Abstract
Objectives. Diastolic dysfunction is a major cause of morbidity in heart transplant recipients. A reliable, non-invasive marker of left ventricular (LV) filling pressure would simplify follow-up in these patients. We aimed to test the validity of echocardiographic indices of LV filling pressure in a contemporary population of heart transplant recipients. Design. Eighty-three patients were examined by right-sided heart catheterisation and echocardiography one year after heart transplantation. We explored the association between echocardiographic parameters of LV filling pressure and invasively measured pulmonary capillary wedge pressure (PCWP). Results. Peak early mitral flow velocity divided by septal early mitral relaxation velocity (E/e’septal) was the echocardiographic parameter that best correlated with PCWP (r = 0.47; p < 0.001). At a cut-off value of 22, E/e’septal could identify patients with a PCWP above 12 mm Hg with a sensitivity of 56% and a specificity of 95%. Conclusions. The E/e’ index was moderately associated with LV filling pressure in heart transplant recipients. Echocardiographic parameters of diastolic function should be interpreted with caution when estimating left ventricular filling pressures in this population.
Introduction
The survival after heart transplantation has been steadily increasing (Citation1). The 50% survival rate in heart transplant (HTx) recipients in Norway now exceeds 12 years (Citation2), in line with international results (Citation1). These patients require regular follow-up. The main cardiac complications in HTx recipients are cardiac allograft rejection and vasculopathy, the detection of which require repeated catheterisations (Citation3), incurring patient discomfort and risk (Citation4). Transthoracic echocardiography is a primary tool in the investigation of allograft function, but whether or not echocardiography holds the potential for detecting allograft rejection remains unresolved (Citation5,Citation6).
Left ventricular (LV) diastolic dysfunction is common in HTx recipients (Citation7). In the allograft, diastolic function may be impaired due to preoperative ischaemia (Citation8), low-grade inflammation (Citation9) and fibrosis (Citation10), whereas extensive cardiac allograft vasculopathy is seldom seen as early as one year after transplantation (Citation11). Elevated LV filling pressure on catheterisation, i.e. end diastolic pressure > 16 mmHg or pulmonary capillary wedge pressure (PCWP) > 12 mmHg, is often used as a measure of diastolic dysfunction (Citation12).
Tissue-Doppler-derived measurements of mitral annular relaxation velocity (e’) and particularly the index of peak early mitral inflow velocity (E) to e’ (E/e’) have been suggested as indices of LV filling pressure (Citation13). In 1998, Sundereswaran and co- workers found the latter parameter to predict LV filling pressures in their population of HTx recipients (Citation14). The validity of this index in other groups of patients has been questioned (Citation15), but it remains the most widely acknowledged non-invasive marker of LV filling pressure (Citation12).
Over the last 15–20 years, most centres have changed their method of HTx surgery from the atrial anastomoses to the bicaval technique (Citation16). The latter method preserves atrial integrity. However, it is not known to which degree echocardiographic measures of LV filling pressure, many highly dependent on left atrial function, reflect filling pressures in HTx patients with bicaval anastomoses. Our aim was to assess the validity of common echocardiographic indices of LV filling pressure, including E/e’, by comparison with invasively measured PCWP, in a contemporary population of HTx recipients.
Methods
In Norway, all orthotopic HTx surgery and follow-up are performed at Oslo University Hospital, Rikshospitalet. In 1998, the surgical technique was switched from the atrial technique to the bicaval technique. By routine, both right-sided heart catheterisation and echocardiography are performed at follow-up one year after heart transplantation.
Material
Data from 187 patients from our HTx database, approved by the Institutional Review Board, were reviewed. Individual patient consent was not deemed necessary for this retrospective study. The patients were consecutive, adult HTx recipients, transplanted between August 22nd 2004 and December 3rd 2010. Patients who had been examined by both echocardiography and right-sided heart catheterisation on the same day at routine follow-up one year after transplantation were included. Exclusion criteria were surgical technique using atrial anastomoses; and atrial fibrillation at follow-up. Twenty-one patients died before their scheduled one-year follow-up; 81 patients had their follow-up echocardiography and right-sided heart catheterisation performed on two different days; one patient was in atrial fibrillation; and in one case, data from the right-sided heart catheterisation were incomplete. Thus, 83 patients fulfilled the inclusion criteria and were included in the current report.
