. In clinical lung transplantation with heart-beating donors (HBDs) heparin is routinely infused prior to organ harvesting since it is believed to improve pulmonary grafts by minimizing thrombosis formation in the pulmonary grafts. Here, we raise the question of whether or not the use of heparin in HBDs improves the quality of the pulmonary grafts. Methods. Twelve landrace pigs were divided into two groups of six animals; heparin was given prior to lung harvesting in one group, while the other group did not receive any heparin. The lungs were evaluated using an ex vivo lung perfusion (EVLP) method. Results. No significant difference in arterial oxygen partial pressure (PaO2) was observed between the two groups at an inspired oxygen fraction (FiO2) of 1.0 (mean 69.2 kPa, range 46.1–77.0 in the non-heparin group, and 61.6 kPa, range 47.9–71.4 in the heparin group, p = 0.44), neither in pulmonary vascular resistance: mean 543 ((dyne × s)/cm5) (range 280–615) in the non-heparin group and 533 ((dyne × s)/cm5) (320–762) in the heparin group (p = 0.99). Conclusions. Heparin did not seem to improve pulmonary graft function in our animal model using conventional HBDs.
Introduction
Lung transplantation is an established option for the treatment of patients with terminal lung disease (Citation1). However, it is necessary to reduce the incidence of ischemia–reperfusion injury which may lead to graft failure (Citation2). One strategy being employed to increase the donor pool is the procurement of organs from non-heart-beating donors (NHBDs) (Citation3–7). According to the Maastricht criteria, donors are classified according to the location of cardiac death. Controlled donors are defined as those in which cardiac arrest is expected after the withdrawal of life support, but before they are declared brain dead (Category 3), and those in which cardiac arrest occurs after brain death (Category 4). Uncontrolled NHBDs, Categories 1, 2, and 5, are donors who die in the prehospital environment or suffer unexpected cardiac arrest in hospital (Citation3,Citation8).
Lung preservation remains a major issue in both heart-beating donors (HBDs) and NHBDs (Citation6,Citation9–13). In clinical lung transplantation today the donor routinely receives intravenous heparin prior to lung harvesting, with the aim of avoiding thrombosis in the pulmonary grafts. Since heparin administration is routine in organ harvesting from HBDs it has been suggested that the same routine be employed in NHBD organ harvesting. However, there is an ongoing debate as to whether it is ethically acceptable to give a patient suffering from acute cardiac arrest after unsuccessful heart resuscitation heparin, and administer heart compression to circulate the heparin after death has been declared, but before permission for donation has been obtained (Citation14). Heparin at such high doses is clearly not given for the benefit of the patient, and some professionals are concerned that its administration may hasten death (Citation14). Some investigators have even suggested that heparin has a negative effect on pulmonary grafts (Citation15), some investigators indicate that heparin might not been needed in the NHBD situation (Citation16,Citation17), while some investigators indicate the opposite; that heparin is needed (Citation18).
Here, we investigate whether the infusion of heparin improves pulmonary grafts from HBDs or not. If heparin does not improve lung grafts from HBDs, there is reason to believe it might not influence the quality of pulmonary grafts from a NHBD. To clarify further, this study is mainly to elucidate the effect of heparin in lung preservation for lung transplantation.
Material and methods
Animal preparation
Twelve Swedish landrace pigs with a mean weight of 61 kg were fasted overnight with free access to water. The study was approved by the Ethics Committee for Animal Research, Lund University, Sweden. The experimental protocol for this study was approved by the Ethics Committee for Animal Research, Lund University, Sweden (No. M 172-11). All animals received care according to the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes, as well as to the Principles of Laboratory Animal Care of the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals.
