Abstract
Objectives. This study investigates the effect of aerobic interval training on diastolic function at rest and during exercise in stable heart transplant (HTx) recipients. Design. Twenty-three stable HTx recipients (74% males, mean age 50 ± 14.9 years) were recruited to a training programme. Intervention was 8 weeks intensive training or control in a randomized controlled design. Results. At baseline, participants had normal or mild diastolic dysfunction at rest. During exercise, mean E/e′ increased from 9.0 (± 2.8) to 12.8 (± 7.7) (p = 0.09), E/A increased from 2.1 (± 0.6) to 2.6 (± 0.7) (p = 0.02), and deceleration time decreased by over 50 ms, all markers of increased filling pressure. There were no correlations between diastolic function and VO2peak at baseline. After intervention VO2peak increased from 23.9 (± 4.5) to 28.3(± 6) ml/kg/min in the training group (difference between groups p = 0.0018). No consistent pattern of improvement in diastolic function at rest or during exercise was seen. Conclusion. The study does not support a role of diastolic dysfunction in the limited exercise capacity of HTx recipients and suggests that in these patients peripheral factors are of greater importance.
Introduction
In heart transplant (HTx) recipients exercise performance (VO2peak) is generally limited to approximately 50–70% of age-predicted value despite normal left ventricular systolic function (Citation1). Chronotropic incompetence due to cardiac denervation and diastolic dysfunction are believed to be the major causes (Citation1–3). The first signs of cardiac impairment present during physical stress and stress echocardiography are becoming an important method to unmask diastolic dysfunction in patients with dyspnoea (Citation4,Citation5). Echocardiographic diastolic parameters E/e′ have been shown to correlate with pulmonary capillary wedge pressure (PCWP) in HTx patients at rest and could predict “day to day” changes in PCWP (Citation6). Only one recent study has focused on diastolic function in HTx patients and the effect of training. Nytroen et al. performed high-intensity interval training in stable HTx patients and found that training was highly efficient in improving exercise capacity, but did not modulate left ventricular function (Citation7). A sub-study investigated exercise stress echo and found no effect of training on left ventricular function during exercise (Citation8). Diastolic function can be improved with modulation of E/e′, left atrial size and deceleration time (Dec.time) by exercise intervention in patients with heart failure with preserved ejection fraction and in untrained elderly subjects (Citation9). We have previously shown that intensive exercise training improves VO2peak and endothelial function, decreases blood pressure and has beneficial effects on inflammatory markers and quality of life in stable HTx recipients (Citation10,Citation11). The aims of this study were (Citation1) to investigate left ventricular diastolic function at rest and during exercise in stable HTx recipients, (Citation2) to relate the findings to exercise capacity before and after an intensive 8 weeks training programme and (Citation3) to evaluate if any echocardiographic parameter at rest or during exercise is predictive of the effect of training.
Materials and methods
We recruited stable HTx recipients from the outpatient transplantation clinic at the department of Cardiology, Copenhagen University Hospital, Rigshospitalet. Inclusion criteria were age above 18 years; more than 1 year post-transplantation and capable of performing exercise training. Exclusion criteria were plasma creatinine above 200 μmol/l or on dialysis; rejection episodes (greater than 1R) in the previous 3 months; severe cardiac allograft vasculopathy (CAV) or malignant disease, multi-organ transplantation or re-transplantation. The randomization was performed by an impartial person as an envelope randomization after stratification by gender. This study is an echocardiographic substudy to the study by Hermann and that no specific power calculation was performed (Citation11).
Exercise testing/training
Cardiopulmonary exercise testing (CPET) with measurement of VO2peak was performed as a maximal symptom limited bicycle ergometer test (Via Sprint 150P, Ergoline, Germany). Breathing gases were collected and analyzed (Jaeger, Master Screen CPX vers.5.21, Cardinal Health, Germany). A respiratory exchange ratio greater than 1.10 indicated a valid test. The patients were not asked to discontinue beta-blocker treatment before the test.
