Abstract
Background. The aim of our study was to assess the relationship between cigarette smoking and epicardial fat in a cohort of patients with metabolic syndrome (MetS) at risk for coronary artery disease. Methods. We studied, in primary prevention, 54 subjects diagnosed with MetS. According to their smoking habits, the subjects were divided into two groups: smokers and non-smokers. Besides anthropometric characterization and screening laboratory tests, the subjects had a multidetector computerized tomography scan, which allowed epicardial fat quantification and calcium score (CS) evaluation. Results. Compared with non-smokers, smokers showed older age (61.6 ± 1.8 vs 56.8 ± 1.2 yrs; p < 0.05). Also, the smokers displayed increased epicardial fat volume (138 [123; 150] vs 101[79; 130] ml; p < 0.01) as well as higher CS (94 [3; 301.5] vs 0 [0;10.2]; p < 0.001), in comparison with non-smokers. Notably, CS was positively correlated with smoking habit (rs 0.469; p < 0.01), epicardial fat (rs 0.377; p < 0.01), age (rs 0.502; p < 0.001) and uric acid (rs 0.498; p < 0.01). Accordingly, the associations between both CS or epicardial fat and cigarette smoking were still maintained after adjustment for age (r 0.317; p < 0.05; r 0.427; p < 0.01). Finally, multiple regression analysis showed that smoke was the variable that best predicted CS (R2 0.131; β 0.362; p < 0.05) and epicardial fat (R2 0.177; β 0.453; p = 0.01). Conclusions. Our findings suggest that, in subjects with MetS, cigarette smoking is an independent predictor of increased epicardial fat volume and higher CS.
Introduction
Overwhelming evidence indicates that cardiovascular disease (CVD) is strongly associated with obesity and metabolic syndrome (MetS) (Citation1). Epicardial fat, which covers approximately 80% of the heart surface, emerges as an important fat depot due to its anatomical localization and functional relationships with coronary arteries and myocardium. Besides the lipid-storing capability, epicardial fat displays endocrine functions by producing hormones, cytokines and chemokines that, in turn, may play a role in modulating vascular endothelial function and promoting coronary atherosclerosis (Citation2). Therefore, epicardial fat is currently seen as an important and independent cardiovascular and metabolic risk factor, which may contribute to the development of coronary atherogenesis (Citation3–5).
In this scenario, the importance of non-invasive techniques for evaluating the expansion of such a fat depot appears counterintuitive. Cardiac multidetector computerized tomography (MDCT) has been proposed as a reliable and reproducible tool for clinical evaluation of EF volume in humans (Citation6).
It has also become clear that cigarette smoking represents another important determinant of the cardio-metabolic risk, along with insulin resistance, abdominal adiposity, hypertension and dyslipidemia (Citation7). Despite these constraints, the association between epicardial fat and smoke in patients with metabolic dysfunctions has not yet been widely investigated (Citation8).
In large prospective studies, the coronary artery calcium score (CS) has been shown to be associated with the risk of future cardiovascular events. In addition, including CS in a prediction model based on traditional risk factors significantly improved the prediction of future coronary heart disease events.
Accordingly the aim of current study was to assess the relationship between cigarette smoking and epicardial fat in patients diagnosed with MetS (Citation9).
Methods
We retrospectively studied 54 adult patients (M/F: 31/23; age: 60 ± 15 years) diagnosed with MetS who underwent cardiac MDCT for the CS evaluation. According to the Third Adult Treatment Panel ATP III, National Cholesterol Education Program, the subjects were diagnosed with MetS in the presence of three or more of the following criteria: 1) waist circumference to 102 cm or higher for men and 88 cm for women; 2) triglyceride levels higher or equal to 1.7 mmol/L; 3) high-density lipoprotein cholesterol (HDLc) levels lesser than 1.03 mmol/L for men and 1.29 mmol/L for women; 4) systolic blood pressure (BPs) higher than, or equal to 130 mmHg, or diastolic blood pressure (BPd) higher than or equal to 85 mmHg; and 5) fasting blood glucose higher than or equal to 6.1 mmol/L (Citation10). Moreover, in order to avoid selection bias, patients with a history of over type 2 diabetes were excluded from the study.
