Abstract
Objectives. Reduced arterial vasodilatatory capacity is a marker of coronary heart disease. The aim was to investigate if the difference between the vasodilatory response before and after exercise, as assessed by non-invasive methodology, is related to endothelial and inflammatory biomarkers. Design. Post-ischemic hyperemia after 5 min of arterial occlusion was examined before and after a bicycle test with strain-gauge plethysmography (measuring peak reactive hyperemia in the forearm) and peripheral arterial tonometry (PAT hyperemia ratio: measuring pulse waves in the index finger relative to the contra-lateral index finger) in 30 healthy males. A low PAT hyperemia ratio or a low peak reactive hyperemia reflects endothelial dysfunction. Inflammatory and endothelial biomarkers were assessed. Results. A low peak reactive hyperemia and a low PAT hyperemia ratio before the bicycle test was associated with a high percentage increase in peak reactive hyperemia after exercise (r = − 0.68, p < 0.001; r = − 0.35, p = 0.06, respectively). Asymmetric dimethylarginine and interleukin-10 were associated with the percentage increase in peak reactive hyperemia in multiple linear regression analyses (β: 165 (confidence interval [CI], 34–296), p = 0.02; β: 19 (CI, − 0.5–39), p = 0.06, respectively). Conclusions. The difference in the vasodilatory response before and after exercise, as assessed by non-invasive methodology, is related to endothelial and inflammatory biomarkers in healthy males.
Introduction
Endothelial dysfunction is regarded as the initial step in the atherosclerotic process (Citation1). A considerable amount of factors is associated with endothelial dysfunction including hypertension, high cholesterol, smoking, and infections. These factors transform the endothelium into a pro-coagulative and pro-inflammatory state. Asymmetric dimethylarginine (ADMA) inhibits the enzyme endothelial nitric oxide synthase (eNOS) and decreases the production of NO and is an important marker of endothelial dysfunction (Citation2). ADMA is related to both cardiovascular risk factors and is a predictor for cardiovascular disease (Citation2,Citation3). von Willebrand factor (vWF) is released during endothelial injury and is a risk factor for cardiovascular disease and stroke (Citation4,Citation5). Pro-inflammatory markers like C-reactive protein (CRP), osteoprotegerin (OPG), soluble tumor necrosis factor receptor inhibitor (sTNFRI), P-selectin, and interleukin-8 (IL-8) indicate a higher risk for adverse cardiovascular events even if all these markers are not sufficiently documented (Citation6–10). Estimation of flow-mediated dilatation (FMD) with brachial ultrasound is regarded as gold standard for diagnosing endothelial function but requires an experienced operator. Invasive methods with the use of vasodilatatory substances like NG-monomethyl L-arginine (L-NMMA), acetylcholine, and sodium nitroprusside are unpractical in order to diagnose endothelial dysfunction.
Strain-gauge plethysmography and peripheral arterial tonometry (PAT) are non-invasive and easy to use. They measure volume changes in response to ischemic stimuli in the forearm and index finger, respectively. However, most studies performed with strain-gauge plethysmography have included invasive administration of endothelium-dependent vasodilatatory agents to detect endothelial dysfunction. The use of exercise may be an alternative to invasive agents to reveal endothelial dysfunction. While exercise training has improved the endothelial function both in healthy males and in patients with coronary artery disease or heart failure, there are few studies addressing the use of an exercise test to reveal endothelial dysfunction (Citation11–13).
Our hypothesis was that a moderate exercise effort used in combination with strain-gauge plethysmography or PAT could identify individuals with a reduced endothelial function and that these individuals were likely to have higher levels of endothelial and inflammatory markers.
Methods
We included 30 apparently healthy males aged 21–72 years. The study was conducted in a quiet clinical laboratory with stable temperature (20–24°C) and with the individual in the supine position according to the recommendations made by the International Task Force studying the clinical application of arterial stiffness (Citation14). The subjects refrained from food and smoking for at least 3 h. Only two men declared health issues or were on some form of medications. The protocol was approved by the Regional Ethical Committee South East (S-04194) and all participants gave their informed consent. Blood pressure was measured with a sphygmomanometer (Colin Electronics Co., Ltd, Japan).
Strain-gauge plethysmography
We used mercury-in-silastic strain-gauge plethysmography (Hokanson EC6, Bellevue, WA, USA) to measure forearm blood flow (mL/min/100 mL tissue) in the left arm. The left arm was elevated above the heart level. A cuff was placed around the upper arm. Mercury-in-silastic strain gauge was applied around the widest and most muscular part of the forearm. First, the baseline forearm blood flow was measured. A venous occlusion pressure of 50 mmHg was used to measure the baseline forearm blood flow, and five consecutive measurements were performed to calculate the baseline forearm blood flow. The coefficient of variation was 14.3%. Second, the cuff around the upper arm was inflated to suprasystolic pressures in 5 min to induce ischemia. Immediately after deflation, the peak reactive hyperemia was measured and forearm blood flow was recorded for 3 min thereafter. Ten minutes after a bicycle test was performed at a moderate intensity (15 min at 150 watt), we repeated the measurement of baseline forearm blood flow and thereafter peak reactive hyperemia.