Echocardiography
Echocardiographic examinations one year after cardiac transplantation were performed with Vivid 7 ultrasound scanners (GE Vingmed Ultrasound, Horten, Norway), using phased array transducers. Cine loops were digitally stored and later analysed offline using Echo-Pac version 7.0.0. (GE Vingmed). For the current report, the examinations were re-analysed by KB, and approximately one-quarter of the examinations were independently assessed by AA. All echocardiographic analyses were performed by blinding to other patient data.
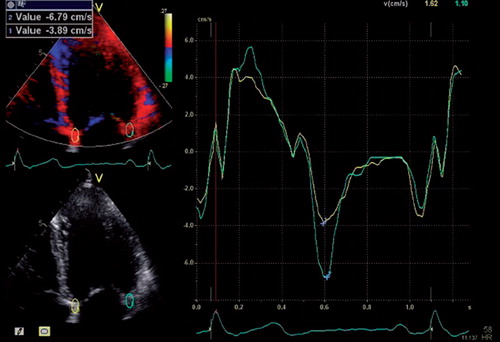
Two-dimensional parameters and conventional Doppler parameters were obtained according to current recommendations (Citation17,Citation18). All Doppler measurements were averaged over ≥ 3 heart beats. Valvular regurgitations were graded as mild, moderate or severe by visual assessment. Left ventricular ejection fraction (LVEF) was measured by Simpson's biplane method. Early mitral inflow velocity was measured by pulsed-wave Doppler at the tip of the mitral leaflets. As pulsed-wave tissue Doppler recordings were unavailable in a proportion of our patients, e’ was derived from colour-coded tissue Doppler imaging (TDI) images (110 ± 25 frames/second), in which 5 by 10 mm regions of interests were positioned at the septal and lateral insertion of the mitral valve, respectively (). Early mitral inflow velocity was divided by e’ measured at the trough of the first negative deflection after the isovolumetric relaxation phase in the septum (e’septal) and lateral wall (e’lateral) in the four chamber view (Citation14) to obtain E/e’septal, E/e’lateral and E/e’average, respectively.
Figure 1. Representative colour-coded TDI 4-chamber view of the left ventricle in which 5 × 10 mm regions of interest were placed at the insertion of the anterior and posterior mitral leaflets, respectively. Time-dependent annular tissue velocities are depicted on the right. Peak early mitral relaxation velocities, e’, (blue crosses) were measured in the septum and lateral free wall.
Catheterisation
Right-sided heart catheterisation was performed using a Swan–Ganz pulmonary artery thermodilution catheter (Baxter Health Care Corp, Santa Ana, CA). Patients were not required to fast. As a rule, we do not administer sedatives or muscle relaxants during catheterisations. Intra-cardiac pressures, including the mean PCWP, were recorded and averaged over several breathing cycles. The wedge position was verified by observing the typical changes in wave forms, and by fluoroscopy. Cardiac output was measured by the thermodilution technique, averaging at least three separate measurements. Cardiac index was calculated by dividing cardiac output by body surface area. Original recordings were reviewed by an experienced invasive cardiologist (EG) who did not have access to the echocardiographic data.
Statistical analysis
Continuous variables are presented as mean values ± standard deviation or as median values (interquartile range) as appropriate. Approximate normality was confirmed by visual analysis of distribution plots. Associations between echocardiographic variables and invasively measured PCWP were analysed by linear, least-squares regression analysis. We assessed the corresponding receiver-operating characteristic (ROC) curves to find optimal cutoff values for predicting a PCWP of above 12 mm Hg. Echocardiographic variables with a univariate association with PCWP statistically significant at a p-level of < 0.15, as well as key clinical variables, were analysed in a multiple linear regression model (enter), with PCWP as the dependent variable. When two or more parameters were interdependent, all but one of the parameters were excluded from the multivariate analysis. We used the heart rate measured at echocardiography for the statistical analyses. All statistical analyses were performed in SPSS version 18 (SPSS Inc. Chicago, IL).
Results
A total of 83 HTx recipients (65 men and 18 women) were included. The average age at the time of heart transplantation was 53 (range: 20–66) years. All patients were haemodynamically stable and in sinus rhythm at follow-up 366 ± 17 days after transplantation. One patient presented with a prolonged PQ interval (340 ms). Twenty-seven patients (33%) had a complete right bundle branch block, none had left bundle branch block. Clinical characteristics are presented in . The heart rate was similar at echocardiography and at right-sided heart catheterisation (85 ± 12 vs 85 ± 12 beats per minute, p for difference 0.86; correlation coefficient: 0.76).
Table I. Population characteristics one year after heart transplantation.