All the subjects were recruited from those that consecutively referred to the outpatient clinics for Prevention of Cardiovascular Diseases of the “Fatebenefratelli Hospital-Isola Tiberina” of Rome (Italy). According to their smoking habits at the moment of MDCT, the subjects were divided into two groups: smokers and non-smokers. The smokers group included individuals who smoked more than 10 cigarettes per day for the last 10 years, while the non-smokers category included either subjects who did not smoke cigarette or who had smoked less than 100 cigarettes in their entire lifetime. Inclusion and exclusion criteria are summarized in . Because of the retrospective nature of the study, written informed consent was not required.
Table I. Inclusion and exclusion criteria.
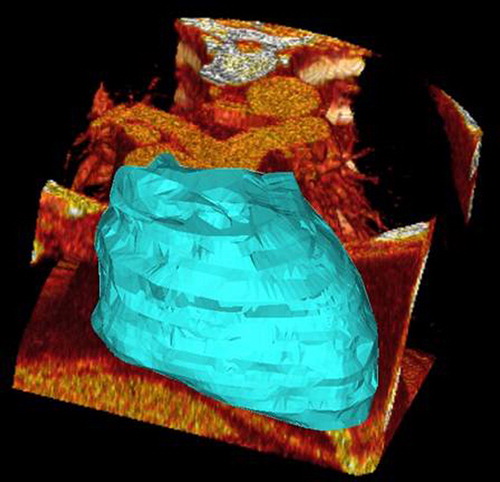
A cardiac MDCT scan was performed according to the protocol used for CS. This method is a non-invasive approach for research and quantification of epicardial fat (). The imaging parameters for cardiac MDCT were: prospective sequential ECG gating; 64 channel detectors along the z-axis, scan field of view 15–21 cm (depend on the patient size), matrix 512 × 512, by means of axial scans detector collimation 3 mm; reconstruction 2,5 mm, gantry rotation time 0.25 ms, tube 9 current range 50–100 mA (depending on the patient size and with automated modulation), 100 kV or 120 kV (according to Body Mass Index [BMI]). CT images were reconstructed with a slice thickness of 2.5 mm and with a slice distance of 0.5 mm with an overlap of 0.5 mm, in Coronary Computed Tomography Angiography images. The magnitude of radiation exposure depends, among other factors, upon electrocardiographic (EKG) triggering, and previous works quoted a radiation dose of up to 21.4 mSv without the use of EKG pulsing, as compared to a mean of 5.6 mSv for diagnostic catheter angiographies (Citation11). Epicardial fat is the adipose tissue accumulated between the visceral pericardium and the myocardium, without a structure or fascia separating it from the myocardium and the epicardial vessels (Citation6). For the quantification of EF, Digital Imaging and COmmunications in Medicine images were transferred to a research workstation. Epicardial surface was computed using an interactive procedure previously developed at the CNR Institute of Clinical Physiology. The epicardial fat volume in the workstation was manipulated using tools around any axis and the adjacent structures were cut. The epicardial fat volume was determined and the epicardial fat volume was automatically measured. In order to evaluate the correlation between individuals (Citation10), every single cardiac silhouette and the subsequent volume were analyzed in a blinded fashion by two radiologists with respectively 4 and 10 years’ experience on thorax CT imaging. We decided to quantify the EF as a volumetric measurement to obtain higher reproducibility and inter-observer agreement compared to a distance measurement (Citation12). The calculation of the CS was performed using the Agatston score (Citation13).
Figure 1. Three-dimensional representation of epicardial fat volume (result of the semiautomatic computation).
Anthropometric measurements
Body height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body weight was measured to the nearest 0.01 kg using calibrated electronic digital scales in barefoot subjects. BMI was calculated by dividing the weight (expressed in kg) by the square of the height (expressed in m2). Waist circumference was measured at the level of the umbilicus in standing position. The calculation of body surface area was done using the Mosteller formula 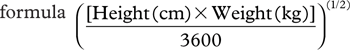 (Citation14).
(Citation14).
Blood pressure
Blood pressure (BP), BPs and BPd, was measured after a 20-min rest period using a validated automatic sphygmomanometer. During measurements, patients lay bare-armed in a bed. For each subject, the average value from two different measurements temporally separated by at least a 3-min interval was calculated.
Biochemical analysis
Blood samples were collected into 8-ml tubes containing heparin, and immediately centrifuged at 3000 rpm for 10 min at 4°C. Serum HDLc and triglyceride (TG) levels were determined using the semiautomatic chemical analyzer Ekem Control Lab. Blood glucose level were measured using Cobas c111 automated chemistry analyzer (Roche Diagnostics GmbH, Mannhein, Germany). Plasma TG concentrations were determined by means of the enzymatic method using a TG kit and plasma free fatty acids were measured using the colorimetric method. Low-density lipoprotein (LDL)-cholesterol was calculated according to Friedewald's formula (Citation15).
