Abstract
Objectives. Mesenchymal stem cells are sensitive to hypoxia under myocardial micro-environment of ischemia and reperfusion. Ischemic postconditioning, which is cardioprotective against ischemia–reperfusion injury, enhances in-vivo survival and therapeutic effects of transplanted stem cells. In this study, we investigated the effects of coronary effluent from postconditioned rat hearts on proliferation and survival of mesenchymal stem cells in vitro under hypoxia. Design. Isolated perfused rat hearts were divided into three groups (n = 6): the Sham group—receiving a 90 min perfusion; the Control group—receiving a 30 min global ischemia followed by a 60 min reperfusion; the ischemic postconditioning group—before sustained reperfusion, 3 cycles of 30 s reperfusion and 30 s ischemia were performed. Inflammation-related factors in coronary effluent were assessed by ELISA. Mesenchymal stem cells from bone marrow of Sprague–Dawley rats were cultured with coronary effluent under hypoxia (95% nitrogen, 5% carbon dioxide, and < 1% oxygen) for 6- or 18 h. Cell proliferation was determined by methyl thiazolyl tetrazolium. Survival rate was measured by Annexin V/PI. Results. Compared with ischemia–reperfusion treatment alone, postconditioning treatment increased the level of interleukin-10 and decreased the level of tumor necrosis factor-α and interleukin-1β in coronary effluent (P < 0.01). Stem cells cultured with postconditioned effluent, compared with those with ischemia–reperfusion effluent, had a higher proliferation (optical density value), more surviving cells, and less necrosis (P < 0.01). Conclusions. Coronary effluent from postconditioned hearts may promote the proliferation and survival of mesenchymal stem cells under hypoxia, and the suppression of inflammation may be involved in this process.
The transplantation of mesenchymal stem cells has been developed as a promising therapy to treat heart failure by repairing infarcted myocardium (Citation1). However, the host myocardial micro-environment, which includes ischemia and reperfusion injury, may lead to serious inflammatory reaction. This adverse environment may weaken the survival of transplanted cells (Citation2–5) and thus limit the benefits of stem cell therapy (Citation6–8). Ischemic postconditioning (POST), induced by a short series of repetitive cycles of reperfusion and ischemia applied immediately at the onset of sustained reperfusion, is cardioprotective against ischemia–reperfusion injury (IRI) through inhibiting the inflammatory reaction (Citation9,Citation10).
Recently, we reported that in an in-vivo rat model, POST may improve the survival of transplanted bone marrow-derived mesenchymal stem cells (BMSCs) and enhance their repair of infarcted myocardium through the modification of cardiac micro-environment by attenuating IRI (Citation11). However, little attention has been paid to the protective mechanisms by which postconditioning improves the survival of BMSCs after transplantation into a damaged heart. We therefore investigated the effects of coronary effluent from POST rat hearts on proliferation and survival of BMSCs under in-vitro-hypoxia conditions, which mimic in-vivo ischemic micro-environment.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats (SHANGHAI SLAC LABORATORY ANIMAL CO. LTD, China) were used in this study. The study complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Experimental Animal Care Committee of the Affiliated Union Hospital, Fujian Medical University.
Preparation of perfused hearts
An isolated and perfused heart model was established according to previous studies (Citation12,Citation13). Briefly, all rats, weighing 250–300 g, were sedated with 75 mg/kg ketamine and 7.5 mg/kg diazepam intraperitoneally. The animals were then sacrificed and their hearts were excised quickly to establish perfusion with Langendorff technique. The aorta was rapidly cannulated and perfused with Krebs-Henseleit bicarbonate buffer (in mmol/L: 11.0 glucose, 118.5 NaCl, 4.75 KCl, 1.19 MgSO4, 1.18 KH2PO4, 25.0 NaHCO3, and 1.4 CaCl2) at pH 7.4. The buffer was bubbled with 95% O2 and 5% CO2 at 37°C, and perfusion was performed under a hydrostatic pressure of 100 cm H2O. The ventricle was paced at a constant rate of 280 bpm. A polyvinyl-chloride balloon was inserted into the left ventricle and expanded to produce a physiologic end-diastolic pressure of 5 mmHg. Then, heart rate (HR) and the left ventricular systolic pressure and end-diastolic pressure (LVSP and LVEDP) were measured, and LV developed pressure (LVDP) was calculated as LVDP = LVSP-LVEDP (Citation12).