In 5 of 83 patients, the echocardiographic image quality was deemed poor. In the other 78 patients, the image quality was good or excellent. Mitral E-wave velocity was measurable in all patients and e’septal in all patients but one. There were no exclusions for poor echocardiographic windows. Inter- and intra-observer repeatability was high, with intra-class correlation coefficients (ICC) for LVEF 0.86 and 0.91, respectively. Inter-observer ICCs for E and e’septal were 0.98 and 0.90, respectively, while intra-observer ICCs were 0.99 and 0.97, respectively.
Key echocardiographic parameters are presented in . Of note, the average LVEF and LV internal diameter in end diastole were within the normal range. Fourteen patients had an LVEF below 50%. A moderate or severe tricuspid regurgitation was present in four patients, one of whom also had a moderate mitral regurgitation. One patient displayed an isolated, moderate mitral regurgitation. Apart from this, no significant valvular pathology was detected. In 16 patients, fusion of the early and atrial mitral inflow waves (E/A fusion) rendered determination of E deceleration time and the ratio of early to atrial mitral inflow velocity (E/A ratio) impossible.
Table II. Key hemodynamic parameters one year after heart transplantation.
Haemodynamic parameters obtained by right-sided heart catheterisation are presented in . There was a wide range in PCWP (2–26 mmHg). Eighteen patients (22%) had an elevated LV filling pressure as defined by a PCWP above 12 mm Hg.
Associations between echocardiographic parameters of LV filling pressure and invasively measured PCWP are shown in . No single echocardiographic parameter was strongly associated with PCWP. Notably, neither E deceleration time nor the estimated transtricuspid pressure gradient was significantly associated with PCWP in our patients. The parameter best correlated with PCWP was E/e’septal, closely followed by E (). The value of E/e’ is known to be limited in patients with mitral valve disease and/or wide QRS complexes (Citation12). However, repeated analyses excluding patients with mitral regurgitations or QRS width of > 120 ms yielded almost identical results (Supplementary Table I - which is only available in the online version of the journal. Please find this material with the following direct link to the article: http://informahealthcare.com/doi/abs/10.3109/14017431.2014.981579.).
Figure 2. The relationship between invasively measured PCWP and (a) LVEF, (b) peak early mitral inflow velocity (E), (c) E deceleration time and (d) E divided by the septal early mitral relaxation velocity (E/e’septal).
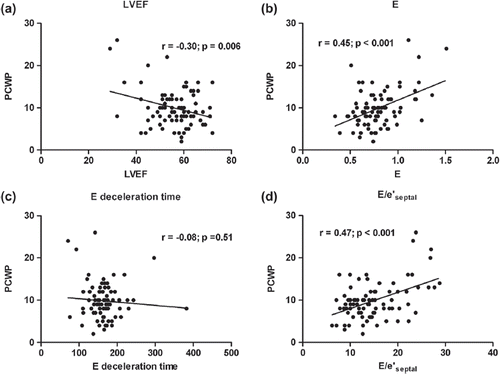
Table III. Echocardiographic predictors of PCWP.
When applying a cutoff value of 15 for E/e’septal, we identified patients with a PCWP above 12 mm Hg with a sensitivity of 67%, and a specificity of 69%. From visual analysis of the corresponding ROC curve (Supplementary Figure 1 – which is only available in the online version of the journal. Please find this material with the following direct link to the article: http://informahealthcare.com/doi/abs/10.3109/14017431.2014.981579.), the area under which was 0.75 (95% confidence interval: 0.60–0.90), we found a cutoff value for E/e’septal of 22 to provide optimal discriminatory power. At this cut point, we could identify patients with PCWP above 12 mm Hg with a sensitivity of 56% and a specificity of 95%. The corresponding negative predictive value (the ability to predict a PCWP of ≤ 12 mm Hg) was 88%, whereas the positive predictive value (the ability to predict a PCWP of > 12 mm Hg) was 77%.
As the relationship between the E/A ratio and LV filling pressure is non-linear, simple correlation analysis cannot be assumed to uncover a potential association between the E/A ratio and filling pressure. We therefore assessed the relationship between the E/A ratio at different cut points and elevated LV filling pressure, defined as PCWP > 12. The area under the corresponding ROC curve was 0.59 (95% confidence interval: 0.40–0.79). All patients with an E/A ratio above 3 had an elevated PCWP, (p < 0.001) but only five patients were in this category, and the sensitivity was therefore low at 28%.
