Abstract
Objectives. It is currently recommended that the left ventricular (LV) lead be placed at the posterolateral or lateral wall of heart during cardiac resynchronization therapy (CRT). The aim of our study is to evaluate the influence of interlead distance on immediate and mid-term response to CRT with altered right ventricular (RV) pacing site. Design. A total of 35 consecutive patients underwent CRT for standard indications. RV pacing site was altered from RV outflow tract (RVOT) to RV apex (RVA) in the course of implantation, permitting assessment of a “poorer response” and a “better response” site based on intraprocedural aortic velocity time integral (aVTI). LV–RV interlead distances were compared between these sites during operation. We also made a comparison of the interlead distances between responders and non-responders 6 months after CRT. Results. In the process of CRT implantation, the better response site showed significantly larger interlead distance (16.5 ± 4.4 cm vs.12.4 ± 5.6 cm, p = 0.001) as well as its vertical component (9.8 ± 4.8 cm vs. 4.3 ± 2.8 mm, p = 0.001) on lateral fluoroscopy view as compared with the poorer response site. Furthermore, RVA proved more likely to be the “better response” site than RVOT (91% vs. 9%, p = 0.001). At 6-month -up, responders demonstrated larger direct interlead distance (18.1 ± 4.5 cm vs. 14.8 ± 3.5 cm, p = 0.011) and horizontal interlead distance (14.1 ± 6.6 cm vs. 8.3 ± 6.1 cm, p = 0.004) on the lateral radiograph in comparison with non-responders with great significance. Conclusions. Larger interlead distance on lateral fluoroscopy view is associated with more favorable immediate and mid-term response to CRT. Use of these findings may help to maximize the benefit derived from CRT.
Introduction
Nowadays cardiac resynchronization therapy (CRT) is widely performed in patients with pharmacological refractory heart failure, and has been proved to be quite effective in appropriately selected patients (Citation1,Citation2). However, about 20–40% of the patients show poor response to CRT (Citation3). A number of factors including etiology of heart failure, renal function, atrial fibrillation and QRS morphology play an important role in the clinical outcome of CRT (Citation4–7). With regard to left ventricular (LV) stimulation, a posterolateral or lateral tributary of the coronary venous system has been reported to be the most optimal pacing site (Citation8,Citation9). Given that CRT is intended to improve ventricular synchrony by coordinating LV and right ventricle (RV) contraction, efforts to maximize RV to LV electrode separation have been advocated (Citation10). Since the LV lead positioning is often restricted due to anatomical variations, unacceptable electrical parameters, phrenic nerve stimulation or other technical problems, can we choose an appropriate RV lead position to maximize CRT response, which could be more easily selected by operators? The goal of the present study is to investigate the association of immediate and mid-term response to CRT with LV–RV lead distances.
Methods
Study population
Thirty-five consecutive patients admitted for CRT from January 2011 to June 2012, were studied. All patients had New York Heart Association (NYHA) heart failure functional Class III or IV with reduced LV ejection fraction (≤ 35%) and prolonged QRS duration (≥ 120 ms) after optimal medical therapy for at least 3 months, including β-blocker, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), aldosterone antagonist, diuretics and digoxin if necessary. Etiologies included ischemic and non-ischemic cardiomyopathy. Before device implantation, all subjects underwent history inquiry, physical examination, 12 lead-electrocardiogram (ECG) and transthoracic echocardiography. A written informed consent was also obtained from each patient before the implantation.
12 Lead-electrocardiogram
All ECGs were recorded at a 50 mm/s sweep speed. QRS duration was measured using hand-held calipers, on the lead showing the widest complex (Citation11). Paced QRS duration was measured from the pulse signal to the end of complex, on the lead with the widest complex. Complete left bundle branch block (CLBBB) is defined as a QRS duration of ≥ 120 ms with a monophasic QS or RS complex in lead V1 and a monophasic R wave in lead V6, and complete right bundle branch block (CRBBB) is defined as a QRS duration of ≥ 120 ms with a secondary R wave (R’) in lead V1 which is greater than the initial R wave (rsR’) and a wide deep terminal S wave in leads II, V5 and V6 (Citation7).