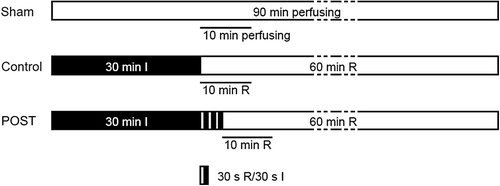
After 10 min of stabilization, isolated hearts were divided at random into three groups (n = 6 each) (): Sham, receiving a 90 min perfusion; Control, receiving a 30 min global ischemia followed by a 60 min reperfusion; POST, after 30 min of ischemia, three episodes of 30 s of global ischemia, each separated by 30 s of reperfusion, which has been shown cardioprotective in the previous study (Citation12), and then a 60 min reperfusion. Global ischemia was induced by stopping the perfusate inflow. The extent of irreversible myocardial damage was determined by assessing the content of MB isoenzyme of creatine kinase (CKMB) and lactate dehydrogenase (LDH) released into effluent at baseline (before ischemia), 30 min and 60 min reperfusion. For each perfused heart, coronary effluent during the first 10 min reperfusion was collected for volume calculation, and then 2 ml of effluent were stored at − 20°C for the assessment of inflammation-related cytokines. In each group, the coronary effluent from 6 rats was mixed with equal volume (10 ml) from each rat. After a sterilization process of filtration (0.22 um filter), the mixed effluent was stored at − 20°C for BMSCs culture under hypoxia conditions. Since the POST cycles were an interventional process, not the starting of sustained reperfusion, the coronary effluent was not collected during the POST cycles ().
Figure 1. Animal experimental protocols and groups. All rats were divided into 3 groups (n = 6 per group): Sham, receiving 90 min perfusion; Control, exposed to 30 min ischemia (I, dark bar) followed by 60 min reperfusion (R, open bar); POST given three episodes of 30 s of global ischemia, each separated by 30 s of reperfusion before 60 min reperfusion. The coronary effluent over 10 min perfusion or reperfusion (straight line) was collected for stem cell culture.
Assessment of myocardial damage and ELISA assay
The contents of CKMB and LDH (Randox Laboratories Ltd, UK) were assessed by immunodepression and rate method, respectively (BECKMAN CX7, USA). Proinflammatory cytokines [tumor necrosis factor (TNF)-α and interleukin (IL)-1β] and antiinflammatory cytokine (IL-10) were quantitatively determinated using Quantikine ELISA kits (R&D Systems, USA) according to the manufacturer's instructions in a blinded manner.
BMSCs isolation, identification, and culture under hypoxia
BMSCs were prepared from rats weighing 60–80 g as described previously (Citation11). The bone marrow cells were cultured in low glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Hyclone, USA). The adherent cells (BMSCs) at the third passage were used for in-vitro identification. To identify the potential of multilineage transdifferentiation, BMSCs were incubated in adipogenic medium (1 umol/L Dexamethasone, 10 mg/L Insulin, 0.5 mmol/L 1-methyl-3-Isobutyl xanthine, and 100 umol/L Indometacin) or in osteogenic medium (0.1 umol/L Dexamethasone, 10 mmol/L β-Sodium Glycerophosphate, and 50 ug/ml Vitamin C) for 3–4 days, and adipogenesis was indicated by 2% oil red O staining, and osteogenesis by 0.1% alizarin red staining. To identify the superficial markers, BMSCs were analyzed by flow cytometry (FACScan, Becton-Dickinson, USA) after being incubated with fluorescence-labeled antibodies (anti-CD34 from Santa Cruz, USA; anti-CD44 from Serotec, UK; anti-CD45, anti-CD90 and anti-CD11b from Biolegend, USA).
