Abstract
Objectives. Alterations of collagen metabolism present in heart failure promote the fibrotic substrate for the development of atrial fibrillation (AF). Myocardial collagen I synthesis and degradation can be assessed indirectly by circulating biomarkers such as the carboxy terminal propeptide (PICP) and carboxy-terminal telopeptide (CITP), respectively. Design. We examined myocardial collagen type-I metabolism in 143 patients with systolic heart failure (New York Heart Association Class 2–4) in relation to coexisting AF. Results. Mean age was 75 years, blood pressure 134/80 mm Hg, ejection fraction 34%, serum PICP 81 μg/L and CITP 8.3 μg/L, and median plasma brain natriuretic peptide 215 pg/L; 77 were in AF. PICP and CITP were related to left atrial diameter (r = 0.22, P = 0.013, and r = 0.26, P = 0.003) and CITP to pulmonary capillary wedge pressure and C-reactive protein (r = 0.19, P = 0.044, and r = 0.29, P = 0.003). A logistic regression suggested that PICP (odds ratio per 1 μg/L change 1.01, P = 0.012) and left ventricular end-diastolic volume (odds ratio per 1 mL change 0.98, P < 0.001) were independently associated with coexisting AF. Conclusion. Collagen type-I metabolism is associated to left atrial size. Heart failure patients with coexisting AF exhibit more altered collagen type-I metabolism than patients in sinus rhythm. This might represent more severe atrial and ventricular fibrosis.
Trial registration: ClinicalTrials.gov identifier: NCT01671995.
Introduction
Heart failure and atrial fibrillation (AF) often coexist, resulting in a worse prognosis than either condition alone. The incidence of AF increases with the severity of heart failure. Excessive accumulation of collagen in the heart leads to increased myocardial fibrosis in heart failure (Citation1,Citation2). Myocardial fibrosis reduces left ventricular (LV) compliance, increases filling pressures and atrial load, and promotes the development of atrial fibrosis and AF. Interstitial fibrosis in the atria can cause regional conduction abnormalities, which may increase the vulnerability for AF (Citation3).
Myocardial fibrosis is the result of enhanced synthesis of collagen type-I and III, and unchanged or reduced collagen degradation. This can be assessed indirectly by circulating biomarkers (Citation4). Serum carboxy-terminal propeptide of procollagen type I (PICP), a biomarker of collagen type-I synthesis (Citation5) and serum carboxy-terminal telopeptide of collagen type-I (CITP), a biomarker of collagen type-I degradation (Citation6), both associate with severity of myocardial fibrosis in heart failure patients. However, the utility of these peptides to predict a fibrotic substrate in AF is controversial, as studies with small numbers of patients only with heart failure and coexisting AF have reported contradicting results for PICP and CITP (Citation7–9). Biomarkers of collagen type-I metabolism have not previously been used to investigate the severity of myocardial fibrosis in a larger population heart failure patients and coexisting AF. Thus, we aimed to assess myocardial collagen type-I metabolism in this group of patients.
Material and methods
The optimizing congestive heart failure outpatient clinic project (OPTIMAL) included patients 60 years or older hospitalized with symptomatic (Class 2–4 according to New York Heart Association; NYHA) systolic heart failure (LV ejection fraction < 45%; using echocardiography) (Citation10). The primary aim was to compare a nurse-led hospital outpatient clinic to traditional primary health care outpatient care. Exclusion criteria were an acute coronary syndrome within three months, valvular stenosis, dementia, or severe concomitant disease. After stabilization, clinical assessment, echocardiography, and blood sampling were performed by the time of discharge. No patient presented chronic liver disease, renal failure, or metabolic bone disease associated with alterations in circulating levels of the biomarkers studied. The regional Ethics Committee approved of the study, which was performed according to the Helsinki Declaration, and all participants gave their informed consent.
All patients underwent 24-h long-term ambulatory ECG recording by the time of discharge. All recordings were analysed with a manual overview. For the purpose of this study patients with less than 17 h of analysable recordings or with pacemaker rhythm were excluded. Long-term ambulatory ECG recordings and blood samples analysed for myocardial fibrosis were available in 143 patients. In a primary analysis, patients were considered to have AF if present for more than half of the recorded time. The remaining patients were considered to have sinus rhythm. In a secondary analysis AF was considered present if unequivocally evident during the index hospitalization from medical records, on a standard 12-lead ECG, during echocardiography, or on routine telemetric monitoring; also patients with paroxysmal AF were included in this group. The remaining patients were considered to have sinus rhythm.
