Abstract
Objectives. Coronary artery bypass grafting (CABG) using bilateral internal thoracic artery (BITA) has been proven to improve survival. Many surgeons use the composite Y-graft which is made of left ITA (LITA) and right ITA (RITA) grafts. The LITA is typically anastomosed to left anterior descending artery (LAD). However, we have used RITA for LAD instead of LITA and reviewed the patency of ITA grafts and their clinical outcomes. Methods. We analyzed 48 patients who underwent CABG using a BITA composite Y-graft from 2002 to 2012. In 30, LITA was anastomosed to LAD (Group L). The other 18 had RITA to LAD anastomosis (Group R). Results. The mean age of Group R was higher than that of Group L (p = 0.009). Postoperative angiography was performed in 35 patients (73%). Two patients in Group L and none of the patients in Group R had an ITA graft failure. The incidence of ITA graft failure and new adverse cardiovascular events were not different between the two groups. Conclusion. The clinical outcome of RITA to LAD anastomosis is comparable with anastomosis of LITA to LAD in CABG using BITA composite Y-grafts. This technique may be useful when longer and larger ITA grafts are needed.
Key words::
Introduction
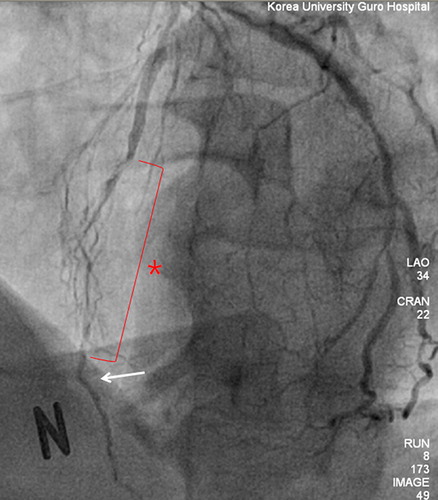
Coronary artery bypass grafting (CABG) using the bilateral internal thoracic artery (ITA) provides better long-term survival than CABG using single ITA (Citation1–3). One of the popular configurations of bilateral ITA is a composite Y-graft using in situ left ITA (LITA) and free right ITA (RITA) grafts (Citation4,Citation5). In CABG using bilateral ITA composite Y-grafts, the LITA graft is usually anastomosed to the left anterior descending (LAD) artery and the RITA graft is anastomosed to a coronary artery other than the LAD artery (Citation6,Citation7). This grafting strategy has been routinely used in many centers and shown to have excellent outcomes (Citation5,Citation6,Citation8). However, the suitable area of the LAD artery for anastomosis can often be distal (). The ITA graft becomes small in its distal portion and rich in smooth muscle. Thus, the use of distal ITA increases the risk of vasospasm as well as technical errors (Citation9,Citation10). We have used RITA grafts for the LAD artery when longer or larger ITA grafts are needed. Herein, we compare the surgical outcome of RITA-to-LAD anastomosis to that of LITA-to-LAD in CABG using composite Y-grafts.
Methods
From January 2002 to April 2012, we identified 48 patients who underwent CABG with BITA. This clinical study was reviewed and approved by the Korea University Guro Hospital Institutional Review Board.
Patient profile
During the study period, 48 patients underwent CABG with BITA. Composite Y-grafts with free RITA and in situ LITA were used in all patients. There were six patients (13%) who had preoperative percutaneous coronary intervention. None of the patients had previous cardiac surgery. The preoperative ejection fraction was moderately or severely depressed (< 45%) in 11 patients (23%). All procedures were electively performed. Thirty patients (63%) underwent LITA-to-LAD anastomosis (Group L) and 18 patients (38%) underwent RITA-to-LAD anastomosis (Group R).
Surgical technique
Operations were performed using standard primary median sternotomy, hypothermic cardiopulmonary bypass with single or double venous cannulas, and antegrade and/or retrograde cold crystalloid cardioplegia for myocardial protection. Before cardiopulmonary bypass, the LITA was taken down in a pedicled or skeletonized fashion. The RITA was always harvested in a skeletonized manner. The radial artery or saphenous vein was harvested according to the number of distal targets. After the RITA was harvested, it was anastomosed to the side of the LITA to construct a composite Y-graft. This was performed before distal anastomosis. Cardiopulmonary bypass was not used in three patients (6%). Thus, off-pump CABG was not our routine practice. In general, the LITA was the conduit of choice for the LAD artery. When a longer or larger graft for the LAD artery was needed, RITA grafts were used. There were two concomitant cardiac operations performed, aortic valve replacement and mitral valve repair.
Outcome measures
Coronary angiography was performed by either conventional angiography or computed tomographic (CT) angiography. ITA graft failure was defined as more than 50% stenosis or complete occlusion of the ITA graft. New cardiovascular events were defined as recurrent angina or myocardial infarction due to new native coronary artery or graft disease, any readmissions due to cardiovascular intervention, and cardiac death including sudden death. In-hospital mortality was defined as death in the same hospital admission as CABG surgery. Thirty-day mortality was defined as death within 30 days after surgery. Survival status was collected from medical records and telephone interviews.