Echocardiography
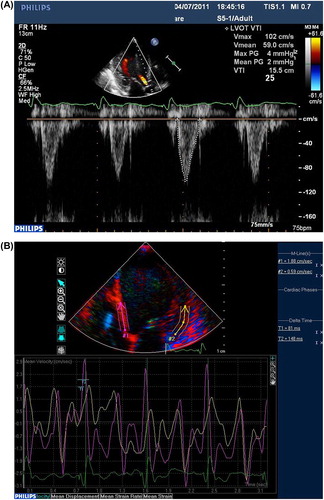
All the echocardiographic parameters were obtained by one echocardiologist using Phillip IE 33 (S5-1) cardiac ultrasound machine. LV end-diastolic diameter (LVEDD) and end-systolic diameter (LVESD) were measured by M-mode echocardiography in the parasternal long-axis view. As for LV ejection fraction (LVEF), we first derived the left ventricular end- diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV), LVEF was calculated from the conventional apical 2- and 4-chamber images, using the biplane Simpson's technique. The severity of tricuspid or mitral valve regurgitation was graded semi-quantitatively from color flow Doppler in the parasternal long axis and apical four-chamber images. The regurgitation was graded on a 3-point scale: mild (jet area/atrial area < 20%), moderate (jet area/atrial area 20–45%) and severe (jet area/atrial area > 45%) (Citation12). In order to evaluate LV dyssynchrony, tissue Doppler imaging (TDI) was acquired in 12 basal- and mid-ventricular segments from the apical 2-, 3- and 4-chamber views with high frame rate (> 80 frames/s). Myocardial velocity curves were obtained and the time to peak velocity during ejection phase (Ts) was measured in each segment, using QRS onset as a reference. Then the standard deviation (SD) of Ts of all 12 segments (Ts-SD) was computed to assess globle LV synchronicity (Citation13,Citation14). The aortic velocity was recorded just above the aortic valve using pulsed wave Doppler and the aortic velocity time integral (aVTI)of at least three beats was measured and averaged, with the purpose of gaining hemodynamic assessment () (Citation15,Citation16).
CRT protocol and acute hemodynamic assessment
Pacemaker implantation was performed with standard techniques via left subclavian vein, and devices were manufactured by Medtronic, Biotronik or St Jude Cooperations. Intraoperatively, coronary sinus angiography was performed using a balloon catheter and a target vein was identified. We placed the LV lead in a lateral or posterolateral tributary of coronary vein as far as possible. If there was no accessible lateral or posterolateral tributary, an anterior or inferior lead position was selected. Wherever the LV lead was placed, it produced an acceptable pacing threshold without phrenic nerve stimulation. The atrial lead was routinely placed in right auricle. The RV lead was first placed at right ventricular outflow tract (RVOT) septum, getting a satisfactory pacing threshold. Then we paced LV and RV simultaneously in VOO mode at a rate of 90 bpm with a suitable output which assured that both ventricles were captured. Afterwards, the 12-lead ECG was performed. Transthoracic echocardiography was performed after 3 min of pacing when a relatively stable hemodynamic parameter could be obtained. The Ts-SD was calculated as asynchrony of LV, and aVTI was measured as the acute hemodynamic effect of CRT. After that, we preserved the fluoroscopy in a posteroanterior (PA) and a left lateral view, with the atrial lead, RV lead and LV lead contained. Then the RV lead was placed at right ventricular apex (RVA) with optimal pacing threshold. Then the same procedure was carried out as above. Both the ECG and echocardiography were carried out with the caution that the operation site should not be contaminated. In the end, we implanted the RV lead at the place where a higher aVTI was acquired, which was defined as a “better response”site. In contrast, another RV pacing site which provided a lower aVTI was defined as a “poorer response”site. Finally all leads were connected to the pulse generator conventionally.