The training programme lasted 8 weeks and was designed as high intensity training with three supervised sessions weekly at a single dedicated Cardiac Rehabilitation Clinic at Bispebjerg University Hospital. Baseline levels of VO2peak, maximal heart rate (HR) and workload along with BORG scale were used to identify interval intensity for each participant. Each exercise session was introduced by a warming up period, followed by 42 min high-intense exercise programme. The interval blocks were 4 min/2 min/30 s at 80%, 85%, 90% of VO2peak and recovery periods of 3 min/1 min/30 s. Finally 10 min of running up staircase at 80% of VO2peak.
Echocardiography
Images were obtained on Phillips IE33 (Philips Medical Systems, Andover, MA, USA), by 2 experienced echocardiographers. To standardize conditions before and after the intervention, all stress exercise echocardiography was performed 1 h after the CPET. Study participants were allowed 200cc of water between the two examinations. Recordings were obtained at rest, on a semi supine bicycle (Lode, the Netherlands) during bicycling at two levels, corresponding to 30% and 60% of the individual's maximum load, and at recovery. Each step included left ventricle inflow velocities (E, A and Dec.time) measured with pulsed Doppler at the tip of the mitral valve, and pulse-wave tissue Doppler images at septal, lateral, anterior and posterior mitral annulus with measurement of early (e’) and late (a’) diastolic and systolic (s’) movement.
Recordings were analyzed off-line using the Xcelera software (Philips, the Netherlands) by two experienced echocardiographers unaware of patient status. Mean of three successive heartbeats were used. Left ventricular mass (LVM) was calculated from 2D images using the formula: LVM = 0.8*(1.04*(LVEDD + PWTd + SWTd)3 − (LVEDD)3) + 0.6 g, where LVEDD is left ventricle end diastolic diameter, PWTd is posterior wall thickness in diastole and SWTd is septum wall thickness in diastole, and indexed (LVMi) to body surface area (BSA) calculated by Du Bois’ formula (BSA = 0.007184 × weight kg0.425 × height cm0.725). Systolic function was assessed by left ventricular ejection fraction calculated with biplane Simpson at rest and s’ (mean of septal, lateral, anterior and posterior) at rest and during exercise, diastolic function by E/A, E/e’ (using mean of e′ from septal, lateral, anterior and posterior mitral annulus) and Dec.time.
Validation
To validate exercise stress echocardiography in our echo lab, we performed a reproducibility study of stress echocardiography on 10 healthy individuals. One stress recording was obtained and analysed off-line using two analysers. Inter-analyser variation and limits of agreement were calculated according to the method of Bland–Altman (Citation12) for E, A and e′ lateral in all stages (rest, exercise and recovery); the limits of agreement all contained 0 indicating no systematic difference in measurements (). Coefficient of variation (COV), calculated as the standard deviation of the within-subject difference divided by the mean of the observations, at rest was between 7.4 and 20.3% with the highest values seen for A. This inter-analyser agreement did not systematically increase with exercise and is similar to what is reported in the literature (Citation13).
Table I. Validation study: Inter-analyser variability and measurements of repeatability by the method of Bland Altman.
Statistical analysis
All analyses were performed on Stata (StataCorp. 2009. Stata statistical software: release 11.1 College Station, Texas, USA). Quantitative variables are presented as mean ± standard deviation and categorical variables as percentages. Chi square test was used to compare categorical data. To evaluate effect of the intervention on the echocardiographic parameters, multi-level models with the change in echocardiographic parameter from baseline to follow-up as dependent variable and the stress-echocardiography response (four levels: rest, 30%, 60% and recovery) as explanatory variables were used. To determine whether any effect was unmasked during exercise, a two-way interaction term (between intervention-group and stress-echocardiography response) was introduced and tested against another multi-level model without this interaction term by a likelihood ratio test. Model control for both fixed and random effects were performed. Associations were explored by using linear regression with VO2peak as dependent variable and echocardiographic parameters as explanatory variables.
The Danish National Committee on Biomedical Research and the Danish Data Protection Agency approved this study. All participants provided written informed consent prior to the study. The investigation was conducted in accordance with Declaration of Helsinki.