Premedication was performed with an intramuscular injection of xylazine (Rompun® vet. 20 mg/ml, Bayer AG, Leverkusen, Germany, 2 mg/kg) mixed with ketamine (Ketaminol® vet. 100 mg/ml, Farmaceutici Gellini S.p.A., Aprilia, Italy, 20 mg/kg) while the pig was still in its stable. Peripheral intravenous access was then established in the ear. The pig was then transferred to the laboratory and placed on the operating table in the supine position. Oral intubation was performed using a 7.5 mm endotracheal tube after the induction of anesthesia with sodium thiopental (Pentothal, Abbott Laboratories, North Chicago, Illinois, USA) and pancuronium bromide (Pavulon, N.V. Organon, Oss, the Netherlands). Anesthesia was maintained by infusions of ketamine (Ketaminol® vet), midazolam (Midazolam Panpharma®, Oslo, Norway), and fentanyl (Leptanal, Lilly, France). Fluid loss was compensated for by continuous infusion of Ringer's acetate. Mechanical ventilation was established with a Siemens- Elema ventilator (Servo Ventilator 300, Siemens, Solna, Sweden) with an inspired oxygen fraction (FiO2) of 0.5, a frequency of 15 breaths/min, a minute ventilation of 6 l/min, and a positive end-expiratory pressure (PEEP) of 5 cm H2O.
Preservation of HBD lungs
The pigs were randomly assigned to one of two groups: one receiving heparin sodium, and the other not receiving heparin sodium. A median sternotomy was performed. Heparin sodium (Heparin LEO; 400 IE/kg, LEO Pharma AB, Malmö, Sweden) was administered intravenously to one group of animals. The pulmonary artery was cannulated via the right ventricle with a 28 F cannula secured with a purse string suture placed in the outflow tract of the a. pulmonalis. A clamp was put on the v. cava superior, and another clamp was put on the v. cava inferior. A clamp was then put on the ascending aorta. The left atrial and v. cava inferior were then opened. The right and left pleurae were filled with ice slush to cool the lungs.
The lungs were perfused antegradely with 2 l of cold Perfadex® containing 1.0 ml isotonic trometamol (Addex-THAM 3.3 mmol/ml, Fresenius Kabi AB Uppsala, Sweden), 2 ml calcium chloride (0.45 mmol/ml), and 3 ml nitroglycerine (5 mg/ml, BMM Pharma AB, Stockholm, Sweden) at a low perfusion pressure (< 20 mmHg). The cannula was then removed from the pulmonary artery. The lungs were harvested en bloc in a standard fashion, and weighed. A segment (˜8 cm) of the descending aorta was also excised. The lungs, together with the aortic segment, were then immersed in cold Perfadex and kept in cold storage at 8°C for 4 h.
Ex vivo lung perfusion
EVLP was performed using the Medtronics Ex Vivo Lung Evaluation Set extracorporeal perfusion circuit by Medtronics (Medtronic AB, Kerkrade, the Netherlands; Ex Vivo Lung Evaluation Set) (). The system was primed with albumin (500 ml, 50 g/l, and 200 ml 200 g/l; Albumin Baxter, Baxter Medical, Kista, Sweden) and 2 units of autologous blood, withdrawn previously from each donor. Imipenem (0.5 g, Tienam, Merck Sharp and Dohme, Sollentuna, Sweden), insulin (20 IU, Actrapid, Novo Nordisk, Bagsvaerd, Denmark), and heparin (10,000 IU, Leo Pharma, Malmö, Sweden) were added, and isotonic trometamol (Addex-Tham, Kabi, Sweden) was used to buffer the mixed solution to a temperature-adjusted pH of 7.4.
Figure 1. EVLP was performed using the Medtronics Ex Vivo Lung Evaluation Set extracorporeal perfusion circuit by Medtronics (Medtronic AB, Kerkrade, the Netherlands; Ex Vivo Lung Evaluation Set).