Statistical analysis
Descriptive statistics for categorical variables are reported as frequency and percentage while continuous variables are reported as mean ± standard deviation (range) as appropriate. Categorical variables were compared between Groups L and R using the Chi-squared test or Fisher's exact test and continuous variables were compared using the two sample t-test or Wilcoxon rank sum test where appropriate. All statistical tests were two-sided with the alpha level set at 0.05 for statistical significance.
Results
The mean age of the patients was 58 ± 7.62 years and 40/48 (83%) were male. Twenty patients (42%) had diabetes mellitus. There were no emergency operations. Twenty-one (44%) patients had both left main and triple coronary artery disease. There was no difference in patient characteristics between Groups L and R except for age at the time of operation. Patients in Group L were younger than those in Group R. The average of preoperative ejection fractions was mildly depressed to 52 ± 10.6% (range, 20–63). Details of patients’ characteristics are shown in .
Table I. Patient characteristics.
There were no operative complications such as reoperation for bleeding, deep sternal infection, or low cardiac output syndrome. One patient died of aspiration pneumonia and hypoxic brain damage 158 days after surgery. Follow-up coronary angiography was performed in 18 patients during the same hospital admission (median days after surgery: 10.5) and 17 patients after discharge (median days after surgery: 394). ITA graft failure was found in two patients who had LITA–LAD anastomosis (Group L). One patient had 80% stenosis at the distal LITA–LAD anastomosis site. The other patient had complete occlusion of the RITA graft which was connected to an obtuse marginal branch. There was no ITA graft failure in Group R. There were five new cardiovascular events involving two patients in Group L and three in Group R. Two patients in Group L had recurrent angina due to ITA graft failures. In Group R, two of the three patients had recurrent chest pain caused by occlusion of a non-bypassed coronary artery and saphenous vein graft occlusion. The other patient had pericardial effusion requiring pericardiocentesis. No late deaths were noted during the follow-up. Details of operative and postoperative outcomes are described in .
Table II. Operative and postoperative outcomes.
Discussion
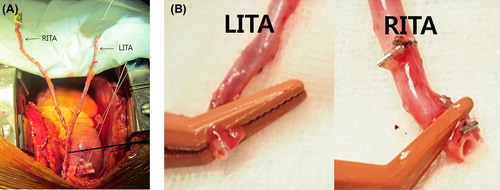
The impact on late survival of CABG using BITA has been demonstrated in previous studies (Citation1–4). However, the configuration of the BITA was variable (Citation4–6). While the LITA is almost always used as an in situ graft, the RITA can be used as a free or in situ graft. In situ RITA grafts can extend to the proximal LAD artery, right coronary artery, or proximal branches of the circumflex artery. If the RITA is used as a free graft, many surgeons make a composite Y- or T-graft. In using a composite Y-graft, the LITA is traditionally anastomosed to the LAD artery. Although LITA–LAD anastomosis has been the gold standard, it has three potential limitations. The first is the limited length of the LITA. Longer LITA grafts can be obtained by skeletonization and proximal dissection. There are many circumstances, such as a large heart, distal location of the critical stenosis, or emphysematous lungs, which require longer conduits. The second limitation is the small diameter of the ITA graft. The size of the ITA is quite variable and the ITA becomes small in diameter in its distal area. Therefore, the use of the distal part of the ITA can produce a flow limitation and higher risk of technical errors. Lastly, the distal one-third of the ITA is known to be rich in smooth muscle and prone to spasm (Citation10). Because the LAD is the most important coronary artery, we avoided using the distal LITA for the LAD artery. Thus, RITA–LAD anastomosis can be a viable option if a longer and larger ITA conduit is needed. When the RITA is harvested for the LAD artery, dissection of only the proximal two-thirds of the RITA is necessary. The distal end of the RITA graft is not rich in smooth muscle and has a large enough diameter for the LAD artery. In addition, there is less risk of spasm, technical problems, or stretched ITA grafts ().
Figure 2. A composite Y-graft using the BITA: The RITA is long enough to reach the distal left anterior descending artery (A). The diameter of the RITA is larger than that of the LITA because the end of the RITA is usually within two-thirds of the entire RITA (B).The more proximal and larger portion of the RITA can be used for the left anterior descending artery rather than the LITA.
Our indications of RITA–LAD anastomosis were LAD stenosis further than the mid-portion and small diameter of the distal LITA graft. During the study period, we observed two changes in our surgical strategy. One was that we have become more aggressive in using BITA for older patients. The other was that we have used RITA–LAD anastomosis more liberally than before, since we have realized its merits which are a large distal ITA end, longer length of graft, and less risk of spasm. Those can explain that patients in Group R were older than those in Group L in preoperative characteristics. Other than age, there were no significant preoperative characteristics between Groups L and R.