Results
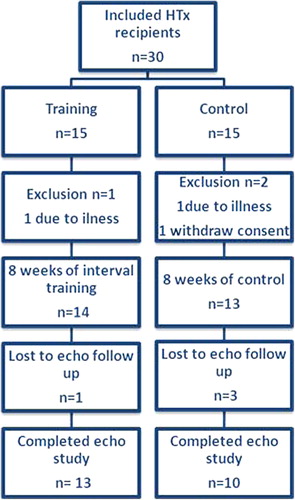
The study population consisted of 23 HTx recipients with full echocardiographic examinations (). shows baseline characteristics. Mean age of study participants was 50.1 years (range 20–71), 83% were male and mean time since transplantation 7.4 years (range 1.6–18.1). Mean BMI was 25.7 (21.1–29.0) and mean VO2peak was 24.2 ml/kg/min (13.8–38.5) corresponding to 78.6 (70.2–86.9) % of the age- and gender-predicted values (Citation14).
Table II. Baseline patient characteristics.
Echocardiography
Mean LVEF was 60.3 (± 10.1)%, study participants had normal or mild diastolic dysfunction: one patient had E/e′ above 15 at rest, while 12 patients (57%) were in ‘grey zone area’ between 8 and 15 and the remaining 10 (43%) had normal E/e′ (< 8) (Citation15). 9/23 (39%) showed signs of restrictive filling pattern with E/A greater than 2. 3/23 (13%) had increased Dec.time (> 240 ms) and 3/23 (13%) had shortened Dec.time (< 140 ms). There were no differences between the two groups at baseline in any of the parameters given in with the exception of LVmass (76 vs. 106, p = 0.01) and s’(p = 0.008).
Diastolic function at baseline in relation to exercise capacity
In univariate analyses, peak HR and BMI were correlated to VO2peak, whereas age and time since transplantation did not. No correlation between VO2peak and measures of systolic or diastolic function during rest was found nor did VO2peak correlate to LVEDD or LVMI.
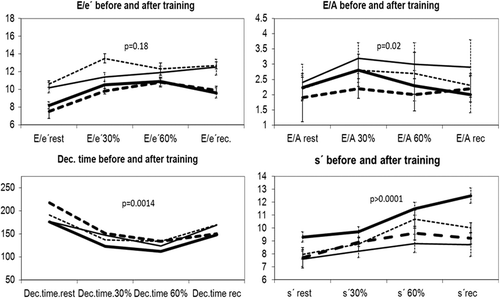
During baseline stress echocardiography E/e′ increased from 9.0 (± 2.8) at rest to 12.8 (± 7.7) at 60% of VO2peak (p = 0.09) and E/A increased from 2.1 (± 0.6) to 2.6 (± 0.7) (p = 0.02), indicating elevated filling pressure during exercise. Dec.time decreased with exercise from mean 166 (± 32) ms at rest to 110 (± 17) ms at 60% of maximum workload (). However, diastolic parameters during stress or changes in parameters from rest to stress did not correlate to VO2peak.
Figure 2. Echocardiographic parameters during exercise echocardiography, at rest, at 30%, 60% of maximum workload and in recovery. Thick lines are training group, thin lines control group. Continuous lines values before training, dotted lines values after training. Vertical lines are S.E of the mean. P-values refer to the results of likelihood test of multi-level models.
Effect of intervention on systolic and diastolic left ventricular function
The intervention was highly efficient in improving exercise capacity and workload and the intervention group had a significant reduction in systolic blood pressure (). Dec.time increased and E/A decreased significantly with intervention (p = 0.001, p = 0.02 respectively). No other diastolic parameter changed at rest or during exercise (E, p = 0.23; A, p = 0.72; E/e′, p = 0.18), (, ). S′ decreased significantly in the training group both at rest and during exercise (p < 0.0001). No baseline measures of systolic or diastolic function during rest or exercise were predictive of improvement in VO2peak.
Table III. Effect of training on hemodynamic parameters and body mass index.