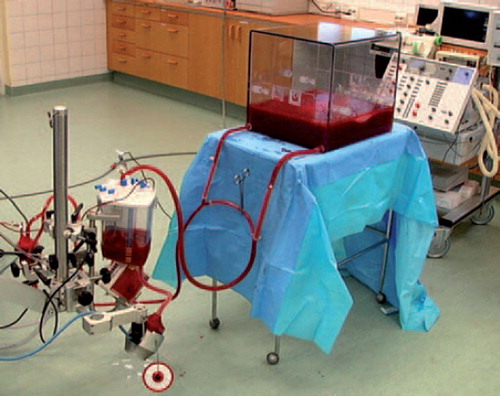
Gas was supplied to the membrane oxygenator; first oxygen and CO2 during the reconditioning phase, and then 93% nitrogen and 7% CO2, during the testing phase, creating a normal venous blood gas in the perfusate to the pulmonary artery (in other words, the oxygenator was used to deoxygenate the perfusate). Before starting perfusion the pulmonary artery was extended using the excised segment of the descending aorta to facilitate cannulation. The pulmonary artery cannula was then connected to the corresponding tube of the extracorporeal circuit, the air was removed, and the shunt of the circuit was clamped. An endotracheal tube was secured in the trachea with a cotton band and connected to the ventilator. The remnant of the left atrium was left open, preventing obstruction of the pulmonary outflow, since the perfusion solution flowed directly out into the lung reconditioning box, and the left atrium pressure was therefore 0 mmHg.
Low-flow perfusion at 25°C was initiated through the lungs. The lungs were gradually warmed by increasing the temperature of the perfusate. When the temperature reached 32°C, ventilation was started with an inspired oxygen fraction of 0.5 and a minute volume of 1 l/min, and no PEEP. The pump flow was gradually increased, but the pulmonary arterial pressure was never allowed to exceed 20 mmHg. With each 1°C increase in temperature, the ventilation was increased by a minute volume of 1 L. Normothermia was reached after 20–30 min, and PEEP was added to fully expand the lungs and eliminate atelectasis. Blood gases were analyzed during perfusion and full ventilation with decreasing fractions of inspired oxygen. The lungs were then disconnected from the EVLP equipment. A collapse test was performed for final evaluation of the lungs by disconnecting the lungs from the ventilator at the end of inspiration. The lungs were then weighed once again. The pulmonary arterial branches were macroscopically studied for thrombotic material by opening the arteries as far distally as possible.
Calculations and statistics
Calculations and statistical analysis were performed using GraphPad 4.0 software. Statistical analysis was performed using Wilcoxon's test to compare the lung function from the HBDs with and without heparin infusion. A level of p < 0.05 was considered statistically significant, and p > 0.05 was considered not significant (n.s.). The results are presented as median and range or mean and standard error of the mean (SEM). Pulmonary artery flow (l/min), that is cardiac output (CO) in the ex vivo model and pulmonary artery pressure (PAP) were measured continuously. The pulmonary artery flow was not allowed to exceed 4 l/min, and the PAP was not allowed to exceed 20 mmHg. Pulmonary vascular resistance (PVR) was calculated using the formula: PVR (dyne × s/cm5) = [(80 * PAP) – LAP]/CO, where PAP is the mean pulmonary artery pressure, LAP is the left atrium pressure, and CO is the cardiac output.
Results
Study groups
No significant differences were observed in animal weight in the two groups (61 ± 2 kg in the HBD non-heparin group and 60 ± 1.5 kg in the HBD heparin group (p > 0.05). Neither were there any differences in arterial oxygen partial pressure (PaO2) at an inspired oxygen fraction of 1.0 (62.4 ± 3.4 in the non-heparin group and 66.1 ± 4.4 in the heparin group). No anatomical anomalies, signs of infection, or malignancy were found in any of the animals at autopsy.
Pulmonary graft function
Arterial and venous blood gases
The arterial blood gases and venous blood gases at the different fractions of inspired oxygen are presented in . No significant differences were observed between the two groups.
Table I. The EVLP data expressed as median and range HBD receiving heparin and those without heparin for inspired oxygen fractions of 1.0, 0.5, and 0.21.
Pulmonary vascular resistance
The pulmonary vascular resistance was calculated at the different fractions of inspired oxygen and is also presented in . Again, no significant difference was seen between the two groups.