Interlead distance measurements
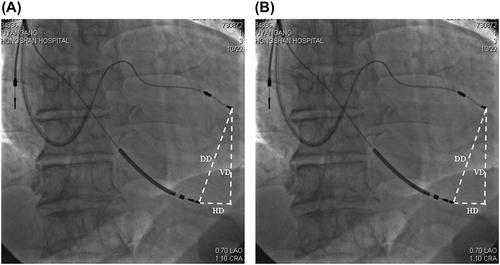
As is mentioned above, we preserved the fluoroscopy in a PA and a left lateral view for both “better response” and “poorer response” sites for each patient. The interlead distance was measured on a digital radiology work station by one cardiologist who was unaware of the hemodynamic results of the study. The direct RV-LV electrode tip distance and its horizontal and vertical components (defined as horizontal distance and vertical distance) were measured from the PA and left lateral fluoroscopy views, taking a tip-ring distance of atrial lead for reference, which was provided by the manufacturer. All patients underwent preprocedure PA chest radiography for measurements of a cardiothoracic ratio (CTR), which was defined as the ratio of the width of the cardiac silhouette to the width of the thoracic cavity measured at the level of the diaphragm. All interlead distances, including direct distance (DD), horizontal distance (HD) and vertical distance (VD), were divided by the cardiothoracic ratio to account for different cardiac and thoracic sizes between patients, thus creating the corrected interlead distances (Citation3,Citation10,Citation17). Theoretically, vertical interlead distance ought to be of the same value whether measured from PA or lateral views. Slight differences between VD measurements may be attributed to respiration and cardiac contraction of the patients (). In addition, the LV lead tip position was defined as anterior (within the great cardiac vein), lateral or posterolateral (within the posterolateral cardiac vein) and inferior (within the middle cardiac vein) according to the PA and left anterior oblique (LAO) fluoroscopy views.
Mid-term follow-up
All patients were interviewed 6 months after CRT implantation. Physical examination, ECGs, pacemaker interrogation, and transthoracic echocardiography were performed. Response was defined as a decrease of ≥ 15% in LVESV measured by echocardiographic ultrasound at 6-month follow-up. The remaining patients were considered as non- responders. A comparison of the interlead distance was made between responders and non-responders.
Statistical analysis
Continuous variables are expressed as mean ± SD. Categorical data are summarized as frequencies and percentages. The categorical characteristics were compared using χ2 and Fisher's exact tests for cell count < 5. The paired Student's t-test was used to compare the continuous data between the “better response” and “poorer response” sites in CRT procedure. Differences between variables in responders versus non-responders were evaluated by grouped Student's t-test. For all analysis, p < 0.05 was considered statistically significant. SAS 8.2 (SAS Institute Inc, USA) was applied to accomplish all the analysis.
Results
Patient characteristics
Baseline characteristics of the 35 patients included in this study are summarized in . Eight cases suffered from ischemic cardiomyopathy and 27 from non-ischemic cardiomyopathy. All baseline ECGs showed sinus rhythm and a CLBBB QRS morphology with a mean QRS duration of 164 ± 28 ms. Patients had severely depressed LV function with a mean LVEF of 29 ± 7% and obvious LV dyssynchrony, as indicated by a mean Ts-SD of 42 ± 12 ms, thus meeting the typical indications for CRT. Almost all patients received an ACEI or ARB (97%) and 94% were on beta blocker therapy. In addition, CRT-ICD (CRT-D) was implanted in 22 patients while CRT-P was implanted in 13 patients. No significant difference was noted between responders and non-responders on baseline clinical features.
Table I. Baseline characteristics of responders versus non-responders (n = 35).
