Abstract
Objectives. Ghrelin is an anabolic hormone that is elevated in heart failure (HF), with resistance to its anabolic effects. This resolves after heart transplantation (HTx). Ghrelin exists in acylated and des-acyl forms, with the acylated form being primarily responsible for endocrine actions. We tested the hypothesis that ghrelin derangements in HF are due to inadequate acylation and that this resolves post transplantation. Design. Plasma levels of des-acyl and acylated ghrelin and acylated/total ratios were assessed in HF (n = 20), post-HTx (n = 35), and healthy controls (n = 4), and correlated with each other and with clinical parameters. Results. Median (interquartile range) of des-acyl ghrelin level, was 167 (121–195) pg/ml in HF versus 149 (130–223) pg/ml in post-HTx, p = NS. Acylated ghrelin level was 76 (51–99) pg/ml versus 13 (0–30) pg/ml, p < 0.001. Acylated/total ratios were 0.33 (0.20–0.47) versus 0.08 (0–0.13), p < 0.001. The correlation between acylated and total ghrelin levels was greater in HF than that in HTx. Acyl ghrelin correlated inversely with body mass index in HF, but not in HTx. Conclusion. Acylated ghrelin and the acylated/total ratio were dramatically higher in HF compared with those in HTx. Acylation rather than secretion of ghrelin is upregulated in HF and the resistance to ghrelin's anabolic and appetite-stimulating effects is not at the level of acylation, but downstream at the ghrelin-receptor level.
Introduction
Severe heart failure (HF) is associated with a catabolic state and often cardiac cachexia (Citation1). Heart transplantation (HTx) is associated with weight gain. This weight gain has been attributed to glucocorticoid therapy, but it is more dramatic than that after other solid organ transplants, despite similar steroid regimens, and also unrelated to steroid dose (Citation2).
A potential alternative mechanism involves resistance to the anabolic and appetite-stimulating hormone ghrelin in HF, which resolves post-HTx (Citation3). Ghrelin is a 28-amino-acid peptide hormone primarily secreted from the X/A-like enteroendocrine cells (also called P/D1 cells in man) of the stomach, duodenum, and ϵ-cells of the pancreas (Citation4) in response to fasting and weight loss (Citation5). Ghrelin correlates inversely with body mass index (BMI) (Citation6), and has received attention mainly as a centrally acting appetite stimulant (Citation7). However, it also stimulates growth hormone (GH) release and may have cardiovascular effects including inotropy and vasodilation (Citation8). Total plasma ghrelin level is elevated in HF, but caloric intake reduced; ghrelin declines post-HTx, while caloric intake increases (Citation3). Elevations in ghrelin levels in HF may represent not only a catabolic state and resistance to its anabolic and appetite-stimulating effects (Citation3), but may also be a compensatory response to the GH resistance that has been described in HF (Citation9), or to impaired cardiac function.
Ghrelin is produced in an unacylated (des-acyl) form and post-translationally modified in the endoplasmic reticulum of secreting cells by fatty acid chain addition to serine-3 in acylated (generally octanoylated) ghrelin, which is responsible for most endocrine actions (Citation10,Citation11). Levels of des-acyl and acylated ghrelin in HF and post-HTx are unknown. Thus, we hypothesized that a potential resistance to ghrelin in HF could be explained by impaired acylation, and that this resolves post-HTx.
Methods
Clinical assessment and fasting blood sampling were performed in a cross-sectional cohort of patients with HF (n = 20), post-HTx (n = 35; age and gender matched to HF), and healthy controls (n = 4). In HF, ejection fraction (EF) and peak VO2 were also assessed. The study was approved by the institutional review board of Columbia University, conformed to the declaration of Helsinki; subjects provided written informed consent.
The study subjects reported in the fasting state to the lab in the morning, and blood samples were collected through a catheter placed in the antecubital vein. Plasma acylated and total ghrelin levels were measured in duplicate using radioimmunoassay (RIA) kits according to manufacturer's instructions (LINCO Research, St.Charles, MO, USA). Blood samples were collected in chilled Ethylenediaminetetraacetic acid (EDTA) tubes and immediately centrifuged for 15 min at 4°C. Plasma was separated into chilled microtubes and for acylated ghrelin analyses, 50 μl of 1N HCl and 10 μl of 10mg/ml phenylmethylsulfonyl fluoride were added for each mL of plasma, and immediately stored at − 70°C. Acylated ghrelin level was measured using RIA with an I125-labeled ghrelin tracer and a guinea pig antibody against the acylated form of ghrelin that has less than 0.1% cross-reactivity with des-acyl ghrelin. The lower limit of detection for this assay was 8 pg/ml, with 7–10 and 10–16% interassay coefficients of variation. Total ghrelin level was measured using RIA with an I125-labeled ghrelin tracer and a rabbit antibody. The lower limit of detection for this assay was 100pg/ml, with 4–10 and 15–17% interassay coefficients of variation. Des-acyl ghrelin levels were calculated by subtracting acylated level from total level as described previously (Citation12). Because ghrelin may exist in numerous fragment forms, the des-acyl ghrelin calculations may be slightly overestimated; however, this would affect samples and patient groups similarly. Different ghrelin forms were correlated with each other. In HF, acylated ghrelin was correlated with BMI, New York Heart Association (NYHA) class, EF, and peak VO2, and in HTx with BMI.