Table IV. Echocardiographic parameters before and after training.
HR and VO2peak
The study participants had elevated resting HR and maximal HR of 84% of the age-predicted resulting in reduced HR reserve. At baseline there was a significant correlation between maximum HR and VO2peak (p = 0.006), but not with resting HR (p = 0.49). After 8 weeks of intensive interval training both resting (p = 0.4) and maximal HR remained unchanged (p = 0.7) and there was still a significant correlation between VO2peak and maximal HR (p = 0.018 for the intervention group, 0.011 for the control).
Discussion
Stable HTx recipients benefit from an exercise training programme in terms of exercise capacity, cardiovascular risk factors and quality of life (Citation7,Citation16,Citation17). Our main result is that, unlike what has been reported in other patient groups, diastolic function was not a limiting factor for exercise capacity and despite considerable improvement of VO2peak, intensive exercise training was not associated with improvements in diastolic function at rest or during stress.
Diastolic function at baseline
Diastolic dysfunction is believed in part to explain the reduced VO2peak in HTx recipients. It is assumed that the main mechanism by which diastolic dysfunction influences VO2peak in HTx recipients is that end-diastolic pressure is increased both at rest and during exercise, implying that the Starling mechanism is less effective in these patients. This is caused by defective beta adrenergic signalling and disturbed intracellular calcium handling resulting in impaired relaxation of the ventricle. The majority of the patients in the current study had E/e′ of 8–15, which was interpreted as in the ‘grey zone’. In the study by Sundereswaran et al. (n = 50) E/e′ greater than 8 identified HTx patients with PCWP more than 15 mmHg. In the plot depicting E/e′ versus PCWP in that study, the relation seems to be linear, but distribution around the line makes the interval 8–15 a ‘grey zone’ similar to what is described in other heart patients (Citation6). E/e′ and E/A increased and Dec.time decreased during exercise unmasking diastolic dysfunction in the majority (Citation18). However, diastolic parameters either at rest or during exercise were not limiting for VO2peak at baseline.
Diastolic function is thought to be a limiting factor for exercise capacity in different populations (Citation5). Roten et al. found that stable HTx recipients (N = 39) with E/e′ less than 10 had significantly higher VO2peak than patients with E/e′ greater than 10 (p = 0.001), and that e′, e′/a′, and E/e’ (r: 0.56, 0.50, and − 0.36, respectively) but not E/A or LVEF were correlated to VO2peak. The study population was similar to ours in age, time since transplantation, systolic and diastolic function and VO2peak and discrepancies could be explained by the fact that study size of both studies is small (n = 39 and n = 23), which increases the risk of type II errors. Larger studies are needed to elucidate the physiological mechanisms behind the limitations of exercise capacity in HTx patients (Citation3).
Diastolic function after training
Dec.time increased significantly with intervention (p = 0.002), but remained within the normal range. E/A decreased (p = 0.02), but did not normalize. Despite 18% improvement in VO2peak the intervention did not have a significant effect on other diastolic parameters (E, p = 0.23; A, p = 0.72; E/e′, p = 0.18). These results are in concordance with the only previous trial of the effect of intensive interval training on diastolic function at rest or during exercise in stable HTx recipients. In this trial, VO2peak increased from 27.7 ± 5.5 to 30.9 ± 5.3 ml/kg/min (p = 0.001) in the intervention group, but this was not accompanied by changes in diastolic or systolic function among 29 patients with echocardiography assessment (Citation8). The study observed a small increase in E/e′ after intervention in the training group; this was attributed to increased plasma volume and thereby increased preload, resulting in an increase in E, but not e′. There was no difference in E/e′ in between group analyses and no changes in echocardiographic parameters during exercise between the groups among the 29 patients with echocardiography assessment. This was supported by NT-proBNP, which did not change after the intervention (Citation8). In contrast, several studies have found that exercise training improves diastolic parameters, both in elderly untrained, heart failure patients with reduced ejection fraction and also in heart failure patients with preserved ejection fraction (Citation9,Citation19). Further, Sandri et al. found that ΔE/e′ at rest and ΔVO2peak after a training programme were inversely correlated both in elderly untrained (n = 30) and in heart failure patients with reduced ejection fraction (n = 30) (Citation20). However, Smart et al. examined the effect of exercise training in patients with diastolic dysfunction (n = 18) and found no correlation between improvement in VO2peak and diastolic function (Citation21). We found that only BMI and maximum HR correlated to VO2peak. This is consistent with a study by Nytroen et al. showing that in stable HTx recipients (n = 51) age, sex, muscular exercise capacity, body fat, blood creatinine and HR reserve accounted for 70.8% of the variance in VO2peak (Citation7).