Statistics
Statistical comparisons between groups (smokers and non-smokers) were analyzed by two-samples Student's t-test or Mann–Whitney U test for parametric or non-normally distributed data (Kolmogorov–Smirnov test), respectively. Moreover, when the variances of the two populations were not equal the Aspin-Welch, Unequal-Variance test was used. Bivariate correlations were assessed using Spearman's (rs) and Pearson's (rp) r correlation, while multivariate relationships were analyzed using multiple regression models. Data are shown as mean ± SEM or median (25th percentile; 75th percentile). P values less than 0.05 were regarded as statistically significant. Statistical analysis was carried out using the NCSS software (Kaysville, UT, USA) (Citation16).
Results
The characteristics of the study population are shown in . As noted, smokers’ subjects were older than those of non-smokers. The occurrence of the MetS dysfunctions was balanced between the groups. Indeed, in both the groups 25% of subjects met three factors of the MetS, while more than three criteria were found in 75% of the whole study population. Impaired fasting glucose was seen in 37% and 32% of SMO and NSMO, respectively. Moreover, BMI, waist circumference, BP, fasting blood glucose, insulin levels, lipids, and uric acid concentrations did not differ significantly between the groups.
Table II. Principal characteristics of the study population.
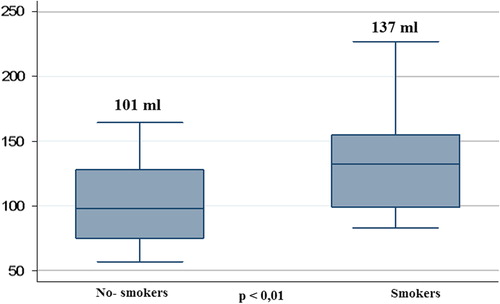
Of note, in comparison with non-smokers, smokers displayed significantly higher Epicardial fat volume (138 ml [123; 150] vs 101 ml [79; 130]; p < 0.01) and CS (94 [3; 302] vs 0 [0; 10]; p < 0.001; and ). Moreover, co-variance analysis showed that the differences in epicardial fat and CS between smokers and non-smokers were independent of age (p < 0.01).
Figure 2. Epicardial fat differences between smokers and non-smokers. Boxes show median, 25th and 75th percentile; whiskers are 5th and 95th percentile.
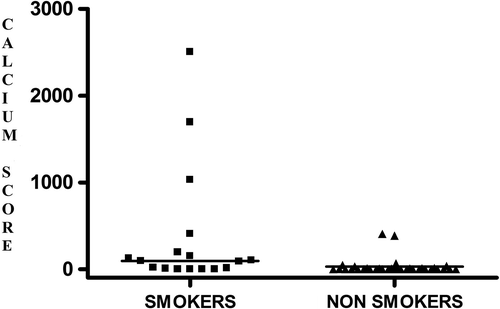
CS was positively correlated with smoking habit (rs 0.469; p < 0.01), epicardial fat (rs 0.377; p < 0.01), age (rs 0.502; p < 0.001), and uric acid (rs 0.498; p < 0.01). Accordingly, the associations between both CS or epicardial fat and cigarette smoking were still maintained after adjustment for age (r 0.317; p < 0.05; r 0.427; p < 0.01). Remarkably, multiple regression analysis showed that smoke was the variable that best predicted either CS (R2 0.131; β; 0.362; p < 0.05) () or epicardial fat (R2 0.177; β; 0.453; p = 0.01; ).
Table III. Multiple regression model for the prediction of calcium score (dependent variable).
Table IV. Multiple regression model for the prediction of epicardial fat (dependent variable).
Discussion
The main finding of our study was that, in a population of subjects with MetS, smokers displayed increased epicardial fat volume as well as higher CS, in comparison with non-smokers. Moreover, we found that cigarette smoking was an independent predictor of increased epicardial fat volume and higher CS.