Statistical analysis was performed using IBM SPSS Statistics 20 (Somers, NY, USA). Continuous data are shown as median (interquartile range) and categorical data as n and percent. Clinical data and ghrelin levels were compared between 2 groups using the t-test for parametric and the Wilcoxon–Mann– Whitney test for non-parametric data (continuous) and Fischer's exact test (categorical). Overall comparisons between 3 groups were performed with parametric and non-parametric (Kruskal–Wallis) one-way analysis of variance (ANOVA) (continuous) and Fischer's exact test by the Monte Carlo method (categorical). Normality testing was done using Shapiro–Wilk test and normal probability plot. A 2-sided p value < 0.05 was considered statistically significant. Correlations were assessed using Spearman's (rank) correlations.
Results
lists patient data. In HF and HTx, age was 57 (50–64) and 58 (52–67) years, respectively, and 20% were women in both groups. In HF, EF was 23 (20–29) % and peak VO2 was 16.5 (13.9–18.3) ml/kg/min. In HTx, median weight gain over 39 (5–72) months post-HTx was 4.3 (−0.6–15.9) kg.
Table I. Patient characteristics and ghrelin types and levels.
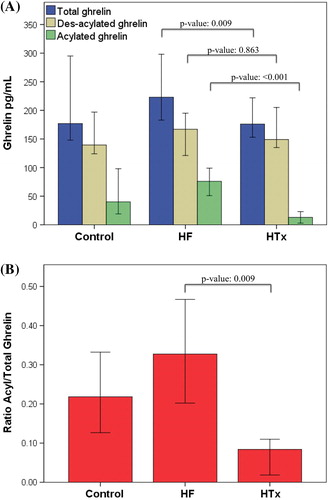
and depict plasma levels (pg/ml) of different forms of ghrelin and ratios. Des-acyl ghrelin levels were similar between the groups: HF, 167 (121–195); HTx, 149 (130–223); control, 140 (126–185), respectively; p overall = 0.85; p HF versus HTx = 0.86. In contrast, total ghrelin level was slightly but significantly higher in HF: 223 (183–298) versus 176 (144–246) versus 177 (149–272), p overall = 0.03; p HF versus HTx = 0.01. Acylated ghrelin and acylated/total ratios were dramatically higher in HF versus the other two groups and lower in HTx compared with controls—acylated ghrelin: HF, 76 (51–99); HTx, 13 (0–30); control, 40 (20–88), p overall, < 0.001; p HF versus HTx, < 0.001; p HTx versus control = 0.06—ratios: 0.33 (0.20–0.47) versus 0.08 (0–0.13) versus 0.22 (0.14–0.32); p HF versus HTx, < 0.001; p HTx versus control, 0.03.
Figure 1. Ghrelin forms and levels (A) and acylated/total ratios (B) in heart failure, heart transplantation, and controls. Bars are median and whiskers are 95% confidence interval; p-values using non-parametric (Kruskal–Wallis) one-way ANOVA. HF, heart failure; HTx, heart transplantation.
lists Spearman's correlation coefficients between different forms of ghrelin. Acylated ghrelin correlated with total ghrelin more in HF than in HTx, and not with des-acyl ghrelin in HF or HTx. shows Spearman's correlations between acylated ghrelin and BMI. In HF () but not HTx (), acylated ghrelin correlated inversely with BMI. There were no correlations between acylated ghrelin and EF or peak VO2 in HF.
Figure 2. Correlations between acylated ghrelin and BMI in heart failure (A) and post heart transplantation (B). Correlations and p-values by Spearman's rank correlation. BMI, body mass index; HF, heart failure; HTx, heart transplantation.
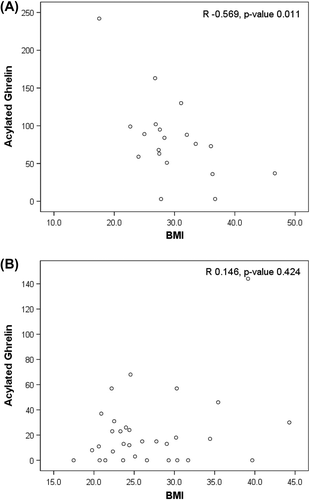
Table II. Ghrelin correlations.