CRT/CRT-D implantation
Device implantation was successful in all patients without procedure-related complications being reported. Though the desired LV lead position was the posterolateral vein, the LV pacing leads were positioned in the lateral or posterolateral region in 32 patients (91%). Two patients received an LV lead in the inferior region and one patient in the anterior region due to anatomical variations of cardiac vein or other technical difficulties. The RV lead position was determined by intraprocedural aVTI, which was placed where a higher aVTI could be acquired. As a result, 32 patients had a RV lead at RVA, while only 3 patients had a RV lead at RVOT septum. In the latter three patients, LV leads were positioned in the inferior and anterior regions.
Immediate echocardiographic assessment and interlead distance during implantation
As is mentioned, the “better response” and “poorer response” sites were defined according to intraprocedural aVTI. The better response site had a significantly larger direct interlead distance (which had been corrected) on the lateral film (p = 0.001), so was the corrected vertical interlead distance on both lateral and PA films (). In the present study, a better response was achieved with the RV lead more frequently localized at RVA (p = 0.001). Only three patients showed a better response with RVOT pacing, in whom the LV lead was anteriorly or inferiorly positioned. Though previous studies showed that RV apical pacing produced a wider QRS duration than RVOT septum, this is not the case with the combination of RV and LV pacing. The better the response was, the narrower the QRS complex seemed to be, in accordance with interlead separation (p < 0.05).
Table II. Comparison of interlead distance with different RV lead positioning.
Response to CRT at 6-month follow-up
At 6 months of follow-up, 18 (51%) patients were rated as responders with a reduction in LVESV ≥ 15%. No patient died 6 months after CRT. Compared with non-responders, responders significantly had a larger corrected interlead distance, together with its horizontal component, on the lateral X-ray. In the PA radiograph, no significant difference of interlead distance was found between responders and non- responders ().
Table III. Comparison of interlead distance between responders and non-responders at 6-month follow-up.
Discussion
As is widely known, response to CRT is associated with the diminishing of the septal-to-lateral delay, which is determined, in a great part, by the LV lead position. Though several studies (Citation3,Citation10,Citation17) have revealed that interlead distance on the lateral thoracic X-ray correlates with CRT response, most of those interlead distance diversities resulted from different LV lead positions and were measured postprocedurally on thoracic X-ray. While in the present study, we altered the RV lead position intraprocedurally and carried out immediate hemodynamic assessment by means of echocardiography. To the best of our knowledge, the present study is the first of this kind to investigate the relationship between LV–RV interlead distance and immediate CRT response more accurately with the exclusion of baseline cardiac function, long-term medication therapy during follow-up and unsuitable life patterns. As a result, we not only found the relationship between the LV–RV interlead distance and immediate hemodynamic response, but also put it into clinical use just in the course of implantation.
Previous studies have elicited that LV–RV interlead distance on lateral radiograph, especially its horizontal component, is closely associated with both acute and mid-term hemodynamic response to CRT. Our results are in consistence with the available studies concerning mid-term response to CRT. However, the immediate hemodynamic effect in the present study proved to be mostly correlated with the vertical component of interlead distance (no matter from the PA view or from a lateral view) and a direct interlead distance on lateral fluoroscopy view. Whereas we should take into consideration that in previous studies, the horizontal interlead distance on the lateral film became progressively greater due to the anterolateral to the lateral to the posterolateral LV lead positions compared with the anteriorly positioned RV lead in different cases. It is not surprising that such kind of association could be found given that a number of studies have demonstrated improved outcomes with lateral and posterolateral LV pacing compared with anterior pacing sites (Citation18–20). While in our study we altered RV pacing site for each individual during the procedure, resulting in different interlead distances especially on the vertical plane instead of horizontal plane. Hence, a larger vertical interlead distance could be observed with the “better response”site in contrast with the aforementioned studies. From this viewpoint, we could conclude that the LV–RV interlead distance on the lateral chest radiograph correlated well with hemodynamic response to CRT, no matter it was driven by its horizontal or vertical component.
It is worth mentioning that neither immediate nor mid-term CRT response had correlation with the interlead distance on PA view, including its horizontal component. Now that a large part of left ventricle is just behind the right ventricle on PA radiograph, their spatial distance may overlap to a certain extent. Therefore, this projection scarcely reflects the real location. In the present study, even a shorter horizontal distance on a PA view tended to get a better response (not significantly).