Echocardiography was performed as described elsewhere (Citation10,Citation11). Maximum early transmitral flow velocity in diastole/tissue Doppler early mitral annulus velocity measured in the LV septal and lateral walls was assessed (E/E'mean), and estimated pulmonary capillary wedge pressure (ePCWP) was calculated as ePCWP = 1.90 + 1.24 (E/E'mean) (Citation12). Fasting blood samples were collected on ice after 30 min of supine rest, centrifuged (+ 4° C, 1500 G for 15 min), and serum and plasma were frozen at –70° C until analysis, as previously described (Citation10). Serum PICP was measured using a sandwich enzyme-linked immunosorbent assay (Quidel Corporation, San Diego, CA, USA). Serum CITP was determined using a quantitative enzyme immunoassay (Orion Diagnostica, Espoo, Finland). Inter-assay and intra-assay coefficients of variation were 6.3% and 6.4% for PICP, and 7.5% and 9.8% for CITP, respectively.
Results are reported as mean values ± SD or median values and interquartiles, as appropriate, Groups were compared by Student's t-test, and the χ2 test, as appropriate, and linear regressions were evaluated by Pearson's correlation coefficients. Skewed variables (brain natriuretic peptide, C-reactive protein, and isovolumic relaxation time) were log transformed. A probability (P) less than 0.05 was considered significant. JMP v 10 (SAS Institute Inc., Cary, NC, USA) was used.
Results
Patient characteristics are presented in . PICP and CITP were related to left atrial dimensions (). CITP was also related to ePCWP (r = 0.19, P = 0.044) and to signs of inflammation (C-reactive protein: r = 0.29, P = 0.003).
Figure 1. The relations between carboxy-terminal propeptide of procollagen type I (PICP, n = 131; upper panel) and carboxy-terminal telopeptide of collagen type-I (CITP, n = 123; lower panel), and left atrial size.
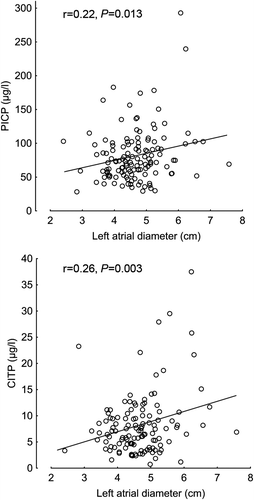
Table I. Clinical characteristics in 143 heart failure patients.
Compared to patients in sinus rhythm, the AF group had lower LV mass, and signs of less impaired LV systolic and diastolic function (). The use of ACE inhibitors/angiotensin receptor antagonists (79 vs. 92%) and spironolactone (17 vs. 20%) was similar, but warfarin and digoxin use was more common in the AF group (82 vs. 51%, and 68 vs. 28%, respectively, both P < 0.001). The ratio PICP (in μg/L)/LV mass index (in g/m2) was higher in AF patients than in the group in sinus rhythm (0.69 ± 0.04 vs. 0.56 ± 0.04, P = 0.007).
A logistic regression analysis including all variables with P less than 0.20 for a difference between the two groups (), that is, age, hypertension, coronary artery disease, diabetes mellitus, brain natriuretic peptide, PICP, LV mass index, and indices for systolic (end-diastolic volume) and diastolic (ePCWP) function (whole model χ2 = 34.1, R2 = 0.18, P < 0.001) showed independent associations to AF for LV end-diastolic volume (odds ratio for 1 mL change 0.98, 95% confidence interval 0.96; 0.99, P < 0.001) and PICP (odds ratio per 1 μg/L change 1.01, 95% confidence interval 1.00; 1.02, P = 0.012). In a secondary analysis patients with AF of any duration were compared to those in persistent sinus rhythm. A logistic regression revealed similar significant results (whole model χ2 = 43.5, R2 = 0.24, P < 0.001) with independent associations to AF for LV end-diastolic volume (odds ratio for 1 mL change 0.98, 95% confidence interval 0.97; 0.99, P < 0.001) and PICP (odds ratio per 1 μg/L change 1.01, 95% confidence interval 1.00; 1.02, P = 0.049). Similar significant results were obtained also when patients with paroxysmal AF were excluded from the analyses (data not shown).