Systolic function after training
LVEF remained unchanged, but s’ decreased significantly in the training group (p < 0.001). This may be related to the marked effect of training intervention on systolic blood pressure, which was reduced from 143 to 127 mmHg. Thus, interestingly, in the recent Norwegian trial of high-intensity interval training in HTx recipients, s’ at rest also decreased following training (from 5.9 to 5.7, for difference between groups p = 0.029). Another study of stable HTx recipients did not find any change in systolic function after a training programme (Citation17). Roten et al. found that systolic function, measured as LVEF, did not correlate to VO2peak in HTx recipients, indicating there are others and more important factors limiting their VO2peak (Citation3). A study of patients with diastolic dysfunction found that exercise training does not change systolic function, measured as s′ and LVEF, despite improvement in exercise capacity (Citation22).
There are several other reasons why HTx recipients have limited exercise performance. Exercise capacity is determined by both central and peripheral factors. In line with other studies, this study indicates that in HTx recipient's HR and peripheral parameters are of greater importance for VO2peak than diastolic function. This denervation of the transplanted heart is responsible for the resting tachycardia and reduced HR reserve HTx recipients have. The circulating catecholamines, stimulated by exercise, are mainly responsible for the increase in HR during exercise. Renervation of the transplanted heart can occur years after transplantation and improves the HR response to exercise and increase VO2peak (Citation23). Other factors limiting exercise capacity include immunosuppressive treatment (Citation24), reduced oxygen delivery and utilization by active muscles, reduced muscle strength caused by myopathy and reduced muscle capillary density in HTx patients (Citation25). CAV is the most important post-transplantation complication and has a significant negative correlation with exercise capacity (Citation26). Training studies examining peripheral effects of interval training in HTx suggest that at least some of the training effect is due to improvement of endothelial function (Citation11) and muscular exercise capacity (Citation17).
One reason why physical training in the current study failed to improve diastolic function might be that duration of the exercise training protocol was fairly short. However, it cannot be ruled out that longer duration of the exercise programme, either through direct cardiac mechanisms or via improvement of endothelial function and reduction in peripheral vascular resistance might have improved also diastolic properties of the donor hearts. However, despite one year of training intervention and only slightly smaller improvement in VO2peak, than in the current study (3.6 vs. 4.4 ml/kg/min), the study from Rustad et al. did not find any alterations in diastolic function (Citation8).
Although this study is similar to previous studies in size, the small number of study participants is a limitation and results should be confirmed in larger, preferably multi-centre trials. With small study samples, the precision of echocardiography measurements is crucial. In this study, COV was similar to what has been described previously during rest and did not increase in stress echocardiography. All the stress echocardiographies were performed 1 h after CPET, and this burst of vigorous exercise could be responsible for reduced systolic and diastolic function, which can be seen in young and healthy subject after a burst of high intensity (Citation27). Although this limitation is important, the controlled conditions standardized preload in the study subjects as much as possible. By chance, the randomization trended towards the control group having higher E/e′ at baseline (9.9 vs. 8.2, p = 0.14) This might be due to hypertrophic myocardium and reduced compliance of the left ventricle, as indicated in the increased LVmass in the control group. This is not likely to have had impact on the results.
In conclusion, diastolic function was not a limiting factor for exercise capacity in these stable HTx recipients. Further, an exercise programme highly efficient in improving VO2peak, endothelial function and quality of life did not improve measures of diastolic function. These results indicate that maximum HR and peripheral factors are of greater importance than diastolic function in determining exercise capacity after heart transplantation.