Discussion
We confirm a previous study that total ghrelin levels are elevated in HF and normalize post-HTx (Citation3). For the first time, we show that acylated ghrelin exhibits the same pattern, whereas des-acyl ghrelin is not affected by HF. Indeed, in HF, total ghrelin levels were slightly elevated, whereas acylated levels were dramatically elevated. Thus, we could not confirm the hypothesis that ghrelin acylation is impaired in HF and normalized post-HTx. In contrast, ghrelin acylation is upregulated in HF and normalized or is possibly downregulated post-HTx. Thus, resistance to the anabolic and appetite-stimulating effects of ghrelin in HF and resolution of ghrelin post-HTx are not due to inadequate acylation of ghrelin in HF and restored acylation post-HTx. In contrast, compensation for HF catabolism occurs primarily at the level of ghrelin acylation rather than secretion and may be a normal response to a catabolic state, and HF may entail resistance to the acylated form of ghrelin and occur downstream, at the level of the receptor. Post-HTx, ghrelin acylation declines and may even be downregulated, possibly as an adaptive compensatory response to weight gain.
In HF, cardiac cachexia and weight loss are harmful regardless of BMI (Citation1). Although cachexia in HF may have become less common in parallel with an increase in BMI in the general population, and the BMI in our HF patients may be considered non-cachectic, HF still involves a catabolic and inflammatory state. This has been attributed to neurohormonal and cytokine activation and growth hormone resistance (Citation9), but ghrelin resistance is another potential explanation (Citation3).
The literature describes elevations of ghrelin in other catabolic conditions, such as anorexia nervosa (Citation13) and renal (Citation14), lung (Citation15), liver (Citation16), and malignant (Citation17) disease, but as in case of HF (Citation3) total ghrelin levels have generally been measured. It has become clear that acylated ghrelin is responsible for most endocrine actions (Citation11). We rule out impaired acylation as an explanation for ghrelin resistance in HF and instead suggest increased acylation as a compensatory response. This may be compensation for the catabolic state, GH resistance, and/or cardiac dysfunction. One study suggests similar ghrelin acylation patterns in cancer cachexia (Citation17), suggesting that not only increases in total plasma ghrelin (Citation3,Citation14–17) but specifically increases in ghrelin acylation rather than secretion may be the universal response to cachexia and catabolism. Post-HTx, the inordinate weight gain (Citation2) may be explained by normalized response to acylated ghrelin. In our study, there was a significant negative correlation between acylated ghrelin and BMI in HF patients. Acylated ghrelin has been associated inversely with BMI also in anorexic and obese women (Citation13), but positively in the metabolic syndrome (Citation18). The absence of correlation between acylated ghrelin and BMI post-HTx suggests that acylated ghrelin may not be adequately suppressed by increases in BMI post-HTx, although other explanations such as leptin resistance (Citation19) may contribute to weight gain. As acylated ghrelin stimulates adrenocorticotropic hormone release (Citation11), the reduction in acylated ghrelin post-HTx may reflect negative feedback from immunosuppressive steroid therapy, although we have previously shown that weight gain post-HTx is independent of steroid use and dose (Citation2).
Ghrelin administration in animal and human models has demonstrated increased appetite and food intake, GH, and lean mass (Citation8); reduced inflammation (Citation20) and apoptosis (Citation21); and reduced sympathetic activation, heart rate, vasoconstriction, and blood pressure (Citation8,Citation22). Appetite stimulation requires the acylated version of ghrelin (Citation5), but des-acyl ghrelin has been suggested to both stimulate (Citation23) and inhibit (Citation24) appetite, and may have other poorly characterized effects, such as vasodilation, cardioprotection, and improved glycemic control (Citation21,Citation25,Citation26). Furthermore, ghrelin is expressed in myocardium; in HF, this expression is reduced, whereas cardiac ghrelin receptors are upregulated (Citation27). Unlike central ghrelin receptors, cardiac ghrelin receptors bind both acylated and des-acyl ghrelin (Citation21), suggesting that cardiac effects may not require acylation. Indeed, preliminary studies in human HF suggest that ghrelin administration may reduce muscle wasting, and improve exercise capacity, left ventricular function, and cardiac output, suggesting responsiveness to the GH stimulating and cardiovascular effects (Citation8,Citation22). These findings require further study but suggest that resistance to the appetite-stimulating effects of ghrelin does not rule out its potential therapeutic role in HF for cardiac function.
Our study and other studies are limited by instability of acylated ghrelin and concerns that the acidification specified in commercial kits may not sufficiently prevent de-acylation (Citation28). Indeed, since our study was performed, newer methods suggest not using acidification (Citation29,Citation30). However, this would affect samples equally and the differences in acylated ghrelin and ratios were highly significant. Elevated acylated ghrelin in the present study is assumed to represent upregulated acylation, but we cannot rule out downregulated breakdown of specifically acylated ghrelin. Several HF parameters, such as EF, were unavailable in HTx patients. Due to the cross-sectional design, we cannot show that heart transplantation is a cause for the changes observed, but only that such an association may exist. The control group was used as a reference but is too small to allow interpretations of differences versus HF and HTx patients.