Pulmonary graft compliance
After evaluation of the lungs they were disconnected from the ventilator and a collapse test was performed. If the lungs do not collapse this may indicate lung injury, lung edema, or pneumonia. All the lungs from both study groups collapsed as they should, showing good compliance. No significant difference was seen between the lungs from the two groups.
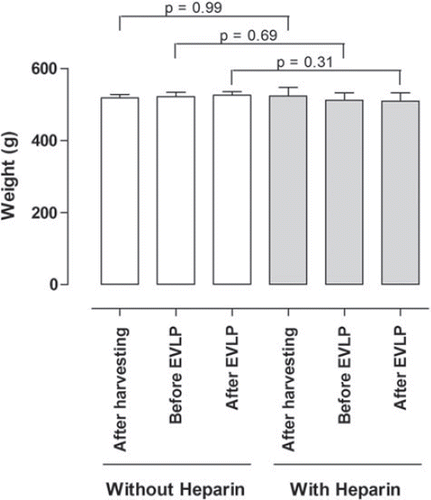
Pulmonary graft weight
The lungs were weighed after harvesting, before EVLP and after EVLP to assess the degree of lung edema. The results are shown in . After harvesting, the mean lung weight in the heparin group was 524 ± 20.5 g and in the non-heparin group 518 ± 9.9 g (p = 0.99). After completion of the lung evaluation the mean lung weight was 509 ± 23.1 g in the heparin group and 526 ± 9.4 g (p = 0.31) in the non-heparin group. Thus no significant differences were found between the two groups.
Figure 2. Mean lung weight (± SEM) after harvesting, before EVLP, and after completion of EVLP for pulmonary grafts from the two groups of pigs: one not treated with heparin, and the other treated with heparin. Statistical analysis was performed using Wilcoxon's test. Significance was defined as p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p > 0.05 (not significant, n.s.).
Hemodynamic data
No significant differences in pulmonary artery flow or PAP were seen between the two groups during the evaluation of the pulmonary graft at the different fractions of inspired oxygen. The data are presented in .
Macroscopic appearance
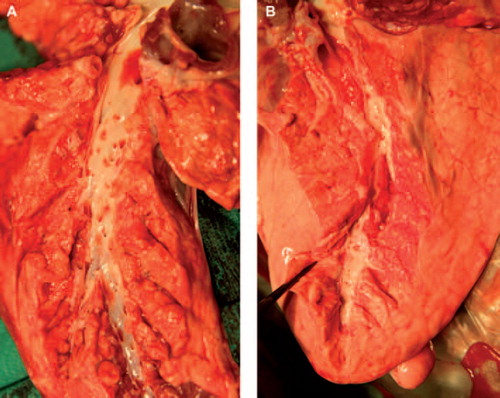
After completing the lung evaluation the pulmonary arterial branches were macroscopically studied for thrombotic material by opening the arteries as far distally as possible (). No thrombotic material was observed in either of the groups.
Figure 3. After lung evaluation the pulmonary arterial branches were macroscopically studied for thrombotic material by opening the arteries as far distally as possible. No thrombotic material was observed in neither of the groups. shows a pulmonary graft not treated with heparin, and a pulmonary graft treated with heparin.
Discussion
Lung transplantation is used to treat patients with a variety of end-stage pulmonary diseases (Citation1). While indications for lung transplantation continue to increase, widespread application of this procedure remains limited by the lack of suitable donor organs (Citation5). Therefore, many investigations have focused on ways of minimizing lung injury during excision, transport and transplantation, and the expansion of the donor pool using organs from NHBDs (Citation3,Citation5,Citation7).
We have recently reported the results of the first six double lung transplantations performed in the world with donor lungs that were rejected for transplantation by the Scandiatransplant, Eurotransplant, and the UK transplant organizations after ex vivo evaluation due to poor arterial oxygen tension (Citation19). The EVLP method, which has been described in detail previously (Citation19–21), has been suggested as a novel method of differentiating between “good” and “poor” pulmonary grafts in this sub-optimal population. When grafts characterized as good using this method were transplanted, the results were no different from those with lungs fulfilling the standard criteria (Citation20). EVLP provides an excellent tool to reveal lung pathology and evaluate lung function prior to decision making regarding lung transplantation, and the number of potential lungs available for transplantation will thus increase. EVLP also serves as an excellent method of evaluating lung function in experimental lung preservation and lung transplantation (Citation22,Citation23).
