Abstract
Background and objectives. The search for novel risk factors of cardiovascular disease (CVD) has provided valuable clinical data concerning underlying mechanism of disease. Increasing evidence indicates a possible involvement of insulin-like growth factor-I (IGF-I) and its binding protein 2 (IGFBP-2) in the pathogenesis of CVD disorders. The aim of this study was to examine the relationship between levels of IGF-I and IGFBP-2 with all-cause and CVD mortality in a prospective study of patients with lower-extremity peripheral artery disease (PAD). Methods and material. Serum IGF-I and IGFBP-2 levels were obtained in 440 patients (257 males) with symptomatic PAD. Patients were followed for a median of 6.1 (IQ 5.1–7.2) years. The relationship between times to lethal outcome and baseline serum IGF-I and IFGBP-2 levels were examined by Cox proportional hazard analysis. The role of IFGBP-2 for prognosis of CVD death was assessed with c-statistic. Results. During follow-up 115 (26%) patients (48 females and 67 males) died, and 53 (12%) died from CVD-related causes. Cox regression analysis revealed that an increase of 100 μg/l of baseline IFGBP-2 were significantly associated with an increased risk for CVD mortality [crude hazard ratio (HR) 1.14 (95% CI (1.05–1.23)), and adjusted HR 1.12 (95% CI (1.01–1.24))]. The receiver operating characteristic (ROC) analysis yielded area under curve of 0.61 (95% CI: 0.51–0.67, p = 0.022). However, the model including IFGBP-2 did not show a significant improvement in accuracy of CVD death prediction [the area under ROC curve 0.73 (0.66–0.80) vs. 0.75 (0.69–0.82), p = 0.696], and net reclassification improvement was 10.3% (p = 0.23). Conclusions. Increased IFGBP-2 concentration was significantly and independently associated with long-term CVD mortality in patients with lower-extremity PAD. However, risk prediction of CVD mortality did not improve by adding IFGBP-2 to a model containing conventional CVD risk factors.
Key words::
Introduction
Recent research has focused on secondary risk prediction in the population of patients with cardiovascular disease (CVD). The lower-extremity peripheral artery disease (PAD) patients are at an exceptionally high risk for adverse CVD events (Citation1). However, traditional atherosclerotic risk factors such as diabetes mellitus, cigarette smoking, advanced age, hyperlipidemia, and hypertension are poor predictors of CVD events for PAD patients (Citation2). Therefore, a current challenge is to identify novel risk markers able to discover who are at high risk for CVD events before clinical syndromes develop.
The insulin-like growth factor (IGF) system has been implicated in ageing and development of age-related diseases such as cancer and CVD. IGF-I is a peptide hormone that is expressed in most tissues, and regulates vascular smooth muscle cell (VSMC) migration, proliferation, apoptosis, and differentiation. The biological activity of IGF-I is regulated by a family of six high-affinity IGF binding proteins (IGFBPs), which in most situations inhibit IGF-I actions. The IGF-system is likely to contribute to the balance between death and survival of VSMCs in atherosclerotic lesions. VSMC apoptosis is a hallmark of unstable atherosclerotic plaques that are predisposed to rupture, leading to acute vascular events (Citation3). Epidemiological studies have reported that low to normal serum IGF-I levels are associated with an increased risk of acute myocardial infarction (MI), ischemic heart disease (Citation4), coronary and carotid artery atherosclerosis, and stroke (Citation5). High serum IGF-I levels have been reported to increase cancer risk, and low IGF-I levels were associated with increased risk for CVD (Citation6), and CVD mortality (Citation7). Furthermore, recent publications reported U-shaped association between serum IGF-I and all-cause mortality (Citation8), and cancer mortality (Citation9).
IGFBP-2 plays a central role in the insulin-IGF system cross talk and is closely linked to insulin resistance, thereby providing a further explanation for the association of the IGF-system with the metabolic syndrome (Citation10). In human epidemiological studies elevated IGFBP-2 levels have been associated with lower fasting insulin, lower fasting glucose, lower adiposity, but with greater mortality in older adults (Citation11), in patients with dilated cardiomyopathy (Citation12), and cancer diseases (Citation13).
Due to the involvement of the IGF-system in the development of CVD and insulin resistance, we found it of interest to examine the association between serum IGF-I and IGFBP-2 and all-cause and CVD mortality in a prospective study of PAD patients.