Peripheral arterial tonometry
Blood flow in the index fingers was measured with PAT (Itamar Medical Ltd, Caesarea, Israel) concomitantly with peak reactive hyperemia both before and after the bicycle test. PAT consists of pneumatic probes with a thimble-shaped sensor cap applied at both index fingers. The constant pressure added to the cap was 10 mmHg below the diastolic blood pressure to maintain a negative venous transmural pressure to prevent venous pooling and blood stasis. Pulsatile volume changes in the distal index finger were measured. The finger probe on the person's right index finger functioned as a reference for the hyperemia testing done on the left index finger, which was performed at the same time as the measurement of peak reactive hypermemia in the left arm with strain-gauge plethysmography. The PAT hyperemia ratio was defined as “the average pulse wave amplitude in the left index finger as compared with the right index finger during the first 5 min after cuff deflation.” PAT measurements were analyzed with a computerized, automated algorithm eliminating intra- or interobserver variability.
Biochemical analyses
Non-fasting blood was sampled into pyrogen-free, pre-cooled vials without additives (serum) or with ethylenediamine tetraacetic acid as anticoagulant (plasma) prior to the measurement of endothelial function. The tubes were centrifuged at 1,000 g for 10 min within 30 min after sampling (plasma) or allowed to clot before centrifugation (serum). All samples were stored at − 70°C and thawed less than three times. Plasma levels of total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured enzymatically on a Roche/Hitachi 917 analyzer (Roche Diagnostics). High-sensitivity (hs)-CRP in plasma was determined by a high- sensitive particle-enhanced immunoturbidimetric assay (Roche Diagnostica). Plasma levels of vWF were determined by enzyme immunoassay (EIA). Serum levels of OPG, P-selectin, IL-8, IL-10, and TNFRI were quantified by EIA using commercially available matched antibodies (R&D Systems, Minneapolis, MN, USA). ADMA was assayed by an enzyme-linked immunosorbent assay using high-pressure liquid chromatography with precolumn derivatization and fluorescence detection (DLD Diagnostika, Hamburg, Germany).
Statistics
Data were analyzed using SPSS 21•0 (SPSS Inc). Baseline forearm blood flow was calculated as the mean of five consecutive measurements in each male both before and after cycling. Peak hyperemia values before and after cycling were corrected for baseline forearm blood flow by subtracting the baseline forearm blood flow before and after cycling from the corresponding peak reactive hyperemia value. Then, the percent change in peak reactive hyperemia was calculated for each male. Differences between peak and baseline forearm blood flow, PAT hyperemia ratio, and blood pressure were analyzed with non-parametric tests. Univariate linear regression analyses and Pearson correlation analyses were performed between age, traditional cardiovascular risk factors, and inflammatory indices, and each of the following parameters: peak reactive hyperemia before and after cycling, the percent change in peak reactive hyperemia, and PAT hyperemia ratio. Covariates with p < 0.10 were included in multiple linear regression analyses. The sample size allowed us to have only three parameters in the multiple linear regression analyses. Due to covariance between systolic and diastolic blood pressure and glucose and HbA1c these parameters were entered separately in the multiple linear regression analyses.
Results
We examined 30 men with a median age of 47 (range, 21–72) years with strain-gauge plethysmography and PAT ( and ). There were four current smokers and three men had smoked earlier. Snuffing was documented in two men; one of them had smoked earlier. Two men had hypertension during the test (> 140/90) but did not have a previous diagnosis of hypertension. Additionally, one had borderline hypertension and was later diagnosed as hypertensive and to have a diabetes mellitus type II (cost regulated). Another used a beta-2 agonist for asthma on occasion (last dose was taken 12 h before the test) and one had been electroconverted for atrial fibrillation three times.
Table I. Patient characteristics and blood indices including inflammatory and endothelial markers (median and range).
Table II. Methodological parameters and blood pressure before and after exercise (median and range).
A low peak reactive hyperemia or a low PAT hyperemia ratio indicates endothelial dysfunction even though there are no established pathological values for strain-gauge plethysmography and PAT hyperemia ratio. Before the bicycle test, the median baseline forearm blood flow was 4.2 (range, 1.5–7.5) mL/min/100 mL tissue, while the median peak reactive hyperemia was 27.4 (range, 13.9–42.7) mL/min/100 mL tissue. After the bicycle test the median baseline forearm blood flow increased to 7.3 (range, 3.8–13.4) mL/min/100 mL tissue while the median peak reactive hyperemia was 29.1 (range, 20.0–44.6) mL/min/100 mL tissue.
