Abstract
Aim. To assess potential additional value of global left ventricular (LV) dyssynchrony markers in predicting cardiac resynchronization therapy (CRT) response in heart failure (HF) patients. Methods. We included 103 HF patients (mean age 67 ± 12 years, 83% male) who fulfilled the guidelines criteria for CRT treatment. All patients had undergone full clinical assessment, NT-proBNP and echocardiographic examination. Global LV dyssynchrony was assessed using total isovolumic time (t-IVT) and Tei index. On the basis of reduction in the NYHA class after CRT, patients were divided into responders and non-responders. Results. Prolonged t-IVT [0.878 (range, 0.802–0.962), p = 0.005], long QRS duration [0.978 (range, 0.960–0.996), p = 0.02] and high tricuspid regurgitation pressure drop [1.047 (range, 1.001–1.096), p = 0.046] independently predicted response to CRT. A t-IVT ≥ 11.6 s/min was 67% sensitive and 62% specific (AUC 0.69, p = 0.001) in predicting CRT response. Respective values for a QRS ≥ 151 ms were 66% and 62% (AUC 0.65, p = 0.01). Combining the two variables had higher specificity (88%) in predicting CRT response. In atrial fibrillation (AF) patients, only prolonged t-IVT [0.690 (range, 0.509–0.937), p = 0.03] independently predicted CRT response. Conclusion. Combining prolonged t-IVT and the conventionally used broad QRS duration has a significantly higher specificity in identifying patients likely to respond to CRT. Moreover, in AF patients, only prolonged t-IVT independently predicted CRT response.
Introduction
Cardiac resynchronization therapy (CRT) is a well-established treatment for patients with symptoms resistant to full medical therapy, who have clear evidence for prolonged depolarization (QRS duration, > 120 ms) and reduced left ventricular (LV) ejection fraction (EF < 35%) (Citation1). CRT has been shown to improve survival (Citation2–3) and quality of life (Citation4); reduce the rate of hospitalization (Citation2,Citation3,Citation5); increase EF (Citation6); and reduce mitral regurgitation (Citation7). Despite that, over 30% of patients receiving CRT do not respond either symptomatically or in cardiac function parameters (Citation6,Citation8). Numerous parameters of LV dyssynchrony using Doppler echocardiography have been proposed, but none has proved a gold standard in predicting CRT response (Citation9–17). Total isovolumic time (t-IVT) was introduced as an indirect global LV dyssynchrony measurement (Citation18,Citation19), which is affected by age (Citation20), but proved a good predictor of exercise capacity in heart failure (HF) (Citation21). Furthermore, patients with atrial fibrillation (AF), commonly seen in HF, constitute a clinical challenge with the lack of predictors of their potential response to CRT (Citation22,Citation23). As a result, such treatment, particularly given to non-responders, carries a potential risk of complications as well as of serious economic burden, with no objective clinical benefit. We hypothesized in this study that markers of global, rather than segmental, LV dyssynchrony should better reflect the extent of such dysfunction and predict response and were set out to study this hypothesis in a group of HF patients who received CRT according to conventional guidelines recommendations.
Methods
Study population
We included 103 HF patients who were all symptomatic in NYHA class III–IV, despite full medical therapy. Patients fulfilled the guidelines criteria for CRT treatment (Citation24); QRS duration of > 120 ms and LV EF of < 35%. Patients underwent CRT between August 2008 and August 2012, after receiving full clinical assessment, NT-proBNP and Doppler echocardiographic examination.
Echocardiographic examination
Patients were examined with Vivid 7 echocardiograph (GE Medical Systems, Horten, Norway) equipped with an adult 1.5- to 4.3-MHz-phased array transducer. Images were obtained with the patient in the left lateral decubitus position, during quiet expiration. LV end-systolic and end-diastolic dimensions and volumes, myocardial wall thickness, fractional shortening and EF, were obtained from the left parasternal cross-sectional recordings of the minor axis with the M-mode cursor positioned by the tips of the mitral valve leaflets. Ventricular long-axis motion was studied by placing the M-mode cursor at the lateral and septal angles of the mitral annulus and the lateral angle of the tricuspid annulus. Total amplitude of long-axis motion was measured as previously described (Citation25). Ventricular long-axis myocardial velocities were also studied using Doppler tissue imaging technique with the sample volume placed at the basal segment of LV lateral and septal wall as well as at the right ventricle (RV)-free wall. Left atrial diameter was measured from the aortic root recordings with the M-mode cursor positioned at the level of the aortic valve leaflets.