Materials and methods
Baseline assessment
Five hundred and five patients with intermittent claudication or chronic critical limb ischemia were enrolled prospectively in the study between November 1999 and February 2004 at the Department of Vascular Surgery, Viborg Hospital, Denmark. The PAD diagnosis was assessed during the clinical examination. The lowest ankle–brachial index (ABI) of the two legs was used in the analysis. Intermittent claudication was defined as functional ischemia rapidly disappearing at rest with ABI below 0.90. Chronic critical limb ischemia was defined as chronic ischemia as rest pain for more than 14 days, ischemic ulcers or gangrene, confirmed by angiography. Acute limb ischemia was defined as sudden onset of rest pain for less than 14 days. Cases with acute lower limb ischemia were not included in the study. The patients with missing data of IGF-I and IGFBP-2, were excluded from the study leaving 440 patients (257 men and 183 women) for the analysis. All participants participated after informed consent. The study was approved by the Research Ethics Committee for North Jutland, Viborg, and Aarhus Counties.
A thorough medical history was recorded in all patients, including details of prior MI, a history of angina pectoris, arterial hypertension, chronic obstructive pulmonary disease (COPD) and prior cerebrovascular disease, smoking status, medications, diabetes mellitus, weight, height, and ankle–brachial and distal blood pressures. The information came from medical records or directly from patients. Patients were considered as smokers if actively smoking or having discontinued smoking within 2 years. Systolic and diastolic blood pressures (SBP and DBP) used for the determination of pulse pressure (PP), defined as the difference between the SBP and DBP (PP = SBP − DBP). Diabetes was defined by history of diabetes mellitus or the use of oral anti-diabetic drugs and/or insulin. Hypertension was defined by any history of hypertension with use of anti-hypertensive drugs for that purpose. A history of cerebrovascular disease combined stroke, transient ischemic attack or carotid revascularization reported in the patient's chart. Obesity was considered when the body–mass index (BMI) exceeded > 30 g/m2.
Laboratory investigation
Serum was collected and stored at –80 C until analysis of IGF-I and IGFBP-2. IGF-I was measured by a validated in-house time-resolved immunoflourometric assay (TR-IFMA) as described by Frystyk et al. (Citation14). Intra- and inter-assay CVs were 5% and 10%. IGFBP-2 was measured by a validated in-house TR-IFMA as described. The within and in-between assay CV for this assay were 5% and 12%, respectively, and the cross-reactivity with other IGFBPs was estimated to less than 0.1% (Citation15).
Serum creatinine (Cr) were analyzed using the Vitros CREA slide on the Vitros 950 Chemistry System (Ortho-Clinical Diagnostics, Rochester, NY, USA). The creatinine clearance (CrCl), expressed in ml/min, was calculated from the equation of Cockroft–Gault as follows: (140 − age) × weight [in kilograms]/serum Cr in μmol/l, multiplied by a constant of 1.25 for men and 1.04 for women.
Serum total cholesterol was measured by enzymatic colorimetric test using Roche/Hitachi analyzer kits.
Follow-up
Patients were followed until December 31, 2008. The median follow-up time for all 440 PAD patients was 6.1 years (IQ 5.1–7.2 years). Deaths from all causes were identified in the Danish National CPR-registry. Death certificates were obtained from Danish Causes of Death Registry. Primary CVD causes of death were identified with International Classification of Disease-10 codes in the I01.0 through I99.9, and sudden death.
Statistical method
Continuous variables are presented as means ± standard deviations (SD); categorical variables are presented as percentages. Student's t-tests or Mann–Whitney U-tests were used for continuous parametric or nonparametric data, respectively. The frequencies were compared using chi-squared tests for categorical variables. The relationships, controlled for age and gender, between BMI, total cholesterol, ABI, PP, and IGF-1, IGFBP-2 were assessed using Pearson's correlation coefficients.