Lung preservation remains a major issue in both HBDs and NHBDs (Citation12). In clinical lung transplantation today organ donors receive heparin before organ harvest with the intention of avoiding thrombosis in the pulmonary grafts. Since heparin administration is routine in organ harvesting from HBDs, it may appear logical to use the same routine in organ harvesting from NHBDs. However, the timing of the heparin administration presents an ethical dilemma. In the case of a brain-dead donor/HBD, the administration of heparin occurs after the declaration of death. In the case of a NHBD, in order for the heparin to circulate through the body, it must be administered before the declaration of death, or given intravenously followed by heart compression after unsuccessful heart resuscitation after death is declared, but before permission has been obtained for donation (Citation14).
In the present study, we have investigated whether or not heparin actually improves the pulmonary function of grafts obtained from HBDs, by comparing the lungs from one group of pigs given heparin according to the standard procedure in organ harvesting, with those from another group not given heparin before lung harvesting. We stored the pulmonary grafts from both groups in cold Perfadex at 8°C for 4 h according to the golden standard. The lungs were then connected to the EVLP circuit, where they were gradually warmed up. When they had reached 37°C, the lungs were evaluated regarding blood gas oxygenation at different fractions of inspired oxygen. We found no significant differences between the two groups when comparing the blood gas oxygenation at the different fractions of inspired oxygen. All the lungs from both groups satisfied the criteria for clinical lung transplantation, regarding blood gas oxygenation at an inspired oxygen fraction of 1.0 for 5 min (> 40 kPa). Blood gases are deemed the most important and easily obtained end-point parameters in the clinical situation in lung donation.
In our setting, we could not detect any thrombus formation in the main pulmonary artery when inserting the pulmonary artery cannula for antegrade flushing with Perfadex in either of the groups. No thrombosis was observed in the large vessels after organ harvesting. We believe that the absence of thrombosis in the non-heparin group is due to the excellent autothrombolytic capacity of the lungs to dissolve thromboses. No problems related to thrombotic occlusions, such as inferior oxygenation, elevated PVR, or areas of visible hypoperfusion were observed in either group during EVLP. Upon completion of EVLP the pulmonary arterial branches were macroscopically studied for thrombotic material by opening the arteries as far distally as possible. No thrombosis was observed in either group.
Our research team and that of Cypel et al. have recently demonstrated in two separate studies that EVLP is a reliable method of predicting graft function in the recipient after transplantation (Citation23,Citation24). Although we realize that the optimal test of lung function is their performance in the recipient after transplantation, we still believe that the results of this study are important.
Some researchers have even implied that heparin may have a negative impact on pulmonary grafts. Bai et al., working on the Toronto lung transplant program, have measured tumor necrosis factor-a mRNA as a marker for ischemia–reperfusion injury in rat pulmonary grafts, and the results indicated that residual heparin in lung tissue increases the risk of ischemia–reperfusion injury in the pulmonary graft.
Conclusion
Our results indicate that pulmonary grafts from HBDs are not dependent on heparin administration prior to lung harvesting, and provide important insight in the issue of whether or not heparin is crucial for lung harvesting in NHBDs. However, heparin is clearly of importance in other organ harvesting procedures such as heart harvesting for heart transplantation, as there is no autothrombolytic effect in the coronary arteries, and not using heparin in heart harvesting for organ transplantation would probably result in acute heart failure.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dilling DF, Glanville AR. Advances in lung transplantation: the year in review. J Heart Lung Transplant. 2011;30: 247–51.
- Knudsen L, Boxler L, Muhlfeld C, Schaefer IM, Becker L, Bussinger C, et al. Lung preservation in experimental ischemia/reperfusion injury and lung transplantation: a comparison of natural and synthetic surfactants. J Heart Lung Transplant. 2012;31:85–93.