Epicardial fat has been previously reported to play an important role in promoting coronary atherosclerosis partly because of its anatomic proximity to the adventitia and the major epicardial coronary arteries (Citation2). Accordingly, two population-based studies, the Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study, identified the expansion of fat depots around the heart as independent risk predictors for CVD (Citation7). Indeed, both epicardial fat thickness and volume were increased in patients with CAD compared to those of patients with normal arteries, as well as in patients with unstable angina as compared to patients with stable angina or atypical chest pain. Importantly, epicardial fat thickness was associated with subclinical markers of atherosclerosis, and patients with a value of epicardial fat thickness higher than 7 mm were found to have increased carotid intima-media thickness and arterial stiffness. Finally, a study performed in women showed a correlation between Epicardial fat thickness and low coronary reserve. In harmony with these data, the results of our study further underscore the importance of epicardial fat volume in the context of high cardiovascular risk, demonstrating the expansion of epicardial fat in a subset of smoking patients diagnosed with MetS.
From a clinical point of view, it is of great importance to have a reliable method for evaluating the amount of epicardial fat. Autopsy and imaging studies indicate that, in Caucasians, the epicardial fat thickness is approximately 4.1 mm (Citation17,Citation18). In our study, the volume of the epicardial fat was ascertained as volumetric measurement because of its higher reproducibility and inter-observer agreement when compared to distance measurements (Citation13). Bastarrika et al. have recently highlighted that patients with significant coronary artery stenosis had higher epicardial fat volume (155 ± 59 ml) as compared to control subjects without significant arterial stenosis (121 ± 82 ml; p = 0.016), while epicardial fat distance failed to show significant differences between the groups (Citation19). In a large cohort of participants of the Framingham Heart Study, Rosito and co-workers found that pericardial and intra-thoracic fat volume, quantified manually, was associated with vascular calcification, suggesting that these fat depots may exert local “toxic” effects on the vasculature (Citation20). A prospective study by Dey et al. showed that, among patients who had a major adverse cardiovascular event (MACE), epicardial fat volume was significantly higher (> 125 ml) compared to that of patients without MACE (Citation21). In line with the results of our study, patients with MetS were reported to have an average epicardial fat volume of 111 ml, lower that that observed in patients without MetS (77 ml) (Citation21). However, the effect of smoke in these studies was only partially considered. In patients with atherosclerotic coronary artery disease, cigarette smoking increases myocardial oxygen demand, but may cause a decrease in coronary blood flow and myocardial oxygen supply. There is increasing evidence that smoking may favor accumulation of visceral fat and contribute to the pathogenesis of insulin resistance, thus increasing the risk of MetS and development of type 2 diabetes (Citation8). Smokers were also shown to have higher fasting plasma cortisol concentrations compared to non-smokers, partly because of the stimulation of sympathetic nervous system activity (Citation22). Moreover, cortisol, in the presence of insulin, may also favor lipid accumulation in visceral fat depots, including the epicardial fat. In addition, sex hormones (i.e., testosterone, estrogens and progesterone) appear involved in the control of regional fat distribution (Citation23), regulating lipid mobilization in a powerful and multifaceted manner. Studies from Khaw indicate that, as compared with non-smokers, smoking women have lower estrogen bioavailability and increased androgen concentrations, even in the absence of hypoestrogenaemia (Citation24). In men, visceral adiposity was previously associated with impaired testosterone availability, and it has been also shown that smoking may reduce testosterone concentrations (Citation25). Nevertheless, nicotine may also down-regulate leptin expression in adipose tissues (Citation26). Based on these data, the expansion of epicardial fat volume observed in smokers with MetS may well be caused by the effects of smoking on adipose tissue expansion, distribution and dysfunction. Indeed, epicardial fat is known to secrete bioactive molecules which may be transported into the adjacent myocardium through vasocrine and/or paracrine pathways (Citation26). The preferential secretion of the pro-inflammatory molecules (i.e., IL-6, IL1b, and MCP-1), as well as the down-regulation of adiponectin, further reinforces the concept that epicardial fat expansion and/or dysfunction may importantly contribute to coronary atherosclerosis beyond the effects of obesity (Citation26). Accordingly, our study shows a significant increase of CS in smokers’. According to the American Heart Association classification system, calcified plaque is type Vb atheroma. This implies that, based on CS, a patient with risk factors may be comparable to a patient with a documented atherosclerosis. Even though the etiopathogenesis of CS is yet not clear, an active process similar to that of bone formation, including similar cells of matrix bond resorption, has been postulated (Citation27). Our study implies a higher severity of coronary atherosclerosis in smokers with elevated CS, suggesting also that, in this population of patients with MetS, smoke represents an independent determinant of CS. The mechanisms underlying such an association appear well circumstantiated by data of the literature showing that, as compared with non-smokers, smokers show increased levels of inflammatory markers (i.e., leucocytes counts, CRP, fibrinogen, etc), as well as a pro-atherogenic lipid's profile (reduced HDL-C, increased TG, RLP-C Remnant lipoprotein—Cholesterol and apolipoprotein B), factors that all may induce an increase of coronary calcifications (Citation27).