In-vitro hypoxia models, which mimic ischemic condition in vivo, were used to study the effects of postconditioned coronary effluent on the biological characteristics of BMSCs. BMSCs were put into a hypoxic incubator in a humidified atmosphere that contained 95% N2 + 5% CO2 (PO2 range in the incubator was maintained at < 1%). BMSCs were divided into five groups: Sham, cultured with coronary effluent from sham-operated heart; Control, cultured with coronary effluent from ischemia– reperfusion heart; Post, with coronary effluent from ischemic postconditioned heart; DMEM/SD, with serum deprivation (SD) DMEM; and DMEM/S, with DMEM and serum (10% FBS). The cells were assessed for proliferation and survival after 6-h- and 18-h culture, respectively.
Proliferation assay by methyl thiazolyl tetrazolium
The cells were plated in 96-well plates (1.25 × 104 cells/well) and synchronized in the G0/G1 phase of the cell cycle in serum-free medium (serum starvation) for 24 h, and then cultured with coronary effluent (0.2 ml/well) or DMEM (0.2 ml/well) under hypoxic conditions for 6- or 18-h. Cell proliferation was measured by methyl thiazolyl tetrazolium (MTT) assay. Briefly, after 6- or 18-h treatment, MTT diluted in PBS was added to the culture medium to a final concentration of 10% and the cells were sequentially cultured for 4 h at 37°C. Then, the medium was removed and the precipitated formazan crystals were solubilized in DMSO. Product formation was measured by the absorbance (optical density value) at 492 nm with a microplate reader (Bio-Rad). The experiment was repeated three times.
Flow cytometric analyses of survival cells
The cells were plated in 24-well plates (5 × 104 cells/well) in serum-free medium for 24 h and then cultured with coronary effluent (1.0 ml/well) or DMEM (1.0 ml/well) under hypoxic conditions. An Annexin-V-FLUOS Staining Kit (Roche) was used to quantitatively determine the percentage of cells undergoing apoptosis and survival according to the manufacturer's instructions. Briefly, cells were collected and resuspended in the binding buffer. Annexin V-FITC and propidium iodide (PI) were added to the suspended cells, and the reaction was incubated in the dark for 10 min. The percentage of apoptotic cells was analyzed by flow cytometry (FACScan, Becton-Dickinson). The experiment was repeated three times.
Statistical analysis
All data were presented as mean ± SD and analyzed with SPSS 16.0 for Windows. The comparison between multiple groups was analyzed by analysis of variance (ANOVA) or a two-factor ANOVA with repeated measure. When the comparison manifested a statistical difference, Bonferroni and Least Significant Difference (LSD) procedure were applied. A value of P < 0.05 was considered statistically significant.
Results
Exclusion of animals and hemodynamic data
Two isolated hearts with HR < 200 bpm and one with LVSP < 75 mmHg before ischemia were excluded. Hemodynamic data are shown in . During 10 min of stabilization, the Sham, Control and Post groups had comparable LVDP, HR and coronary flow (CF). During reperfusion, the Post group had higher LVDP than the Control group [at 10 min, (54 ± 8) mmHg vs. (20 ± 7) mmHg, P < 0.01; at 60 min, (56 ± 8) mmHg vs. (19 ± 6) mmHg, P < 0.01]; there were no significant differences in HR among the Sham, Control and Post groups. Compared with the Control group, the Post group had higher CF during reperfusion [at 10 min, (5.4 ± 0.7) ml/min vs. (7.7 ± 1.1) ml/min, P < 0.01; at 60 min, (5.6 ± 0.7) ml/min vs. (8.0 ± 0.8) ml/min, P < 0.01].
Table I. The hemodynamic variables during the experiments.
Suppressed myocardial damage and inflammation in coronary effluent by POST
At baseline, all three groups had low contents of CKMB and LDH. Compared with the Sham group, after 30 min of reperfusion, the Control and Post group reported significantly higher contents of CKMB and LDH. Compared with the Control group, the Post group showed significantly lower contents of CKMB and LDH at 30 min and 60 min reperfusion, respectively (P < 0.01) ().