- Dominguez-Gil B, Haase-Kromwijk B, Van Leiden H, Neuberger J, Coene L, Morel P, et al. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676–86.
- Egan TM, Lambert CJ Jr, Reddick R, Ulicny KS Jr, Keagy BA, Wilcox BR. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg. 1991;52:1113–20; discussion 20–1.
- Cypel M, Yeung JC, Keshavjee S. Novel approaches to expanding the lung donor pool: donation after cardiac death and ex vivo conditioning. Clin Chest Med. 2011;32:233–44.
- Bolys R, Ingemansson R, Sjoberg T, Steen S. Vascular function in the cadaver up to six hours after cardiac arrest. J Heart Lung Transplant. 1999;18:582–6.
- De Vleeschauwer S, Van Raemdonck D, Vanaudenaerde B, Vos R, Meers C, Wauters S, et al. Early outcome after lung transplantation from non-heart-beating donors is comparable to heart-beating donors. J Heart Lung Transplant. 2009;28:380–7.
- Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc. 1995;27:2893–4.
- Pizanis N, Petrov A, Heckmann J, Wiswedel I, Wohlschlager J, de Groot H, et al. A new preservation solution for lung transplantation: evaluation in a porcine transplantation model. J Heart Lung Transplant. 2012;31:310–7.
- Ingemansson R, Massa G, Pandita RK, Sjoberg T, Steen S. Perfadex is superior to Euro-Collins solution regarding 24-hour preservation of vascular function. Ann Thorac Surg. 1995;60:1210–4.
- Steen S, Kimblad PO, Sjoberg T, Lindberg L, Ingemansson R, Massa G. Safe lung preservation for twenty-four hours with Perfadex. Ann Thorac Surg. 1994;57:450–7.
- Marasco SF, Bailey M, McGlade D, Snell G, Westall G, Oto T, Pilcher D. Effect of donor preservation solution and survival in lung transplantation. J Heart Lung Transplant. 2011;30:414–9.
- Ohsumi A, Chen F, Sakamoto J, Nakajima D, Hijiya K, Motoyama H, et al. Protective effect of pre-recovery surfactant inhalation on lungs donated after cardiac death in a canine lung transplantation model. J Heart Lung Transplant. 2012;31:1136–42.
- Motta ED. The ethics of heparin administration to the potential non-heart-beating organ donor. J Prof Nurs. 2005; 21:97–102.
- Bai X, Fischer S, Keshavjee S, Liu M. Heparin interference with reverse transcriptase polymerase chain reaction of RNA extracted from lungs after ischemia-reperfusion. Transpl Int. 2000;13:146–50.
- Erasmus ME, Verschuuren EA, Nijkamp DM, Vermeyden JW, van der Bij W. Lung transplantation from nonheparinized category III non-heart-beating donors. A single-centre report. Transplantation. 2010;89:452–7.
- Wallinder A, Steen S, Liden H, Hansson C, Hussein AA, Sjoberg T, Dellgren G. Heparin does not improve graft function in uncontrolled non-heart-beating lung donation: an experimental study in pigs. Eur J Cardiothorac Surg. 2013;43: 413–9.
- Sanchez PG, Bittle GJ, Williams K, Pasrija C, Xu K, Wei X, et al. Ex vivo lung evaluation of prearrest heparinization in donation after cardiac death. Ann Surg. 2013;257:534–41.
- Ingemansson R, Eyjolfsson A, Mared L, Pierre L, Algotsson L, Ekmehag B, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87:255–60.
- Lindstedt S, Eyjolfsson A, Koul B, Wierup P, Pierre L, Gustafsson R, Ingemansson R. How to recondition ex vivo initially rejected donor lungs for clinical transplantation; clinical experience from Lund University Hospital. J Transpl. 2011;2011:754383.
- Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83:2191–4.
- Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27: 1319–25.
- Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–40.
- Lindstedt S, Hlebowicz J, Koul B, Wierup P, Sjogren J, Gustafsson R, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact Cardiovasc Thorac Surg. 2010.