Spectral transvalvular Doppler flow velocities were obtained using pulsed and continuous-wave Doppler techniques as proposed by the American Society of Echocardiography (Citation26). Peak LV and RV early (E wave), and late (A wave) diastolic velocities were measured and E/A ratio was calculated. LV filling pattern was considered ‘restrictive’ when E/A ratio was > 2.0; E wave deceleration time, < 150 ms; and the left atrium dilated, > 40 mm in transverse diameter (Citation27).
Mitral and tricuspid regurgitation severity was assessed using colour and continuous-wave Doppler and was graded as mild, moderate, or severe according to the relative jet area to that of the left and right atria, respectively, as well as the flow velocity profile, in line with the recommendations of the American Society of Echocardiography (Citation28). Retrograde tricuspid regurgitation pressure drop (TRPD) > 35 mmHg was taken as evidence for pulmonary hypertension, in the absence of more than mild tricuspid regurgitation. All M-mode and Doppler recordings were made at a fast speed of 100 mm/s with a superimposed ECG (lead II).
LV dyssynchrony measurements
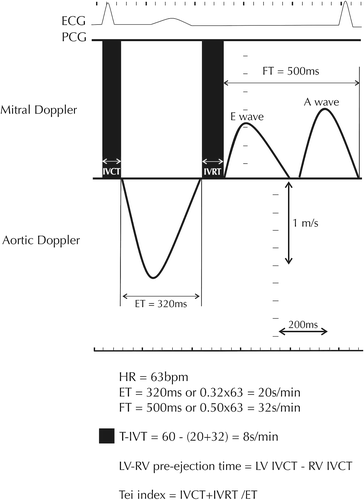
Indirect assessment of LV dyssynchronous function was obtained by measuring t-IVT, Tei index and LV–RV pre-ejection time delay. Total LV filling time was measured from the onset of the E wave to the end of the A wave and ejection time from the onset to the end of the aortic Doppler flow velocity (). t-IVT was calculated as 60 (total ejection time + total filling time) and was expressed in s/min (Citation29). Tei index was calculated as the ratio between t-IVT and ejection time (Citation30). LV–RV pre-ejection times were measured from the onset of the QRS to the onset of aortic spectral flow and pulmonary artery flow, respectively. The difference between these two measurements was considered as LV–RV pre-ejection time delay. In AF patients, the filling and ejections times were measured from the longest cycle with a minimum RR interval of > 700 ms.
Figure 1. Schematic presentation of LV filling and ejection spectral Doppler showing how global markers of dyssynchrony, that is, total isovolumic time (t-IVT), Tei index and LV–RV pre-ejection times, are measured. LV: left ventricle; RV: right ventricle; HR: heart rate; ET: ejection time; FT: filling time; IVCT: isovolumic contraction time; IVRT: isovolumic relaxation time.
NT-pro BNP measurements
Venous blood samples were withdrawn from an antecubital vein into chilled ethylene–diamine–tetra- acetic acid–containing Vacutainer test tubes after 20 min of rest in the supine position. Samples were placed immediately on ice-cold water, and the tubes were then centrifuged at 4000 rpm at 4°C for 15 min. Supernatant plasma was then immediately aliquoted into labelled cryovials. NT-proBNP was determined using a commercially available lectrochemiluminescence immunoassay based on a polyclonal antibody–based sandwich chemiluminescence assay (Roche Diagnostics, Germany) using an autoanalyser (Elecsys 2010).
CRT implantation
All CRT implantations were performed by the intervention team at the Heart Centre of Umeå University Hospital. CRT device implantation was performed in the standard fashion with three trans-venous leads inserted. The right atrial and ventricular (apical) leads were conventionally positioned, and the LV lead was inserted through the coronary sinus into the lateral or posterolateral cardiac vein. All inserted devices were Insync III (Medtronic Inc., Minneapolis, MN, USA).