Remarkably, we also found a close and direct relationship between CS and epicardial fat leading to hypothesize that also epicardial fat may contribute to influence the amount of CS partly through the secretion of pro-inflammatory mediators, as previously outlined. On the other hand, multiple regression analysis indicated that the prediction of smoke for CS or epicardial fat was independent from each other ( and ), further reinforcing the concept that smoke appears an important contributor to the CS above and beyond the epicardial fat thickness, which, in turn, is associated with smoke also independently of CS.
Study limitations
This study has some limitations: The retrospective and cross-sectional nature of the survey does not allow conclusions to be drawn about the true prognostic values and the molecular mechanisms underlying the relationships between CS, EF, and smoke. Moreover, the small sample size may partly explain the lack of differences between groups (type I error). These constraints however have also to be weighed against the strengths of our work, which reside in the representative nature of the population studied, the extensive characterization of the study participants, as well as the adjustment for various confounders using multivariable methods.
Conclusions
In conclusion, our data demonstrate that in patients with MetS, smoke emerges as an important determinant of CS and increased epicardial fat. Further studies are, however, required to better elucidate the molecular mechanisms underlying the effects of smoke on CS and epicardial fat in the setting of MetS.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2012;33:1635–701.
- Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol. 2011;12:879–87.
- Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009; 30:850–6.
- Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis 2010;20:481.
- Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol. 2013;611:388–95.
- Marwan M, Achenbach S. Quantification of epicardial fat by computed tomography: why, when and how? J Cardiovasc Comput Tomogr. 2013;7:3–10.
- Al-Delaimy WK, Manson JE, Solomon CG, Kawachi I, Stampfer MJ, Willett WC, Hu FB. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med. 2002;162:273–9.
- Al-Delaimy WK, Willett WC, Manson JE, Speizer FE, Hu FB. Smoking and mortality among women with type 2 diabetes: the nurses’ health study cohort. Diabetes Care. 2001;24:2043–8.
- Chen NX, Moe SM. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14: 228–37.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
- Dey D, Nazakato R, Li D, Berman DS. Epicardial and thoracic fat Noninvasive measurement and clinical implications. Cardiovasc Diagn Ther. 2012;2:85–93.
- Budoff MJ, Achembach S, Duerinck A. Clinical utility of computed tomography and magnetic resonance techniques for non invasive coronary angiography. J Am Col Cardiol. 2003;42:1867–78.
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 8:307–10.
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8.
- Flüchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W, et al. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity. 2007;15:870–8.
- Bastarrika G, Broncano J, Schoepf UJ, Schwarz F, Lee YS, Abro JA, et al. Relationship between coronary artery disease and epicardial adipose tissue quantification at cardiac CT: comparison between automatic volumetric measurement and manual bidimensional estimation. Acad Radiol. 2010;17:727–34.
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan R, et al. Pericardial Fat, Visceral Abdominal Fat, Cardiovascular Disease Risk Factors, and Vascular Calcification in a Community-Based Sample: The Framingham Heart Study. Circulation. 2008;117:605–13.
- Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–41.
- Pasquali R, Vicennati V. Activity of the hypothalamic-pituitary-adrenal axis in different obesity phenotypes. Int J Obes Relat Metab Disord. 2000;24:S47–9.
- Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999:22: 110–6.
- Khaw KT. Epidemiology of coronary heart disease in women. Heart. 2006;92:2–4.
- Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J. Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med. 1986;315:1305–9.
- Nagayasu S, Suzuki S, Yamashita A, Taniguchi A, Fukushima M, Nakai Y, et al. Smoking and adipose tissue inflammation suppress leptin expression in Japanese obese males: potential mechanism of resistance to weight loss among Japanese obese smokers. Tob Induc Dis. 2012;10:3.
- Ratti C, Chiurlia E, Grimaldi T, Malagoli A, Ligabue G, Modena MG. Coronary calcification in cardiovascular risk stratification. Minerva Cardioangiol. 2006;54:591–601.