A multiple regression analysis was performed in order to isolate independent predictors of PCWP. We included echocardiographic variables with a univariate association with PCWP significant at a p-level of 0.15, but to avoid a collinearity problem, we excluded parameters with interdependence. Early mitral inflow velocity, E/e’lateral and E/e’average were therefore excluded from the analysis, whereas E/e’septal, demonstrating the strongest association with PCWP on univariate analysis, was included as an independent variable. Results of the multiple regression analysis are presented in , and show that E/e’septal is associated with PCWP independent of LVEF, cardiac output by echocardiography and key clinical patient characteristics. Donor characteristics, such as age, gender and total time from cardiac explantation to implantation, did not correlate with LV filling pressure (data not shown). However, E/e’septal was significantly higher in recipients with female versus male donors (17.4 vs. 13.1; p < 0.001) and there was a trend towards an association between E/e’septal and donor age (r = 0.21; p = 0.05).
Table IV. Independent predictors of PCWP.
Early/atrial mitral inflow velocity fusion is a common phenomenon in HTx recipients, partly due to denervation of the allograft and ensuing tachycardia at rest. An elevated PCWP was equally distributed between patients with complete early and atrial mitral inflow wave separation (8/40 patients = 20%); partial E/A fusion (6/27 patients = 22%) and complete E/A fusion (4/16 patients = 25%; p for difference between the three groups = 0.92 by Pearson's Chi-square test). Early/atrial mitral inflow velocity fusion might act as a confounder when analysing diastolic function. We therefore repeated our analyses, excluding patients with absolute or both absolute and partial E/A fusion, respectively. When the 16 patients with absolute E/A fusion were excluded, results were virtually identical to those of the entire cohort (data not shown). In the 40 patients in whom the E and A waves were completely separated, peak E and E/e’septal were associated with PCWP to a similar degree (r = 0.52, p < 0.001 and r = 0.50, p < 0.001, respectively).
Discussion
In the present study, E/e’septal was the echocardiographic parameter that best correlated to LV filling pressure as estimated by PCWP. However, although highly significant and independent of potential confounders, the correlation between E/e’septal and PCWP was but moderate. Based on previous studies (Citation13), the European Society of Echocardiography and the American Society of Echocardiography recommend using E/e’ to diagnose diastolic dysfunction, where values of 15 or higher indicate an elevated LV filling pressure (Citation12). This cutoff value is based on pulsed–wave-derived tissue Doppler velocities, and has not been validated in HTx recipients. In our population of HTx recipients, where e’ was derived from TDI colour images, an E/e’septal ratio of 22 predicted a normal LV filling pressure with acceptable precision. At this cut point, however, the sensitivity was low. In concordance with a previous report (Citation14), we were unable to find close associations between invasively measured PCWP and traditional echocardiographic parameters of diastolic function.
Previous investigations have yielded variable results when trying to detect elevated filling pressures in HTx recipients. Whereas Sundereswaran and colleagues found a very high correlation between invasively measured PCWP and E/e’ (Citation14), Ricards at al. failed to find clinically relevant associations between PCWP and the E/A ratio or the isovolumetric relaxation time (Citation19). Recently, López and colleagues (Citation20) and Odd Bech-Hanssen and associates (Citation21) were able to detect elevated left ventricular filling pressures with reasonable accuracy. Whereas López's group found that E divided by colour M-mode propagation velocity, a relatively little used index, correlated modestly with PCWP (r = 0.615), no cut off value with corresponding sensitivity and specificity was provided. Bech-Hanssen's group, on the other hand, was able to show that either estimated right atrial pressure by echocardiography, or a post-hoc combination of several diastolic indices, either of which was modestly associated with PCWP, could identify patients with elevated LV filling pressure with acceptable precision.
Since first promoted by Nagueh and co-workers in 1997 (Citation22), E/e’ has become a preferred and recommended non-invasive parameter for predicting LV filling pressure (Citation23). Because E depends on LV relaxation as well as filling pressure (Citation24), and e’ is primarily related to LV relaxation (Citation22), the E/e’ index should, in theory, primarily reflect LV filling pressure. However, E is not only related to relaxation and filling pressures, but also to other factors, such as ventricular and atrial compliance, LV systolic function and mitral valve properties (Citation25), Likewise, it has been demonstrated that e’ relates not only to LV relaxation, but also to filling pressure and systolic LV shortening (Citation26). In previous reports, the correlation coefficients for the association between E/e’ and invasively measured PCWP have ranged from 0.18 (15) to 0.8 (14), with most groups reporting intermediate correlations (Citation13,Citation27). Indeed, in the seminal paper by Ommen and co-workers, the correlation coefficient between E/e’ and PCWP was a modest 0.47, comparable to what we found in our HTx recipients.