Figure 2. Assessment of myocardial damage and ELISA assay in coronary effluent. A: Activities of CKMB and LDH at baseline, 30 min and 60 min reperfusion. All values are expressed as mean ± SD (n = 6). *P < 0.01 vs. Sham; †P < 0.01 vs. Control. B: Activities of TNF-α, IL-1β and IL-10. *P < 0.01 vs. Control. LDH, lactate dehydrogenase; CKMB, MB isoenzyme of creatine kinase; R30, 30 min of reperfusion; R60, 60 min of reperfusion; TNF, tumor necrosis factor; IL, interleukin.
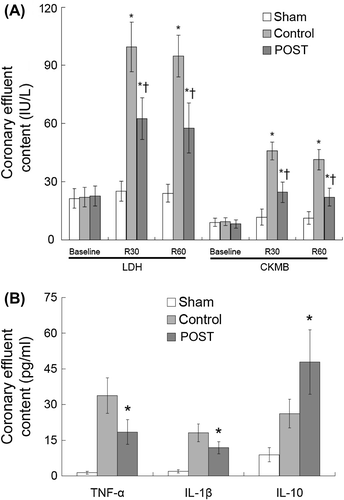
We also investigated the expression of inflammation-related factors in coronary effluent, which reflect the contribution of ischemic postconditioning to myocardial micro-environment. The Sham group and hypoxic incubation medium (DMEM/SD and DMEM/S) had comparable concentrations of TNF-α, IL-1β and IL-10, which were significantly lower than the Control and Post groups (P < 0.01). Compared with the Control group, the Post group showed lower concentrations of TNF-α [(33.7 ± 7.5) pg/ml vs. (18.4 ± 5.2) pg/ml, P < 0.01] and IL-1β [(18.1 ± 3.8) pg/ml vs. (11.8 ± 2.6) pg/ml, P < 0.01], and higher concentration of IL-10 [(26.2 ± 6.0) pg/ml vs. (47.9 ± 13.5) pg/ml, P < 0.01] ().
These data in vitro show that the impaired cardiac micro-environment (i.e. coronary effluent) may be improved by ischemic postconditioning.
Characterization of cultured BMSCs
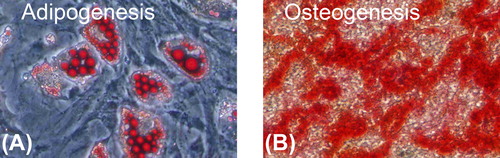
The cultured stem cells were 99.2% pure for CD90, and 99.8% for CD44. The contaminated population of hematopoietic stem cells positive to CD11b, CD45, and CD34 was lower than 1.0%. Cultured BMSCs had a powerful ability to proliferate and differentiate into multiple mesenchymal lineages ().
Figure 3. Multiple differentiation of rat cultured BMSCs (original amplification: × 200). A: After 14 days of induction, adipogenesis was indicated by the accumulation of neutral lipid vacuoles with oil red O staining. B: After 21 days of induction, osteogenesis was indicated by a mass of red calcium salinity accumulation with alizarin red staining.
Promoted proliferation of BMSCs by POST with MTT assay under hypoxia
Quantitative analysis by MTT was performed to determine whether improved cardiac micro- environment by POST promotes the proliferation of stem cells under hypoxia in vitro (). At 6-h and 18-h culture, the DMEM/S group had the highest cell proliferation (optical density value) and the Control group had the lowest (P < 0.01). At both time points, the Post group had a higher optical density value than the Control group [at 6 h, (0.438 ± 0.014) vs. (0.347 ± 0.019), P < 0.01; at 18 h, (0.209 ± 0.005) vs. (0.141 ± 0.006), P < 0.01], and a lower optical density value than the Sham and DMEM/SD groups (P < 0.05). The cell proliferation of all five groups at 18-h culture was significantly lower than that at 6-h culture (P < 0.01).
Figure 4. Quantitative analysis of stem cell proliferation by MTT under hypoxia in vitro. All values are expressed as mean ± SD (n = 3). *P < 0.01 versus 6 h. †P < 0.01 versus Control.