Follow-up
Clinical characteristics at baseline and within 6-month follow-up were obtained from the medical reports. A good response to CRT was taken as a symptomatic improvement of ≥ 1 NYHA class at follow-up.
Statistical analysis
Data are presented as mean ± SD or proportions (percentage of patients). Continuous data were compared with two-tailed unpaired Student t-test and discrete data with chi-square test. Correlations were tested with Pearson coefficients. Univariate analysis and multivariate logistic regression analysis were used to identify predictors of CRT response. Patients were divided according to their CRT response into responders and non-responders, and were compared using the unpaired t-test. Receiver operating characteristic (ROC) curves for identification of responders were analysed for t-IVT, QRS duration and TRPD. Then, we used logistic regression for combining two of these continuous variables for different models, and the results were saved as new parameters. The sensitivity and specificity of these new parameters, for CRT response, was analysed using ROC analyses, and created a ROC curve, respectively. A significant difference was defined as p < 0.05 (2-tailed). All statistical analyses were performed using SPSS Statistics 19.0 (SPSS Inc).
Measurements reproducibility: The interobserver and itraobserver variations were determined for t-IVT, calculating the Cronbach's Alpha reliability coefficient, for a subset of 47 patients.
Results
The mean age of the study patients was 67 ± 12 years, and 83% of them were males; 61 (59%) of the 103 patients had a good response to CRT treatment.
Responders vs. Non-responders to CRT
shows the clinical, biochemical and echo differences between the two groups. Responders to CRT had longer QRS duration, longer t-IVT, higher Tei index and lower TRPD on the baseline echocardiographic examination, compared with non-responders. All other clinical, biochemical and echocardiographic measurements were not different between the two groups.
Table I. Clinical and echocardiography data in study patients and in study groups.
Predictors of CRT response
In univariate analysis, [odds ratio 95% confidence interval] ()], t-IVT [0.874 (range, 0.802–0.953), p = 0.001], Tei index [0.186 (range, 0.041–0.845), p = 0.03], QRS duration [0.981 (range, 0.965–0.997), p = 0.02] and TRPD [1.044 (range, 1.003–1.087), p = 0.03] predicted CRT non-responders.
Table II. Predictors of CRT non-responders in all patients.
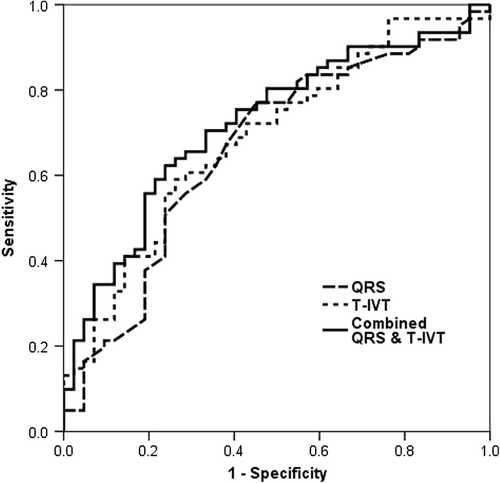
In multivariate analysis [odds ratio 95% confidence interval ()], t-IVT [0.878 (0.802–0.962), p = 0.005], QRS duration [0.978 (range, 0.960–0.996), p = 0.019 and TRPD [1.047 (range, 1.001–1.096), p = 0.046] remained as independent predictors of response to CRT. A t-IVT ≥ 11.6 s/min was 67% sensitive and 62% specific (AUC, 0.69; p = 0.001, ) in predicting CRT non-responders. Respective values for a QRS of ≥ 151 ms were 66% and 62% (AUC, 0.65; p = 0.01, ), and for TRPD < 37.6 mmHg were 65% and 61% (AUC 0.63, p = 0.03). Combined prolonged t-IVT and QRS had a sensitivity of 67%, but higher specificity of 88% in predicting CRT non-responders. Combined prolonged t-IVT and low TRPD had a sensitivity of 67% and a specificity of 77%, and combining prolonged QRS and low TRPD had a sensitivity of 66% and specificity of 76%.