In our study, 91% of the subjects had the LV lead placed in a lateral or posterolateral region, while 9% received the LV lead in a different location. It should be realized that obtaining the LV electrode site for best resynchronization is sometimes difficult because of the characteristics of coronary venous anatomy, unacceptable electrical parameters, phrenic nerve stimulation, or other technical difficulties. Nevertheless, the RV electrode site could be chosen more freely by operators. As is shown in our study, 32 cases displayed a better response with the RV lead placed at RVA, while another 3 cases showed a better response with the RV lead at RVOT septum. Buck et al. (Citation3) also found that non-responders more frequently had the RV lead localized in the RVOT. On the contrary, more studies (Citation21–23) showed no significant positive effect on CRT response of altering the RV lead position. Those studies discussed the impact of RV lead position on a 6–12-month follow-up using unpaired Student's t-test; while in the present study, we made an immediate hemodynamic assessment using a paired Student's t-test for each individual. Besides, previous studies discussed the effect of RV lead position regardless of LV lead location; our study carried out a self-contrast analysis, minimizing the confounding variables. For the 3 cases that showed a better response with the RV lead at RVOT septum, the LV lead happened to be anteriorly or inferiorly positioned, much closer to RV apex. Possibly, a larger interlead separation is created by placing the RV lead in the apex while LV lead is posterolaterally positioned; In contrast, RVOT septum pacing contributes to maximize interlead distance when LV is positioned close to apex. Thus we were able to conclude that CRT outcome resulted from a composite effect of LV and RV pacing site, rather than the LV or RV lead alone. The optimal RV lead location somewhat varied depending on the LV lead.
It needs to be pointed out that most candidates for CRT showed a CLBBB morphology on ECGs. The LV lead position is naturally most important. As mentioned, a lateral or posterolateral LV lead positioning is usually preferable, while an optimal RV lead helps to maximize the interlead distance on a lateral fluoroscopy view, adding to CRT benefits.
Limitations
The main limitation of our study is the small number of patients. Another limitation relates to the non-random selection of the patients. However, paired Student's t-test may have reduced the variance. After all, for individuals, the larger the interlead distance on lateral view is, the better one may respond to CRT.
Conclusions
The present study highlights that larger interlead distance on lateral fluoroscopy view is associated with better response to CRT, both intraprocedurally and at mid-term follow-up. In addition, RVA pacing is generally superior to RVOT pacing during biventricular pacing, especially when the LV lead is posterolaterally or laterally positioned.
Acknowledgments
The echocardiologists of Zhongshan Hospital are acknowledged for help to obtain echocardiographic images and data. We also appreciate the help of specialists at the radiology work station who instructed us in interlead measurements.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49.
- Buck S, Maass AH, Nieuwland W, Anthonio RL, Van Veldhuisen DJ, Van Gelder IC. Impact of interventricular lead distance and the decrease in septal-to-lateral delay on response to cardiac resynchronization therapy. Europace. 2008;10: 1313–9.
- Gasparini M, Mantica M, Galimberti P, Genovese L, Pini D, Faletra F, et al. Is the outcome of cardiac resynchronization therapy related to the underlying etiology?Pacing Clin Electrophysiol. 2003;26: 175–80.
- Díaz-Infante E, Mont L, Leal J, García-Bolao I, Fernández-Lozano I, Hernández-Madrid A, et al. SCARS Investigators. Predictors of lack of response to resynchronization therapy. Am J Cardiol. 2005;95: 1436–40.
- Rickard J, Kumbhani DJ, Gorodeski EZ, Baranowski B, Wazni O, Martin DO, et al. Cardiac resynchronization therapy in non-left bundle branch block morphologies. Pacing Clin Electrophysiol. 2010;33: 590–5.