Discussion
One finding in this study of systolic heart failure patients is the direct relation of circulating levels of PICP and CITP to left atrial size. This confirms the previous findings and suggests that an increase in left atrial size is associated with more advanced myocardial fibrosis (Citation13). Furthermore, C-reactive protein is related to CITP. This suggests that chronic inflammation may contribute to collagen deposition and the development of myocardial fibrosis in heart failure, in support of previous observations (Citation14).
Serum concentrations of PICP are elevated in systolic heart failure of hypertensive origin and relate to the amount of myocardial collagen content (Citation5). Also CITP is elevated in systolic heart failure and dilated cardiomyopathy, and relates to the extent of myocardial collagen deposition (Citation6). We have shown that biomarkers of collagen type-I synthesis and degradation in systolic heart failure relate independently to brain natriuretic peptide and to measures of LV size and diastolic function (Citation15). Our present findings of an independent association of PICP to coexisting AF in patients with heart failure may suggest an increased severity of myocardial fibrosis in patients with heart failure complicated by AF. We did not assess biomarkers of collagen type-III metabolism. However, circulating amino-terminal propeptide of procollagen type III was not reported different between patients with AF and control subjects (Citation16). Biopsy and autopsy specimens have shown increased atrial fibrosis in patients with AF, as compared to subjects in sinus rhythm (Citation17,Citation18). Increased atrial fibrosis is seen in animal models of heart failure (Citation3) and is considered as an important substrate for AF (Citation19). Thus, our results provide circumstantial evidence for a higher degree of atrial fibrosis in heart failure patients with coexisting AF.
Compared to heart failure patients in sinus rhythm, patients with coexisting AF presented with similar NYHA class and BNP levels but smaller LV volumes and less impaired global systolic function, and signs of lower filling pressures. This supports previous findings (Citation20) and suggests that the presence of AF contributes to the symptoms and signs of patients hospitalized with heart failure. These observations support the contention that stimulated myocardial collagen type-I metabolism in patients with heart failure and AF may represent increased fibrosis of the left atrium. However, others have failed to show circulating markers of collagen turnover in severe cardiac disease to reflect the extent of atrial fibrosis (Citation21) or have proposed that AF is associated with increased LV myocardial fibrosis, as assessed using magnetic resonance imaging in patients without heart failure (Citation22). Also, atrial fibrosis in patients with and without AF was reported similar in end-stage heart failure patients undergoing transplantation (Citation23). The current results, however, cannot ascertain whether the increase in markers of myocardial collagen type-I metabolism represent alterations in the left atrium or the LV.
Some important limitations of this study deserve comment. First, we used 24-h long-term ambulatory ECG recordings to define the two study groups. However, patients with paroxysmal AF may have remained in sinus rhythm during the 24-h ECG recording and thus have been misclassified into the control group with sinus rhythm. This could be important as patients with paroxysmal AF may have outstanding fibrosis (Citation24), which would reduce the discrimination between patients in AF and in sinus rhythm. However, a secondary analysis based on all available clinical information on rhythm revealed similar results. Second, we assessed left atrial size by atrial diameter. Left atrial volume indexed for body size may be more accurate, and could change (strengthen) the associations between biomarkers of fibrosis and left atrial size. Third, although the size of this study compares favorably to previous small studies on heart failure with coexisting AF (7-9) the number of patients in the current study was small. Thus, the modest associations and the results of multivariable analyses must be interpreted with great caution.
The combination of heart failure and AF has an unfavorable prognosis. Reducing the potentially harmful effects of increased myocardial fibrosis that may promote the development of AF and heart failure can be important. Blood pressure should be controlled. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers may reduce myocardial fibrosis beyond blood pressure reduction (Citation25), and spironolactone reduces myocardial fibrosis in dilated cardiomyopathy and LV dysfunction (Citation26). Whether preventing the development of myocardial fibrosis or reducing existing fibrosis will translate to improved long-term prognosis in patients with heart failure warrant further study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet; Karolinska Institutet Research Foundations; the Agreement between the Foundation for Applied Medical Research (FIMA) and Unión Temporal de Empresas project Centro de Investigación Médica Aplicada (CIMA); the Red Temática de Investigación Cooperativa en Enfermedades Cardiovasculares (RECAVA) from the Instituto de Salud Carlos III, Ministry of Science and Innovation, Spain [RD06/0014/0008]; and the European Commission [FP7-HEALTH-2010-261409, FP7-HEALTH-2011-278249, FP7-HEALTH-2012-305507, and FP7-HEALTH-2013-602904].