There may be several reasons why the high correlation between E/e’ and PCWP reported by Sundereswaran and co-workers could not be reproduced in the present study. First, there may be differences in patient populations. The choice of surgical technique would be expected to influence atrial function and thus mitral flow dynamics. Second, we used colour-coded TDI to obtain e’ rather than pulsed-wave Doppler. Our results are therefore not immediately comparable to others’, where e’ was measured by pulsed-wave TDI (Citation13,Citation14).
For clinical purposes, it has been difficult to find a simple and reproducible echocardiographic parameter that reflects LV filling pressure. Blood flow and tissue velocities depend on ventricular compliance, filling pressure, heart rate and rhythm, and ventricular geometry (Citation15). These factors vary between individual patients with heart failure, and between groups of patients with different causes of heart failure. Thus, a variable that seems to predict elevated LV filling pressure in one group of patients cannot be assumed to carry the same information in patients with heart failure of a different aetiology.
Heart transplant recipients represent a particular challenge in this respect. In HTx recipients, atrial anatomy is distorted, presumably affecting the pulmonary flow pattern and the atrial contribution to LV filling. Relative to circular contraction, longitudinal contraction in systole, and thus longitudinal lengthening in diastole, is diminished in the allograft (Citation28), possibly owing to atrial tethering or pre-operative ischaemia. Denervation of the allograft leads to an increased heart rate at rest, and repeated biopsy incurs damage to the conducting system with ensuing conduction delays, particularly right bundle branch block. Increased heart rate at rest, as well as prolongation of the PR interval, leads to partial or absolute E/A fusion in a large proportion of HTx recipients. Taken together, these characteristics may substantially alter diastolic physiology in HTx recipients, making direct comparison with the general population difficult. As in the general population, an integrative approach using several echocardiographic parameters should probably be employed when non-invasively assessing LV filling pressure in the cardiac allograft.
Limitations
The present study was retrospective, but the aim of the study was stated a priori and without knowledge of patient characteristics. All echocardiographic recordings were performed according to protocol using high standard equipment. Nevertheless, some parameters, such as isovolumetric relaxation time, pulmonary vein flow velocity and pulsed-wave tissue Doppler recordings, were not routinely obtained. We therefore used colour-coded images to obtain e’. There is a strong correlation between values derived by colour-coded and pulsed-wave TDI (Citation29).
Echocardiography and right-sided heart catheterisation were performed on the same day, but not simultaneously. Thus, one cannot rule out that alterations in intravascular volume and blood pressure may have occurred from one examination to the next, diminishing the apparent association between invasive and echocardiographic measurements. On the other hand, all patients were clinically stable on the day of their one-year follow-up, and any such change in haemodynamics would therefore be expected to be small.
Conclusion
Traditional echocardiographic indices of LV filling pressure could not be used to accurately predict PCWP in our population of HTx recipients. The ratio of E to e’, especially when the latter is derived from the septum, is more closely related to PCWP in these patients. However, the association is moderate, and echocardiographic parameters should be interpreted with caution when seeking information about LV filling pressure in contemporary HTx recipients. Further investigation is required to establish non-invasive parameters that more accurately reflect LV filling pressures in this group of patients.
Supplementary Table I and Figure 1
Download PDF (63.3 KB)Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Funding
No funding was received for the research reported in the article.
References
- Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–64.
- Gude E, Simonsen S, Geiran OR, Fiane AE, Gullestad L, Arora S, et al. Pulmonary hypertension in heart transplantation: discrepant prognostic impact of pre-operative compared with 1-year post-operative right heart hemodynamics. J Heart Lung Transplant. 2010;29:216–23.
- Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56.
- Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–33.
- Palka P, Lange A, Galbraith A, Duhig E, Clarke BE, Parsonage W, et al. The role of left and right ventricular early diastolic Doppler tissue echocardiographic indices in the evaluation of acute rejection in orthotopic heart transplant. J Am Soc Echocardiogr. 2005;18:107–15.
- Mena C, Wencker D, Krumholz HM, McNamara RL. Detection of heart transplant rejection in adults by echocardiographic diastolic indices: a systematic review of the literature. J Am Soc Echocardiogr. 2006;19:1295–300.