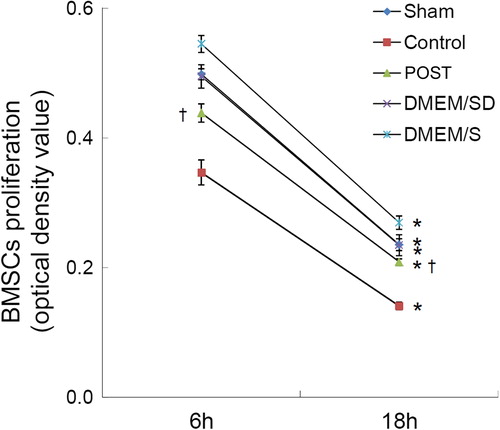
These data indicate that under hypoxic condition, the coronary effluent from the postconditioned heart can promote the proliferation of stem cell in vitro.
Improved survival of BMSCs by POST with FACS analysis under hypoxia
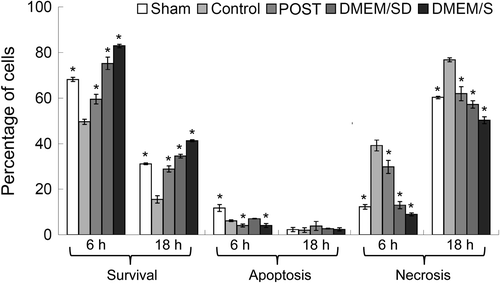
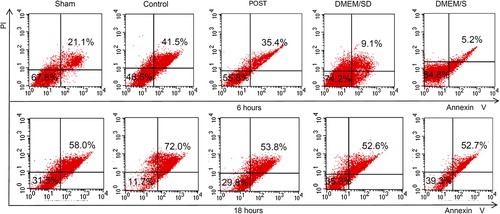
To delineate the contribution of ischemic postconditioning to BMSCs survival, we exposed BMSCs to different coronary effluents for 6 or 18 h. The survival, apoptosis, and necrosis of BMSCs were analyzed by Annexin V-FITC and PI staining ( and ), and the representative charts of flow cytometric analysis were shown in . At 6 h, the percentage of the apoptotic cells (V+/PI-) in the Post group was lower than that of the Control group [(4.1 ± 0.7) % vs. (6.2 ± 0.4) %, P = 0.015], and the Sham group had more apoptosis than any other group; at 18 h, there was no difference in the percentage of the apoptotic cells among the five groups. During culture, the percentage of the necrotic cells (V+/PI+) in the Post group was lower than that of the Control group [at 6 h, (29.8 ± 2.9) % vs. (39.2 ± 2.4) %, P < 0.01; at 18 h, (61.9 ± 3.1) % vs. (76.8 ± 0.9) %, P < 0.01]. Accordingly, the Post group had higher percentage of survival cells (V-/PI-) than the Control group [at 6 h, (59.5 ± 2.1) % vs. (49.8 ± 1.1) %, P < 0.01; at 18 h, (28.9 ± 1.3) % vs. (15.6 ± 1.6) %, P < 0.01]. In line with the MTT results, at both time points, the DMEM/S group had the highest cell survival and lowest necrosis, and the Control group had the lowest survival and highest necrosis (P < 0.05). Compared with those at 6 h, at 18 h, the survival cells decreased (P < 0.01) and the necrotic cells increased (P < 0.05) in all five groups, and the apoptotic cells decreased in the Sham, Control and DMEM/SD groups (P < 0.05), indicating the adverse effect of impaired environment under hypoxia.
Figure 5. Improved survival of BMSCs by POST with FACS analysis under hypoxia. Flow cytometric analysis differentiates normal (living) cells with low Annexin and low PI staining, apoptotic cells with high Annexin and low PI staining, and necrotic cells with high Annexin and high PI staining. FACS, fluorescence-activated cell sorter.
Discussion
The factors, important in determining the fate of transplanted cells, include the types of donor cells, the host micro-environment, and the transplantation timing. Recently, to improve the in vivo survival of transplanted stem cells, efforts have been made to modify host impaired cardiac micro-environment endogenously by POST (Citation11). However, under in-vivo conditions, there exist many confounding factors, such as cell interaction. To directly observe the effects of POST-modified micro-environment on stem cells, in this study, we further performed a novel in-vitro study. Our new findings are that coronary effluent from rat hearts of POST promoted the proliferation and survival of stem cells under in-vitro hypoxic culture, and the inflammatory suppression may be involved in it. To some extent, this current in-vitro study is helpful to understand the cellular mechanisms that may mediate the in vivo protective process of POST.