- Chandra R, Zolty R, Palma E. A left hemiblock improves cardiac resynchronization therapy outcomes in patients with a right bundle branch block. Clin Cardiol. 2010;33: 89–93.
- Wilton SB, Shibata MA, Sondergaard R, Cowan K, Semeniuk L, Exner DV. Relationship between left ventricular lead position using a simple radiographic classification scheme and long-term outcome with resynchronization therapy. J Interv Card Electrophysiol. 2008;23: 219–27.
- Kleemann T, Becker T, Strauss M, Dyck N, Schneider S, Weisse U, et al. Impact of left ventricular lead position on the incidence of ventricular arrhythmia and clinical outcome in patients with cardiac resynchronization therapy. J Interv Card Electrophysiol. 2010;28: 109–16.
- Heist EK, Fan D, Mela T, Arzola-Castaner D, Reddy VY, Mansour M, et al. Radiographic left ventricular-right ventricular interlead distance predicts the acute hemodynamic response to cardiac resynchronization therapy. Am J Cardiol. 2005;96: 685–90.
- Ricci R, Pignalberi C, Ansalone G, Jannone E, Vaccaro MV, Denaro A, et al. Early and late QRS morphology and width in biventricular pacing: relationship to lead site and electrical remodeling. J Interv Card Electrophysiol. 2002;6: 279–85.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2: 358–67.
- Yu CM, Zhang Q, Fung JW, Chan HC, Chan YS, Yip GW, et al. A novel tool to assess systolic asynchrony and identify responders of cardiac resynchronization therapy by tissue synchronization imaging. J Am Coll Cardiol. 2005;45: 677–84.
- Yu CM, Gorcsan J 3rd, Bleeker GB, Zhang Q, Schalij MJ, Suffoletto MS, et al. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol. 2007;100: 1263–70.
- Doltra A, Vidal B, Silva E, Mont L, Tamborero D, Castel MÁ, et al. Comparison of hemodynamic versus dyssynchrony assessment for interventricular delay optimization with echocardiography in cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34: 984–90.
- Bhan A, Kapetanakis S, Monaghan MJ. Optimization of cardiac resynchronization therapy. Echocardiography. 2008;25: 1031–9.
- Merchant FM, Heist EK, Nandigam KV, Mulligan LJ, Blendea D, Riedl L, et al. Interlead distance and left ventricular lead electrical delay predict reverse remodeling during cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2010;33: 575–82.
- Macias A, Gavira JJ, Alegria E, Azcarate PM, Barba J, Garcia-Bolao I. Effect of the left ventricular pacing site on echocardiographic parameters of ventricular dyssynchrony in patients receiving cardiac resynchronization therapy. Rev Esp Cardiol. 2004;57: 138–45.
- Butter C, Auricchio A, Stellbrink C, Fleck E, Ding J, Yu Y, et al. Pacing Therapy for Chronic Heart Failure II Study Group. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation. 2001;104: 3026–9.
- Rossillo A, Verma A, Saad EB, Corrado A, Gasparini G, Marrouche NR, et al. Impact of coronary sinus lead position on biventricular pacing: mortality and echocardiographic evaluation during long-term follow-up. J Cardiovasc Electrophysiol. 2004;15: 1120–5.
- Kristiansen HM, Vollan G, Hovstad T, Keilegavlen H, Faerestrand S. A randomized study of haemodynamic effects and left ventricular dyssynchrony in right ventricular apical vs. high posterior septal pacing in cardiac resynchronization therapy. Eur J Heart Fail. 2012;14: 506–16.
- Thébault C, Donal E, Meunier C, Gervais R, Gerritse B, Gold MR, et al. Sites of left and right ventricular lead implantation and response to cardiac resynchronization therapy observations from the REVERSE trial. Eur Heart J. 2012;33: 2662–71.
- Khan FZ, Salahshouri P, Duehmke R, Read PA, Pugh PJ, Elsik M, et al. The impact of the right ventricular lead position on response to cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2011;34: 467–74.