Kaplan–Meyer and Cox proportional hazard regression analysis were used to analyze the risk of mortality during follow-up period. Hazard ratios (HR) were adjusted for age, gender, current smoking, diabetes mellitus, symptoms of leg ischemia, CrCl, and previous MI. Multivariable regression model of conventional cardiovascular risk factors, included age ≥ 65 years, previous MI, smoking status, CrCl ≤ 60, and diabetes mellitus, with and without IGFBP-2 were composed to predict CVD mortality. We used receiver operating characteristic (ROC) curves to evaluate the overall ability of the models of conventional risk factors with and without IGFBP-2 to predict CVD mortality. Net reclassification improvement (NRI) was based on the reclassification tables, and was calculated from a sum of differences between the “upward” movement in categories for event, and the “downward” movement of non-event subjects (Citation16). Ninety-five percent confidence intervals (CI) were calculated for each comparison. A p value < 0.05 was considered statistically significant. All tests were two-tailed. The Statistics Package for Social Sciences (SPSS for Windows, version 17.0), and MedCalc statistical software (www.medcalc.org) were used for the analyses.
Results
At enrollment, the mean age of the PAD patients was 65.7 ± 9.6 years with a mean BMI of 24.9 ± 3.7 kg/m2, and 58% were males. Median levels of serum IGF-I were 107 μg/l (IQR 80–143), and the level was significantly higher in men than women (p < 0.0001). Median levels of IGFBP-2 were 313 μg/l (IQR 205–468), and did not differ between men and women (p = 0.3). The mean value of ABI was 0.63 ± 0.18. Men and women had similar ABIs.
Relation of IGF-I and IGFBP-2 with CVD risk factors
IGF-I and IGFBP-2 concentrations were inversely correlated with each other (r = − 0.22, p < 0.0001), and when controlled for gender, both variables correlated significantly with age (r = − 0.12, p = 0.015; and r = 0.29; p < 0.0001, respectively), and serum Cr (r = 0.172, p < 0.0001, and r = 0.199, p < 0.0001, respectively). In addition, IGF-I correlated significantly with ABI (r = − 0.12, p = 0.015), and BMI (r = 0.11, p = 0.026). IGFBP-2 correlated negatively with total cholesterol (r = − 0.16, p = 0.001) and BMI (r = − 0.32, p < 0.0001). As we expected, serum IGFBP-2 concentration were significantly lower in patients with obesity (p = 0.023).
The levels of IGF-I were significantly lower (p = 0.004), and the levels of IGFBP-2 (p = 0.001) significantly higher in patients with critical limb ischemia at presentation comparing with patients with intermittent claudication on admission. The patients with severe PAD, defined as an ABI < 0.5 had significantly lower IGF-1 (p = 0.007) and significantly higher IGFBP-2 (p = 0.02), however, this difference disappeared after adjustment for age and gender.
One hundred and fifteen patients (26%) had died by the end of the study. Fifty-four of them (12%) died from CVD causes. As compared with survivors, a higher proportion of decedents had history of angina pectoris, suffered from previous MI, and COPD (). Deceased patients had significantly higher levels of serum IGFBP-2, baseline age, serum Cr, and significantly lower ABI ().
Table I. Baseline characteristics of PAD patients according to survival.
Relation of IGF-I to mortality
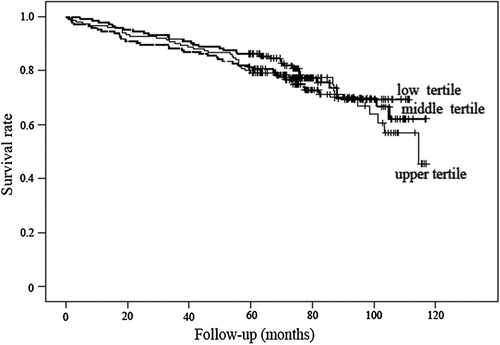
There was no significant difference in concentrations of IGF-I between patients who died from all-causes, and CVD death when compared with survivors (p = 0.89, and p = 0.68, respectively). Kaplan–Meier estimates of the survival for the 440 PAD patients, stratified according to tertiles of serum IGF-I are shown in . Cox regression showed insignificant positive associations of serum IGF-I with all-cause [crude HR 1.06 (95% CI (0.74–1.54)), adjusted HR 1.09 (95% CI (0.74–1.61))] and CVD death [crude HR 1.18 (95% CI (0.70–1.99)), adjusted HR 1.05 (95% CI (0.59–1.85))].