Funding
The study was supported by The Research Fund for Danish Physiotherapist Organization and The Danish National Research Foundation.
Trial Registration: The study was registered at www.clinicaltrials.gov (NCT01028599).
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gullestad L, Myers J, Edvardsen T, Kjekshus J, Geiran O, Simonsen S. Predictors of exercise capacity and the impact of angiographic coronary artery disease in heart transplant recipients. Am Heart J. 2004;147:49–54.
- Quigg R, Salyer J, Mohanty PK, Simpson P. Impaired exercise capacity late after cardiac transplantation: influence of chronotropic incompetence, hypertension, and calcium channel blockers. Am Heart J. 1998;136:465–73.
- Roten L, Schmid JP, Merz F, Carrel T, Zwahlen M, Walpoth N, et al. Diastolic dysfunction of the cardiac allograft and maximal exercise capacity. J Heart Lung Transplant. 2009;28:434–9.
- Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18:63–8.
- Podolec P, Rubis P, Tomkiewicz-Pajak L, Kopec G, Tracz W. Usefulness of the evaluation of left ventricular diastolic function changes during stress echocardiography in predicting exercise capacity in patients with ischemic heart failure. J Am Soc Echocardiogr. 2008;21:834–40.
- Sundereswaran L, Nagueh SF, Vardan S, Middleton KJ, Zoghbi WA, Quinones MA, Torre-Amione G. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998; 82:352–7.
- Nytroen K, Rustad LA, Aukrust P, Ueland T, Hallen J, Holm I, et al. High-Intensity Interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12: 3134–42.
- Rustad LA, Nytroen K, Amundsen BH, Gullestad L, Aakhus S. One year of high-intensity interval training improves exercise capacity, but not left ventricular function in stable heart transplant recipients: A randomisedcontrolled trial. Eur J Prev Cardiol. 2012.
- Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91.
- Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: a randomized, controlled trial. J Heart Lung Transplant. 2012;31:106–7.
- Hermann TS, Dall CH, Christensen SB, Goetze JP, Prescott E, Gustafsson F. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant. 2011;11:536–41.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
- Thorstensen A, Dalen H, Amundsen BH, Aase SA, Stoylen A. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr. 2010;11:149–56.
- ASTRAND I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92.
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–69.
- Squires RW. Exercise therapy for cardiac transplant recipients. Prog Cardiovasc Dis. 2011;53:429–36.
- Haykowsky M, Taylor D, Kim D, Tymchak W. Exercise training improves aerobic capacity and skeletal muscle function in heart transplant recipients. Am J Transplant. 2009;9:734–9.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–93.
- Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–94.
- Sandri M, Kozarez I, Adams V, Mangner N, Hollriegel R, Erbs S, et al. Age-related effects of exercise training on diastolic function in heart failure with reduced ejection fraction: the Leipzig Exercise Intervention in Chronic Heart Failure and Aging (LEICA) Diastolic Dysfunction Study. Eur Heart J. 2012;33:1758–68.
- Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–6.
- Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: a randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012;18: 295–301.
- Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of Sympathetic Reinnervation on Cardiac Performance after Heart Transplantation. N Engl J Med. 2001;345:731–8.
- Petrakopoulou P, Anthopoulou L, Muscholl M, Klauss V, von SW, Uberfuhr P, et al. Coronary endothelial vasomotor function and vascular remodeling in heart transplant recipients randomized for tacrolimus or cyclosporine immunosuppression. J Am Coll Cardiol. 2006;47:1622–9.
- Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, Aranda JM Jr. Effect of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005;95:1192–8.
- Marconi C, Marzorati M. Exercise after heart transplantation. Eur J Appl Physiol 2003;90:250–9.
- Banks L, Sasson Z, Busato M, Goodman JM. Impaired left and right ventricular function following prolonged exercise in young athletes: influence of exercise intensity and responses to dobutamine stress. J Appl Physiol (1985). 2010;108: 112–9.