- Tallaj JA, Kirklin JK, Brown RN, Rayburn BK, Bourge RC, Benza RL, et al. Post-heart transplant diastolic dysfunction is a risk factor for mortality. J Am Coll Cardiol. 2007;50:1064–9.
- Hausdorf G, Banner NR, Mitchell A, Khaghani A, Martin M, Yacoub M. Diastolic function after cardiac and heart-lung transplantation. Br Heart J. 1989;62:123–32.
- de Groot-Kruseman HA, Baan CC, Hagman EM, Mol WM, Niesters HG, Maat AP, et al. Sequential monitoring of intragraft cytokine mRNA expression in relation to diastolic left ventricular wall thickness and function early after heart transplantation. Clin Transplant. 2002;16:433–41.
- Goland S, Siegel RJ, Burton K, De Robertis MA, Rafique A, Schwarz E, et al. Changes in left and right ventricular function of donor hearts during the first year after heart transplantation. Heart. 2011;97:1681–6.
- Prada-Delgado O, Estevez-Loureiro R, Paniagua-Martin MJ, Lopez-Sainz A, Crespo-Leiro MG. Prevalence and prognostic value of cardiac allograft vasculopathy 1 year after heart transplantation according to the ISHLT recommended nomenclature. J Heart Lung Transplant. 2012;31:332–3.
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33.
- Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94.
- Sundereswaran L, Nagueh SF, Vardan S, Middleton KJ, Zoghbi WA, Quinones MA, Torre-Amione G. Estimation of left and right ventricular filling pressures after heart transplantation by tissue Doppler imaging. Am J Cardiol. 1998; 82:352–7.
- Mullens W, Borowski AG, Curtin RJ, Thomas JD, Tang WH. Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation. 2009;119:62–70.
- Aziz TM, Burgess MI, El-Gamel A, Campbell CS, Rahman AN, Deiraniya AK, Yonan NA. Orthotopic cardiac transplantation technique: a survey of current practice. Ann Thorac Surg. 1999;68:1242–6.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–84.
- Richards DR, Gilliland Y, Bernal JA, Smart FW, Stapleton DD, Ventura HO, Cheirif J. Mitral inflow and pulmonary venous Doppler measurements do not predict pulmonary capillary wedge pressure in heart transplant recipients. Am Heart J. 1998;135:641–6.
- Lopez B, Sanchez V, Delgado JF, Casares SF, Mora L, Garcia J, et al. Accuracy of noninvasive estimation of pulmonary wedge pressure by echocardiographic indices in heart transplant recipients. Transplant Proc. 2012;44: 2639–41.
- Bech-Hanssen O, Al-Habeeb W, Ahmed W, Di SG, Pergola V, Al-Admawi M, et al. Echocardiography detects elevated left ventricular filling pressures in heart transplant recipients. Echocardiography. 2014.
- Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30: 1527–33.
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–50.
- Takagi S, Yokota M, Iwase M, Yoshida J, Hayashi H, Sotobata I, et al. The important role of left ventricular relaxation and left atrial pressure in the left ventricular filling velocity profile. Am Heart J. 1989;118:954–62.
- Thomas JD, Newell JB, Choong CY, Weyman AE. Physical and physiological determinants of transmitral velocity: numerical analysis. Am J Physiol. 1991;260:H1718–31.
- Opdahl A, Remme EW, Helle-Valle T, Lyseggen E, Vartdal T, Pettersen E, et al. Determinants of left ventricular early- diastolic lengthening velocity: independent contributions from left ventricular relaxation, restoring forces, and lengthening load. Circulation. 2009;119:2578–86.
- Hadano Y, Murata K, Liu J, Oyama R, Harada N, Okuda S, et al. Can transthoracic Doppler echocardiography predict the discrepancy between left ventricular end-diastolic pressure and mean pulmonary capillary wedge pressure in patients with heart failure? Circ J. 2005;69:432–8.
- Saleh HK, Villarraga HR, Kane GC, Pereira NL, Raichlin E, Yu Y, et al. Normal left ventricular mechanical function and synchrony values by speckle-tracking echocardiography in the transplanted heart with normal ejection fraction. J Heart Lung Transplant. 2010.
- Hummel YM, Klip IJ, de Jong RM, Pieper PG, van Veldhuisen DJ, Voors AA. Diastolic function measurements and diagnostic consequences: a comparison of pulsed wave- and color-coded tissue Doppler imaging. Clin Res Cardiol. 2010;99:453–8.