Relation of IGFBP-2 to mortality
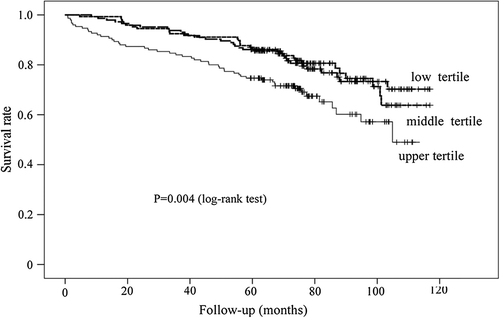
PAD patients who died from all causes as well as CVD causes had significantly higher levels of serum IGFBP-2 on admission comparing with survivors (p = 0.013, and p = 0.012, respectively). Kaplan–Meier estimates of survival for the 440 PAD patients, stratified according to tertiles of serum IGFBP-2 are shown in . The rate of all-cause mortality as well as CVD mortality were significantly higher in patients with baseline IGFBP-2 concentration in the upper tertile compared with patients in two lower tertiles (p = 0.004, and p = 0.031, respectively).
Figure 2. Kaplan–Meier plots showing survival according to tertiles of IGFBP-2 levels in patients with symptomatic peripheral artery disease.
Cox regression analysis revealed that an increase of 100 μg/l of baseline IGFBP-2 were significantly associated with an increased risk for all-cause and CVD mortality [HR 1.09 (95% CI (1.03–1.16))], and [HR 1.14 (95% CI (1.05–1.23))]. After adjustment for age, gender, smoking status, symptoms of leg ischemia, previous MI, diabetes mellitus, and CrCl the association remained significant only for CVD mortality [adjusted HR 1.12 (95% CI (1.01–1.24))], but not for all-cause mortality [adjusted HR 1.05 (95% CI (0.97–1.14))]. ROC analysis revealed that IFGBP-2 provided area under ROC curve (AUC) of 0.61 (95% CI: 0.51–0.67, p = 0.022) for prediction of CVD mortality.
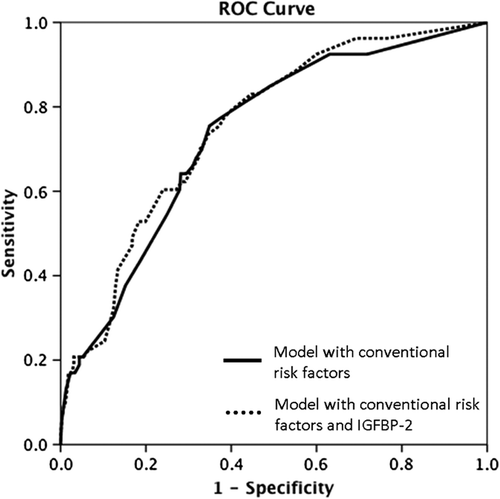
To assess the clinical usefulness of IGFBP-2 in predicting CVD mortality we created two multivariable regression models with and without IGFBP-2. The model with conventional cardiovascular risk factors included age ≥ 65 years, previous MI, smoking status, CrCl ≤ 60, and diabetes mellitus. According to c-statistics, the model including IGFBP-2 did not yield a significant improvement in accuracy of CVD death prediction compared with the model with conventional CVD risk factors [AUC 0.73 (0.66–0.80) vs. 0.75 (0.69–0.82), p = 0.696] ().
Figure 3. ROC curves for cardiovascular death. Curves are based on models’ prediction of risk using conventional risk variables (age ≥ 65 years, previous MI, smoking status, CrCl ≤ 60, and diabetes mellitus) with and without the levels of IGFBP-2.
In reclassification analyses, patients were classified across tertiles of estimated 6-year risk categories as at low, intermediate, or high risk, and estimated risk of less than 7%, 7–28%, and greater than 28%, respectively. The majority of persons remained at the same level of risk after the IGFBP-2 was included as an additional variable in the prediction model. Some persons were reclassified “upward” and some were reclassified “downward”. Of the deceased patients (n = 53), 5 (9.4%) were reclassified “upward” a risk category and 6 (11.3%) “downward” when adding IGFBP-2 to the model, yielding an overall change of − 1.9%, showing that there was a worsening of classification for decedents. For the survivors (n = 387), 77 (19.8%) were downwardly reclassified, whereas 30 (7.7%) upwardly, resulting a relative improvement for survivors of 12.1%. However, the NRI when IGFBP-2 was added to conventional factors was 10.3% for CVD death, however, it was not statistically significant (p = 0.23).