Different apoptotic pattern of stem cells in the coronary effluent culture under hypoxia
It has been known that hypoxia and SD are two important components of ischemia (Citation14). Mesenchymal stem cells are sensitive to hypoxia and SD, which mimic the micro-environment of ischemia and reperfusion injury in vivo (Citation2,Citation4,Citation15). Since the micro-environment of reperfusion injury following ischemia may be more complex than ischemia alone, we included the DMEM/SD and DMEM/S groups in this study. Previous studies (Citation16) have shown that hypoxia/SD induces mesenchymal stem cells apoptosis with a maximal induction within 6 h, and that at 24 h, the population of necrotic cells was much greater than that of survival or apoptotic cells. Given the extremely adverse micro-environment with coronary effluent from reperfusion injury myocardium, which may accelerate the damage of stem cells, in addition to the time point of 6 h, we selected the time point of 18 h instead of that of 24 h to study the change of stem cell survival over time. Our results are generally consistent with those of previous findings. However, we found that the reduction of the apoptotic cells was only shown in the Sham, Control and DMEM/SD groups over time, and the Sham group had the highest percentage of the apoptotic cells. Moreover, at 18 h, there was no difference in the percentage of the apoptotic cells among the five groups. This indicates that the apoptotic pattern of stem cells in the coronary effluent culture in this study may be different from that of DMEM culture in previous study (Citation16). Therefore, the time point of the peak apoptosis induction may appear beforehand (i.e. at 18 h), and the reduction in apoptosis from postconditioned effluent seen at 6 h is lost by 18 h.
Important role of the balance between stimulatory and inhibitory cytokines
Clinical and experimental studies have documented that the inflammatory response to myocardial ischemia and reperfusion injury is associated with the induction of cytokines such as TNF-α, and IL-1β (Citation17,Citation18). To date, the effect of inflammation on BMSCs is poorly understood. Recently, Miettinen et al. (Citation19) demonstrated that TNF-α may enhance the cell growth in a human bone marrow-derived stem cell. However, Skyschally et al. (Citation20) showed that TNF-α is responsible for both progressive contractile dysfunction and delayed protection against infarction. It has been known that the balance between stimulatory and inhibitory cytokines plays an important role in in-vitro expansion of hematopoietic progenitor cells (Citation21).
The current in-vitro model showed POST can increase CF (due to coronary vasodilation) and improve LVDP. Moreover, similar to in vivo studies (Citation9–11,Citation22), the present study also documented that POST can protect the heart against reperfusion injury indicated by lower contents of CKMB and LDH in coronary effluent during 60 min reperfusion, and the impaired cardiac micro-environment (i.e. coronary effluent) can be improved by POST via reducing the release of TNF-α and IL-1β, and increasing the release of IL-10, and the hypoxic incubation medium had very low concentration of TNF-α, IL-1β and IL-10. Therefore, we speculate that the observed decreased inflammatory reaction by POST, with inflammation-related cytokines at an optimal level, may contribute to the survival of the cultured BMSCs under hypoxia. In this study, during hypoxic incubation period, BMSCs themselves may also modulate the inflammatory process by secreting soluble factors, similar to in vivo environments (Citation11).
Potential cardioprotective factors released in the coronary effluent by POST
The coronary effluent, which was used for cell culture in this study, may contain many kinds of bioactive molecules less than 0.22 um in diameter because we used 0.22 um filter to sterilize them. However, we did not identify the effective components. In fact, some studies have reported the cardioprotective factors released in the coronary effluent by POST or ischemic preconditioning (another endogenous cardioprotective mechanism) are thermolabile hydrophobic substances with molecular weights of 3.5–30 kDa (Citation23–25).