In conclusion, acylated ghrelin and the acylated/total ratio were dramatically higher in HF compared to HTx. This indicates that acylation rather than secretion of ghrelin is upregulated in HF and the resistance to ghrelin's anabolic and appetite- stimulating effects is not at the level of acylation, but downstream at the ghrelin-receptor level. Future studies should address regulation of ghrelin acylation and ghrelin receptor expression and potential post-translational modifications.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Swedish Research Council [grant 2013-23897-104604-23 to LHL], the Swedish Heart Lung Foundation [grants 20080409 and 20100419 to LHL], the Stockholm County Council [grants 20090556 and 20110120 to LHL], NIH [grants DK064720 and DK073040 to PUF], the Division of Research Resources, General Clinical Research Centers Program, N.I.H. 5 MO1 RR00645 [to Columbia University], the Foundation for Cardiac Therapies [FACT Fund to DMM] and the Altman Fund [to DMM].
References
- Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, et al. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–50.
- Williams JJ, Lund LH, LaManca J, Kunavarapu C, Cohen DJ, Heshka S, et al. Excessive weight gain in cardiac transplant recipients. J Heart Lung Transplant. 2006;25: 36–41.
- Lund LH, Williams JJ, Freda P, LaManca JJ, LeJemtel TH, Mancini DM. Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur J Heart Fail. 2009;11:789–94.
- Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013;2013:518909.
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–4.
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30.
- Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004; 110:3674–9.
- Lund LH, Freda P, Williams JJ, LaManca JJ, LeJemtel TH, Mancini DM. Growth hormone resistance in severe heart failure resolves after cardiac transplantation. Eur J Heart Fail. 2009;11:525–8.
- Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132: 387–96.
- Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, et al. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004; 89:3062–5.
- Stengel A, Goebel M, Wang L, Reeve JR Jr, Tache Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–96.
- Nakahara T, Harada T, Yasuhara D, Shimada N, Amitani H, Sakoguchi T, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64:252–5.
- Buscher AK, Buscher R, Hauffa BP, Hoyer PF. Alterations in appetite-regulating hormones influence protein-energy wasting in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2010;25:2295–301.
- Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:879–82.
- Tacke F, Brabant G, Kruck E, Horn R, Schoffski P, Hecker H, et al. Ghrelin in chronic liver disease. J Hepatol. 2003;38:447–54.
- Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, et al. Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab. 2005;90:2920–6.
- Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, et al. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3935–40.
- Lund LH, Freda P, Williams JJ, LaManca JJ, LeJemtel TH, Mancini DM. Leptin resistance after heart transplantation. Eur J Heart Fail. 2010;12:516–20.
- Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, et al. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109:2221–6.
- Baldanzi G, Filigheddu N, Cutrupi S, Catapano F, Bonissoni S, Fubini A, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002; 159:1029–37.
- Nagaya N, Miyatake K, Uematsu M, Oya H, Shimizu W, Hosoda H, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854–9.
- Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, et al. Des-acyl ghrelin induces food intake by a mechanism independent of the growth hormone secretagogue receptor. Endocrinology. 2006;147:2306–14.
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24.
- Moazed B, Quest D, Gopalakrishnan V. Des-acyl ghrelin fragments evoke endothelium-dependent vasodilatation of rat mesenteric vascular bed via activation of potassium channels. Eur J Pharmacol. 2009;604:79–86.
- Ozcan B, Neggers SJ, Miller AR, Yang HC, Lucaites V, Abribat T, et al. Does des-acyl ghrelin improve glycemic control in obese diabetic subjects by decreasing acylated ghrelin levels? Eur J Endocrinol. 2014;170:799–807.
- Beiras-Fernandez A, Kaczmarek I, Schmoeckel M, Beiras A, Vicol C, Reichart B. Expression of Ghrelin, a novel cardiovascular hormone, and its peptide in the myocardium of patients undergoing heart transplantation. J Heart Lung Transpl. 2007;26:S84–5.
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93: 1980–7.
- Blatnik M, Soderstrom CI, Dysinger M, Fraser SA. Prandial ghrelin attenuation provides evidence that des-acyl ghrelin may be an artifact of sample handling in human plasma. Bioanalysis. 2012;4:2447–55.
- Delhanty PJ, Huisman M, Julien M, Mouchain K, Brune P, Themmen AP, et al. The acylated (AG) to unacylated (UAG) ghrelin ratio in esterase inhibitor-treated blood is higher than previously described. Clin Endocrinol (Oxf). 2014;1–5