CRT for AF patients
Of the 103 studied patients, 27 had AF (mean age, 74 ± 7 years; male, 78%), 13 of whom (48%) were responders, according to the above criteria. Responders had longer t-IVT (p < 0.001) and higher Tei index (p = 0.03), before CRT implantation compared with non-responders. In multivariate analysis [odds ratio 95% confidence interval], prolonged t-IVT [0.745 (range, 0.547–0.968), p = 0.03] was the only independent predictor of good CRT response (). A t-IVT ≥ 11 s/min was 69% sensitive and 79% specific (AUC 0.78, p = 0.015) in predicting CRT non-responders in AF. There was no difference in the prevalence of AF between responders and non-responders, 13/61 responders (21%) vs. 14/42 non-responders (33%; p = 0.18).
Table III. Predictors of CRT non-responders in atrial fibrillation patients.
Effect of CRT on cardiac structure and function
Of the 103 studied patients, 36 had complete follow-up echocardiographic examination, 19 of whom were responders. In responders, CRT shortened t-IVT (14 ± 4 to 11 ± 4 s/min, p = 0.03), decreased LV EDD (from 71 ± 7 to 65 ± 10 mm, p = 0.008) and ESD (63 ± 7 to 54 ± 12 mm, p = 0.002), and increased LV-filling time (328 ± 95 to 475 ± 96 ms, p < 0.001), LV EF (25 ± 7 to 36 ± 13%, p = 0.003). Whereas in the remaining 17 non-responders, only LVESD decreased (from 59 ± 8 to 52 ± 13 mm, p = 0.01) and EF increased (from 26 ± 5 to 36 ± 19%, p = 0.01) after CRT. All other echo measurements including mitral regurgitation did not change significantly in the two groups. In the patient's cohort as a whole, LV filling time correlated with t-IVT (r = − 0.63, p < 0.001). Also the delta changes in t-IVT and in LV filling time with CRT correlated modestly (r = − 0.38, p = 0.02) but the changes in QRS duration did not correlate with those in either t-IVT or filling time.
Reproducibility
Cronbach's Alpha reliability coefficients for inter-observer and intra-observer variability for filling time were 0.96 and 0.98, respectively.
Discussion
Findings
Our results show that independent predictors of response to CRT are prolonged QRS duration, and t-IVT, and raised TRPD, with combined prolonged QRS (> 151 ms) and t-IVT (> 11.6 s/m) having the highest predictive value and a specificity of 88%, among all other combinations. Conventional measurements of LV dimensions and function including ejection fraction did not differentiate between responders and non-responders. In addition, t-IVT remained the only independent predictor of clinical response to CRT in the subgroup of patients with AF. Finally, although responders and non-responders shared a significant fall in LV cavity dimensions and increase in EF with CRT, t-IVT was the main discriminator between the two groups, having shortened significantly, only in responders and not in non- responders, resulting in increased total filling time.
Data interpretation
In this modest group of patients who received CRT for resistant HF symptoms despite full medical therapy, we identified clear markers for worse cardiac function in the responders. Those markers involved electrical measures of prolonged depolarization, conventionally used as a selection criterion for CRT treatment, as well as two other markers of global LV dyssynchrony, t-IVT and Tei index. The two measurements are, in fact, complementary to each other since t-IVT represents the absolute measure of LV dyssynchrony, that is, the total time when the LV is neither filling nor ejecting, with its prolongation caused mainly by increased intracavitary tension, whereas Tei index is the ratio between t-IVT and ejection time. TRPD reflects the severity of systolic pulmonary artery pressure rise, which in this scenario is secondary to increased left atrial pressure, again another marker of either a stiff LV cavity or short LA emptying time due to LV dyssynchrony. These findings came with no surprise, since they are all different facets of the same coin, which is LV cavity rather than individual segment dyssynchrony. In the multivariate analysis, the four variables were filtered up to just three (i.e. QRS duration, t-IVT and TRPD), but the highest specific combination was that of the QRS and t-IVT confirming the serious association between the two measures, although one is purely electrical and the other is a reflection of LV synchronous mechanical function. To our surprise, the comparison of the response of cardiac function to CRT in the two groups confirmed the importance of t-IVT as the main discriminator, having shortened significantly only in the responders compared with that in the non-responders, in contrast to the other cavity dimensions and EF which improved, almost equally, in the two groups. These results provide a clear evidence for a successful treatment that did not only improve patients’ symptoms but also corrected the main underlying dysfunction, causing the patients symptoms, which is the short filling time, which reflects the volume of blood filling the left ventricle. This was clearly shown in our patients by the good inverse relationship between t-IVT and LV filing time we found.