In this study, we observed five new cardiovascular events in 48 patients. There were two ITA graft failures in Group L. One patient had recurrent chest pain 1 year after surgery and was found to have an occluded RITA graft which was anastomosed to an obtuse marginal branch. The other patient also had recurrent angina after surgery. The coronary angiography revealed 80% stenosis at the LITA–LAD anastomosis site. None of the patients in Group R had ITA graft failure. The three other cardiovascular events involved two patients with recurrent chest pain caused by occlusion of a non-bypassed branch of left circumflex coronary artery and occlusion of the saphenous vein graft supplying the right coronary artery. The other patient had massive pericardial effusion after discharge which required pericardiocentesis. There was one in-hospital mortality and no late deaths. This patient was found to have coronary artery disease during the work-up for surgery for gastric cancer. The coronary artery bypass surgery was uneventful, but the patient died from aspiration pneumonia during recovery. Thus, there was no significant difference in the clinical outcomes between Groups L and R.
A potential problem of RITA–LAD anastomosis for composite Y-grafts is that the flow from the LITA crosses the surgical anastomosis site between two ITA grafts. The quality of LITA and RITA anastomoses can affect the flow to the LAD artery. Therefore, LITA and RITA anastomoses should be perfect and done by an experienced surgeon.
There is another limitation to this technique. Because the LITA is not long enough to reach the distal portion of the left circumflex coronary artery, distal branches such as the posterior lateral branch cannot be bypassed with the LITA. In our technique, two ITAs are usually not sufficient to bypass all three coronary arteries because of the short length of LITA. This may increase the need for the third conduit such as saphenous vein, radial artery, or gastroepiploic artery. Thus, RITA–LAD anastomosis may be useful in only specific situations and therefore may not be a standard technique.
This study's retrospective design and small cohort limit the generalizability of our findings. However, the aim of our study was to prove the safety and clinical feasibility of RITA–LAD anastomosis in CABG using a BITA composite Y-graft. We did not find any ITA graft failure in Group R. The incidence of cardiovascular events or mortality was not different between Groups L and R. The timing of postoperative coronary angiography was variable, but was mostly within 1 year after surgery. Therefore, our data only suggest early postoperative patency. Because RITA–LAD anastomosis in composite Y-graft has one more suture line before distal anastomosis, the long-term results of RITA–LAD anastomosis should be investigated in the future.
In conclusion, using the RITA for LAD bypass is a viable option in CABG using composite Y-grafting with BITA. This technique did not increase procedural time or postoperative complications. Either LITA or RITA for the LAD artery did not affect the angiographic patency of the grafts. RITA–LAD anastomosis may be useful in some patients who have distal LAD target lesions or a relatively large LAD artery and small LITA.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lytle BW, Blackstone EH, Sabik JF, Houghtaling P, Loop FD, Cosgrove DM. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg. 2004;78: 2005–12; discussion 12–4.
- Fiore AC, Naunheim KS, Dean P, Kaiser GC, Pennington G, Willman VL, et al. Results of internal thoracic artery grafting over 15 years: single versus double grafts. Ann Thorac Surg. 1990;49: 202–8; discussion 8–9.
- Kurlansky PA, Traad EA, Dorman MJ, Galbut DL, Zucker M, Ebra G. Thirty-year follow-up defines survival benefit for second internal mammary artery in propensity-matched groups. Ann Thorac Surg. 2010;90: 101–8.
- Locker C, Schaff HV, Dearani JA, Joyce LD, Park SJ, Burkhart HM, et al. Multiple arterial grafts improve late survival of patients undergoing coronary artery bypass graft surgery: analysis of 8622 patients with multivessel disease. Circulation. 2012;126: 1023–30.
- Calafiore AM, Contini M, Vitolla G, Di Mauro M, Mazzei V, Teodori G, et al. Bilateral internal thoracic artery grafting: long-term clinical and angiographic results of in situ versus Y grafts. J Thorac Cardiovasc Surg. 2000;120: 990–6.
- Kim KB, Cho KR, Chang WI, Lim C, Ham BM, Kim YL. Bilateral skeletonized internal thoracic artery graftings in off-pump coronary artery bypass: early result of Y versus in situ grafts. Ann Thorac Surg. 2002;74: S1371–6.
- Navia D, Vrancic M, Vaccarino G, Piccinini F, Raich H, Florit S, et al. Total arterial off-pump coronary revascularization using bilateral internal thoracic arteries in triple-vessel disease: surgical technique and clinical outcomes. Ann Thorac Surg. 2008;86: 524–30.
- Kim WS, Lee J, Lee YT, Sung K, Yang JH, Jun TG, et al. Total arterial revascularization in triple-vessel disease with off-pump and aortic no-touch technique. Ann Thorac Surg. 2008;86: 1861–5.
- Lorusso R, Crudeli E, Luca F, De Cicco G, Vizzardi E, D’Aloia A, et al. Refractory spasm of coronary arteries and grafted conduits after isolated coronary artery bypass surgery. Ann Thorac Surg. 2012;93: 545–51.
- He GW, Ryan WH, Acuff TE, Yang CQ, Mack MJ. Greater contractility of internal mammary artery bifurcation: possible cause of low patency rates. Ann Thorac Surg. 1994;58: 529–32.