Discussion
In the present study we examined the association between serum IGF-I and IGFBP-2 levels and the mortality risk for patients with symptomatic PAD. Higher levels of IGFBP-2 were associated with a greater risk for CVD, but not for all-cause mortality. Cox regression analysis revealed that an elevated IGFBP-2 level was an independent risk factor for CVD mortality in patients with PAD. However, AUC for IGFBP-2 was poor, and the model including IFGBP-2 did not show a significant improvement in accuracy of CVD death prediction, and differences in NRI did not reach significance level. No significant association between IGF-I and mortality was revealed in patients with symptomatic PAD.
To the best of our knowledge, this is the first study of PAD patients to investigate the risk of all-cause and CVD mortality based on IGF-I and IGFBP-2 levels. Previous studies identified association between lower IGF-1 and increased risk for CVD mortality among elderly men and women, but no association was found with all-cause mortality (Citation7). In prospective population-based study men with low IGF-I levels had an almost 2-fold higher risk of all-cause, CVD, and cancer mortality compared with men with normal IGF-I levels, whereas no association between IGF-I and mortality was found in women (Citation17). High and low levels of IGF-1 were related to higher mortality in the population of patients with cancer diseases (Citation9), and all-cause mortality in elderly subjects (Citation8,Citation18). Consequently, previous studies provided conflicting results regarding the association of serum IGF-I and mortality. In our study we did not find any significant association between serum IGF-I and mortality. We studied both men and women, and there may exist some overlap between CVD and cancer disease in PAD population, that might influence our results.
In addition, previous research have reported that low serum IGF-I levels are associated with many CVD risk factors, as insulin resistance, the presence of the metabolic syndrome (Citation19), diabetes (Citation5), BMI and obesity (Citation20), and smoking (Citation21). It was suggested that low IGF-I levels may represent an additional risk factors for CVD disease. In agreement with previous studies, we observed that IGF-I levels decreased with age, and were significantly higher in men than women (Citation22). Controlled for age and sex, IGF-I correlated negatively with ABI, and positively with serum Cr and BMI. However, we did not find statistically significant association with obesity and diabetes mellitus as were demonstrated in other studies (Citation23). Furthermore, we found lower IGF-1 and higher IGFBP-2 levels when comparing patients with critical leg ischemia and intermittent claudication. Moreover, we found that patients with severe PAD, defined as ABI < 0.5, had significantly lower IGF-1 and higher IGFBP-2 concentrations, however, these differences disappeared after adjustment for age and gender. In this context previous study indicates that in atherosclerotic plaques the IGF-1 expression is reduced, whereas the expression of IGFBP-2 is elevated (Citation4).
Recent literature reported that serum IGFBP-2 concentration tended to rise with age (Citation24) and is closely linked to insulin resistance (Citation10). In the present study IGFBP-2 levels increased with age and correlated negatively with BMI. A weak correlation was found also with serum Cr, and total cholesterol. IGFBP-2 levels were found to be lower in patients with obesity and it was published previously (Citation25). As previously shown, IGFBP-2 concentrations provide an independent prognostic information in older adults (Citation11), in cancer patients (Citation13), and in patients with dilated cardiomyopathy (Citation12). To date, the present study is the first study investigating the associations between IGFBP-2 and the prognosis of patients with CVD. We found that increasing IGFBP-2 independently predicted CVD mortality in patients with PAD. However, baseline IGFBP-2 levels showed only a poor accuracy estimated by c-statistic. In addition, the model including IGFBP-2 did not yield a significant improvement in accuracy of CVD death prediction comparing with the model with conventional risk factors. Therefore, our results suggest that despite being associated with CVD mortality, IGFBP-2 unlikely to be regarded as useful clinical marker in the population of PAD patients. But these findings have not been confirmed in other studies.
Our study has several limitations and strengths that need to be considered. The relatively small number of CVD deaths (n = 53) limits the statistical power of our analysis. Furthermore, death certificates were not obtained for 2% of the deceased patients. Finally, confounding and information bias might also occur. Although care was taken to adjust for the most powerful and well-known confounders, residual confounding might occur. But, the strengths of our study include prospective design, the large sample size, long duration of follow-up, systematic and complete ascertainment of clinical covariates, and use of multivariable-adjusted analyses.