The second important finding was the accurate t-IVT prediction of response in AF patients. No study has, so far, identified a functional marker that could predict AF responders. It seems therefore that t-IVT remains of significant value in predicting response of AF patients, who constitute about 1/4 of all eligible patients for CRT (Citation18). Thirdly, our results showed that neither EF nor mitral regurgitation could differentiate between responders and non-responders to CRT. These commonly used two parameters of LV function in HF patients have been shown to improve with CRT in many studies (Citation6,Citation7), We concur with those, having demonstrated a significant increase in EF in the group of patients as a whole and a fall in mitral regurgitation with CRT, despite the failure of the two measures to discriminate between groups. Again, this finding was not unexpected since the two measures reflect overall systolic LV function and degree of mitral valve dysfunction rather than the nature and severity of LV dyssynchrony which is the main objective target of CRT electrical treatment.
Clinical implications
The combination of prolonged QRS duration > 151 ms and t-IVT > 11.6 s/min seems to be the most sensitive setting for predicting response to CRT. In addition, failure of prolongation of LV filling time may explain the lack of response to CRT. The two measures are easily obtainable and highly reproducible without a need for sophisticated softwares of limited sampling rate, as is the case with some imaging techniques. In view of the demonstrated limited sensitivity of EF and mitral regurgitation in predicting response to CRT, their use as sole predictors of treatment response in patients with late-stage HF seems not to be fully justified.
Limitations
The main limitation of this study is the relatively small number of patients, particularly in the AF subgroup, which reduced the statistical power for multivariate analysis. The same limitation applies to our inability to run a comprehensive statistics in the subgroups of patients according to the underlying aetiology of HF, ischaemic vs. non-ischaemic. We are unable to compare our findings with segmental measurements of dyssynchrony, previously reported and which failed to prove to have any predictive value, since not all our cohort had them studied before CRT.
Conclusion
Combining prolonged t-IVT, a marker of global LV dyssynchrony, and the conventionally used broad QRS duration has a significantly higher specificity in identifying patients likely to respond to CRT, irrespective of the presence of AF.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiacpacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281–329.
- Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, etTavazzi L; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352: 1539–49.
- Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al.; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–38.
- Veazie PJ, Noyes K, Li Q, Hall WJ, Buttaccio A, Thevenet-Morrison K, Moss AJ. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol 2012;60:1940–4.
- Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119:969–77.
- Brambatti M, Guerra F, Matassini MV, Cipolletta L, Barbarossa A, Urbinati A, et al. Cardiac resynchronization therapy improves ejection fraction and cardiac remodelling regardless of patients’ age. Europace. 2013;5:704–10.
- Vinereanu D, Turner MS, Bleasdale RA, Mumford CE, Cinteza M, Frenneaux MP, Fraser AG. Mechanisms of reduction of mitral regurgitation by cardiac resynchronization therapy. J Am Soc Echocardiogr. 2007;20:54–62.
- Bank AJ, Burns KV, Gage RM, Vatterott DB, Adler SW, Sajady M, et al. Cardiac resynchronization therapy in the real world: comparison with the COMPANION study. J Card Fail. 2012;18:153–8.
- Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16.
- Abdelhadi R, Adelstein E, Vogt A, Gorscan J, Saba S. Measures of left ventricular dyssynchrony and the correlation to clinical and echocardiographic response after cardiac resynchronization therapy. Am J Cardiol. 2008;102:598–601.