References
- Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin. 2000;18:435–42.
- Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev. 2014;19:173–85.
- Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: Atrial remodeling of a different sort. Circulation. 1999;100:87–95.
- López B, González A, Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–54.
- Querejeta R, López B, González A, Sanchez E, Larman M, Martinez Ubago JL, Díez J. Increased collagen type-I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation. 2004;110:1263–8.
- Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, et al. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–8.
- Kallergis EM, Manios EG, Kanoupakis EM, Mavrakis HE, Arfanakis DA, Maliaraki NE, et al. Extracellular matrix alterations in patients with paroxysmal and persistent atrial fibrillation: Biochemical assessment of collagen type-I turnover. J Am Coll Cardiol. 2008;52:211–5.
- Chiu YW, Lo MT, Tsai MR, Chang YC, Hsu RB, Yu HY, et al. Applying harmonic optical microscopy for spatial alignment of atrial collagen fibers. PLoS One. 2010;5:e13917.
- Timonen P, Magga J, Risteli J, Punnonen K, Vanninen E, Turpeinen A, et al. Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int J Cardiol. 2008;124:293–300.
- Mejhert M, Kahan T, Persson H, Edner M. Limited long term effects of a management programme for heart failure. Heart. 2004;90:1010–5.
- Mejhert M, Kahan T, Edner M, Persson HE. Sex differences in systolic heart failure in the elderly: the prognostic importance of left ventricular mass in women. J Womens Health. 2008;17:373–81.
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33.
- Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, et al. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail. 2011;13:1087–95.
- Abulhul E1, McDonald K, Martos R, Phelan D, Spiers JP, Hennessy M, et al. Long-term statin therapy in patients with systolic heart failure and normal cholesterol: effects on elevated serum markers of collagen turnover, inflammation, and B-type natriuretic peptide. Clin Ther. 2012;34:91–100.
- Löfsjögård J, Kahan T, Díez J, López B, Gónzalez A, Edner M, et al. Biomarkers of collagen type i metabolism are related to BNP, left ventricular size and diastolic function in heart failure. J Cardiovasc Med. 2014;15:463–9.
- Shimano M, Shibata R, Tsuji Y, Kamiya H, Uchikawa T, Harata S, et al. Circulating adiponectin levels in patients with atrial fibrillation. Circ J. 2008;72:1120–4.
- Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–79.
- Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–5.
- Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–74.
- Pedersen OD, Sondergaard P, Nielsen T, Nielsen SJ, Nielsen ES, Falstie-Jensen N, et al. Atrial fibrillation, ischaemic heart disease, and the risk of death in patients with heart failure. Eur Heart J. 2006;27:2866–70.
- Neuberger HR, Cacciatore A, Reil JC, Gräber S, Schäfers HJ, Ukena C, et al. Procollagen propeptides: serum markers for atrial fibrosis? Clin Res Cardiol. 2012;101:655–61
- Ling LH, Kistler PM, Ellims AH, Iles LM, Lee G, Hughes GL, et al. Diffuse ventricular fibrosis in atrial fibrillation: Noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60:2402–8.
- Aldhoon B, Kucera T, Smorodinova N, Martinek J, Melenovsky V, Kautzner J. Associations between cardiac fibrosis and permanent atrial fibrillation in advanced heart failure. Physiol Res. 2013;62:247–55.
- Cao H, Xue L, Wu Y, Ma H, Chen L, Wang X, et al. Natriuretic peptides and right atrial fibrosis in patients with paroxysmal versus persistent atrial fibrillation. Peptides. 2010;31:1531–9.
- Díez J, Querejeta R, López B, González A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–7.
- Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, et al. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: A pilot study. Circulation. 2006;112:2940–5.