In conclusion, increased IGFBP-2 concentration was significantly and independently associated with long-term CVD mortality in patients with lower-extremity PAD. However, risk prediction of CVD mortality was not improved by adding IGFBP-2 to a model containing conventional CVD risk factors. Accordingly, serum IGFBP-2 is unlikely to be regarded as useful clinical marker in predicting CVD mortality in the population of PAD patients. Recently characterized IGFBP-2 like other IGFBPs, is still not completely understood, consequently, more experimental studies are required to explain the possible role of IGFBP-2 in the development of CVD disease.
Acknowledgements
Mrs. Kirsten Nyborg Rasmussen, Susanne Sørensen and Joan Hansen are thanked for helping with the IGF-assays.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag. 2007;3:229–34.
- Chi YW, Jaff MR. Optimal risk factor modification and medical management of the patient with peripheral arterial disease. Catheter Cardiovasc Interv. 2008;71:475–89.
- Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24: 435–44.
- Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–44.
- Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol. 2008;69: 347–58.
- Burgers AM, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, et al. Meta-analysis and dose- response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–20.
- Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–20.
- van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJ, Lips P, Drent ML. The association of serum insulin-like growth factor-I with mortality, cardiovascular disease, and cancer in the elderly: a population-based study. J Clin Endocrinol Metab. 2010;95:4616–24.
- Svensson J, Carlzon D, Petzold M, Karlsson MK, Ljunggren O, Tivesten A, et al. Both low and high serum IGF-I levels associate with cancer mortality in older men. J Clin Endocrinol Metab. 2012;97:4623–30.
- Arafat AM, Weickert MO, Frystyk J, Spranger J, Schofl C, Mohlig M, Pfeiffer AF. The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. J Clin Endocrinol Metab. 2009;94:5093–101.
- Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, et al. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. 2009;57:1213–8.
- Hassfeld S, Eichhorn C, Stehr K, Naegele H, Geier C, Steeg M, et al. Insulin-like growth factor-binding proteins 2 and 3 are independent predictors of a poor prognosis in patients with dilated cardiomyopathy. Heart. 2007;93: 359–60.
- Liou JM, Shun CT, Liang JT, Chiu HM, Chen MJ, Chen CC, et al. Plasma insulin-like growth factor-binding protein-2 levels as diagnostic and prognostic biomarker of colorectal cancer. J Clin Endocrinol Metab. 2010;95: 1717–25.
- Frystyk J, Dinesen B, Orskov H. Non-competitive time- resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5:169–76.
- Krassas GE, Pontikides N, Kaltsas T, Dumas A, Frystyk J, Chen JW, Flyvbjerg A. Free and total insulin-like growth factor (IGF)-I, -II, and IGF binding protein-1, -2, and -3 serum levels in patients with active thyroid eye disease. J Clin Endocrinol Metab. 2003;88:132–5.
- Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72.
- Friedrich N, Haring R, Nauck M, Ludemann J, Rosskopf D, Spilcke-Liss E, et al. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab. 2009;94:1732–9.
- Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LO. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160:25–31.
- Saydah S, Ballard-Barbash R, Potischman N. Association of metabolic syndrome with insulin-like growth factors among adults in the US. Cancer Causes Control. 2009;20: 1309–16.
- Onder G, Liperoti R, Russo A, Soldato M, Capoluongo E, Volpato S, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291:E829–34.
- Janssen JA, Stolk RP, Pols HA, Grobbee DE, Lamberts SW. Serum total IGF-I, free IGF-I, and IGFB-1 levels in an elderly population: relation to cardiovascular risk factors and disease. Arterioscler Thromb Vasc Biol. 1998;18: 277–82.
- Probst-Hensch NM, Wang H, Goh VH, Seow A, Lee HP, Yu MC. Determinants of circulating insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations in a cohort of Singapore men and women. Cancer Epidemiol Biomarkers Prev. 2003;12:739–46.
- Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010;33: 2257–9.
- Mattsson A, Svensson D, Schuett B, Osterziel KJ, Ranke MB. Multidimensional reference regions for IGF-I, IGFBP-2 and IGFBP-3 concentrations in serum of healthy adults. Growth Horm IGF Res. 2008;18:506–16.
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H. Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev. 1999;15:314–22.