Since the molecular weight of IL-10 is 35–40 kDa and those of TNF-α and IL-1β are less than 20 kDa, the suppression of pro-inflammatory cytokines (TNF-α and IL-1β) may contribute to improved proliferation and survival of in-vitro stem cells under hypoxia in this study. The variations in TNF, IL-1β, and IL-10 certainly suggest that inflammation plays a role. But because the coronary effluent and the cardiac micro-environment it reflects are complex, these three cytokines reflect only a few of the potentially numerous soluble factors and pathways. Despite this limitation, these data may also demonstrate the importance of maintaining a balance between pro-inflammatory and anti-inflammatory markers as a major determinant of stem cells survival in this study. This study suggests that POST has important effects on the inflammatory cytokine cascade and, as a result, promote the survival and proliferation of BMSCs.
Limitations
There are some limitations. First, we did not identify what relevant factors might have been excluded by a 0.22 um diameter filter. Second, we did not observe the effects of postconditioned coronary effluent on BMSCs grown in normoxia. Moreover, the potential factors secreted by BMSCs to suppress inflammation were not tested in this study.
Conclusion
Using an in-vitro model, this study demonstrates that ischemic postconditioning, as an endogenous cardioprotective mechanism, can promote the proliferation and survival of cultured stem cells under hypoxia, and the suppression of inflammation may be involved in this process.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported in part by the National Natural Science Foundation of China (81100151; 81170196), the Science Foundation for Distinguished Young Scholars of Fujian Province, China (2013J06015), the Science and Technology Project of Education Department of Fujian Province, China (JA12132), and the Youth Foundation of Health Department of Fujian Province, China (2009-1-18).
References
- Arnous S, Mozid A, Martin J, Mathur A. Bone marrow mononuclear cells and acute myocardial infarction. Stem Cell Res Ther. 2012;3:2.
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8.
- Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg. 2008;20:94–101.
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33: 907–21.
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73.
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21.
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209.
- Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, et al. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95: 1983–9.
- Fang J, Chen L, Wu L, Li W. Intra-cardiac remote ischemic post-conditioning attenuates ischemia-reperfusion injury in rats. Scand Cardiovasc J. 2009;43:386–94.
- Fang J, Wu L, Chen L. Postconditioning attenuates cardiocyte ultrastructure injury and apoptosis by blocking mitochondrial permeability transition in rats. Acta Cardiol. 2008; 63:377–87.
- Fang J, Chen L, Fan L, Wu L, Chen X, Li W, et al. Enhanced therapeutic effects of mesenchymal stem cells on myocardial infarction by ischemic postconditioning through paracrine mechanisms in rats. J Mol Cell Cardiol. 2011; 51:839–47.
- Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006;69:178–85.
- Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, et al. Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol. 2006; 101:168–79.
- Chao W, Shen Y, Li L, Rosenzweig A. Importance of FADD signaling in serum deprivation- and hypoxia- induced cardiomyocyte apoptosis. J Biol Chem. 2002; 277:31639–45.
- Li RK, Mickle DA, Weisel RD, Rao V, Jia ZQ. Optimal time for cardiomyocyte transplantation to maximize myocardial function after left ventricular injury. Ann Thorac Surg. 2001; 72:1957–63.
- Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–25.
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword?J Clin Invest. 1985;76:1713–9.
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47.
- Miettinen JA, Pietilä M, Salonen RJ, Ohlmeier S, Ylitalo K, Huikuri HV, Lehenkari P. Tumor necrosis factor alpha promotes the expression of immunosuppressive proteins and enhances the cell growth in a human bone marrow-derived stem cell culture. Exp Cell Res. 2011;317: 791–801.
- Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100: 140–6.
- Imamura M, Zhu X, Han M, Kobayashi M, Hashino S, Tanaka J, et al. In vitro expansion of murine hematopoietic progenitor cells by leukemia inhibitory factor, stem cell factor, and interleukin-1 beta. Exp Hematol. 1996;24: 1280–8.
- Rohilla A, Balakumar P. The infarct size-limiting effect of ischemic postconditioning (IPOC) is suppressed in isolated hyperhomocysteinemic (Hhcy) rat hearts: the reasonable role of PKC-delta. Biomed Pharmacother. 2009; 63:787–91.
- Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–45.
- Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–7.
- Serejo FC, Rodrigues LF Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007; 49:214–20.