- Fung JW, Chan JY, Yip GW, Chan HC, Chan WW, Zhang Q, Yu CM. Effect of left ventricular endocardial activation pattern on echocardiographic and clinical response to cardiacresynchronization therapy. Heart. 2007;93:432–7.
- Steffel J, Milosevic G, Hürlimann A, Krasniqi N, Namdar M, Ruschitzka F, et al. Characteristics and long-term outcome of echocardiographic super-responders to cardiac cardiac resynchronization therapy: 'real world’ experience from a single tertiary care centre. Heart. 2011;97:1668–74.
- Diab IG, Hunter RJ, Kamdar R, Berriman T, Duncan E, Richmond L, et al. Does ventricular dyssynchrony on echocardiography predict response to cardiac resynchronisation therapy? Arandomised controlled study. Heart. 2011;97:1410–6.
- Lim P, Donal E, Lafitte S, Derumeaux G, Habib G, Réant P, et al. Multicentre study using strain delay index for predicting response to cardiac resynchronization therapy (MUSICstudy). Eur J Heart Fail. 2011;13:984–91.
- Sakamaki F, Seo Y, Ishizu T, Yanaka S, Atsumi A, Yamamoto M, et al. Tissue Doppler imaging dyssynchrony parameter derived from the myocardial active wall motion improves prediction of responders for cardiac resynchronization therapy. Circ J. 2012;76:689–97.
- Seo Y, Ito H, Nakatani S, Takami M, Naito S, Shiga T, Ando K, Wakayama Y, Aonuma K, et al.; J-CRT investigators. The role of echocardiography in predicting responders to cardiac resynchronization therapy. Circ J. 2011;75: 1156–63.
- Yu CM, Gorcsan J III, Bleeker GB, Zhang Q, Schalij MJ, Suffoletto MS, et al. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol. 2007;100:1263–70.
- Duncan A, Francis D, Gibson D, Pepper J, Henein M. Electromechanical left ventricular resynchronisation by coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;26:711–9.
- Bajraktari G, Duncan A, Pepper J, Henein M. Prolonged total isovolumic time predicts cardiac events following coronary artery bypass surgery. Eur J Echocardiogr. 2008;9: 779–83.
- Bajraktari G, Lindqvist P, Henein MY. Left ventricular global dyssynchrony is exaggerated with age. Int Cardiovasc Forum. 2013;1:47–51.
- Ibrahimi P, Poniku A, Hysenaj V, Ahmeti A, Jashari F, Haliti E, et al. Combined right ventricular dysfunction and raised left ventricular filling pressures predict limited exercise capacity in reduced ejection fraction heart failure. Int Cardiovasc Forum. 2013;1:37–42.
- Luedorff G, Grove R, Kowalski M, Wolff E, Thale J, Kranig W. Impact of chronic atrial fibrillation in patients with severe heart failure and indication for CRT: data of two registries with 711 patients (1999–2006 and 2007-6/2008). Herzschrittmacherther Elektrophysiol. 2011;22:226–32.
- Delnoy PP, Ottervanger JP, Luttikhuis HO, Elvan A, Misier AR, Beukema WP, van Hemel NM. Comparison of usefulness of cardiac resynchronization therapy in patients with atrial fibrillation and heart failureversus patients with sinus rhythm and heart failure. Am J Cardiol. 2007;99: 1252–7.
- Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, et al.; ESC Committee for Practice Guidelines. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–36.
- Henein MY, Gibson DG. Normal long axis function. Heart. 1999;81:111–3.
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
- Galderisi M, Henein MY, D'hooge J, Sicari R, Badano LP, Zamorano JL, Roelandt JR.; European Association of Echocardiography. Recommendations of the European Association of Echocardiography: how to use echo-Doppler in clinical trials: different modalities for different purposes. Eur J Echocardiogr. 2011;12:339–53.
- Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003; 16:777–802.
- Duncan AM, Francis DP, Henein MY, Gibson DG. Importance of left ventricular activation in determining myocardial performance (Tei) index: comparison with total isovolumic time. Int J Cardiol. 2004;95:211–7.
- Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function - a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66.