After correction for the baseline forearm blood flow there were no overall differences between peak reactive hyperemia before and after exercise (p = 0.27; ), but for each individual there was a large variation in the percent change in peak reactive hyperemia which varied from –47 to 160% (). Both a low peak reactive hyperemia and a low PAT hyperemia ratio before the bicycle test were associated with a high percentage change in peak reactive hyperemia [r = − 0.68, p < 0.001 (); r = − 0.35, p = 0.06, respectively]. Furthermore, the percentage change in peak reactive hyperemia was associated with OPG (r = 0.41, p = 0.03), IL-10 (r = 0.43, p = 0.02), TNFRI (r = 0.37, p = 0.05), and ADMA (r = 0.53, p = 0.003; and ). In multiple linear regression analyses, ADMA was a significant determinator of the percentage change in peak reactive hyperemia after correction for blood pressure and glucose parameters (β: 165 (confidence interval [CI], 34–296), p = 0.02). Additionally, IL-10 seemed to be associated with the percentage change in peak reactive hyperemia [β: 19.4 (CI, − 0.5–39), p = 0.06] together with both systolic blood pressure and glucose ().
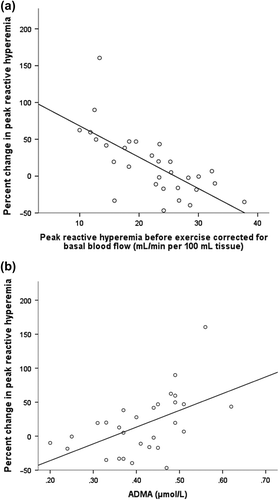
Table III. Determinants of the percentage change in peak reactive hyperemia.
PAT hyperemia ratio was 2.4 (range, 1.6–3.0) before bicycling and decreased to 1.8 (range, 1.4–2.5) after bicycling reflecting that the basal blood flow increased in both index fingers after exercise which consequently attenuated the PAT hyperemia ratio.
No association was revealed between peak reactive hyperemia and PAT hyperemia ratio before exercise with any of the endothelial or inflammatory markers except from a correlation between ADMA and peak reactive hyperemia (r = − 0.45, p = 0.02).
Discussion
The present study demonstrates that a sub-maximal exercise effort in combination with strain-gauge plethysmography identifies those with decreased endothelial function in a population consisting of apparently healthy males. PAT recording combined with electrocardiogram may enhance the diagnostic sensitivity and specificity of coronary atherosclerosis compared with electrocardiogram alone (Citation15). Furthermore, endothelial dysfunction measured with PAT predicted cardiac events in high-risk patients within 5 years (Citation16). Strain-gauge plethysmography has been effective in revealing endothelial dysfunction measured indirectly by forearm blood flow. A decreased forearm blood flow has been demonstrated in patients with high cholesterol, hypertension, smoking, diabetes mellitus, and coronary artery disease (Citation17–21). Most of these studies are performed with the use of invasive agents like acetylcholine, but there is evidence of a correlation between reactive hyperemia and acetylcholine-induced vasodilatation (Citation22). The exercise test will stimulate NO release through increased shear stress. Individuals with endothelial dysfunction will probably have low basal level of NO as reflected by a low peak reactive hyperemia and a low PAT hyperemia before exercise. These individuals will perhaps achieve a substantial higher release of NO or metabolic products during exercise resulting in a higher percentage increase in peak reactive hyperemia. Males with a high peak reactive hyperemia ratio before exercise had a sufficient response to an ischemic stimulus and did not obtain a higher peak reactive hyperemia after exercise. Peak reactive hyperemia is caused by instant relaxation of peripheral resistance arteries mediated by local vasoactive ischemic metabolites like adenosine where contributing factors are NO, prostacyclin, endothelium- derived hyperpolarizing factor, and activation of K+ channels (Citation23,Citation24). In a meta-analysis, NO explained about half of the FMD in studies using L-NMMA (Citation23). During physical activity other metabolites are essential for arterial vasodilatation and will have an impact on peak reactive hyperemia measured after exercise. For instance, ATP is metabolized by ectonucleotidases to adenosine, which dilates arterioles in a NO-independent mechanism while prostaglandins like PGE2 and PGI2 accumulate in the interstitium during exercise and contribute to exercise hyperemia (Citation24). We believe that in individuals with reduced endothelial function, contributing factors other than NO will be of increased importance to achieve sufficient hyperemia during exercise and serve as compensating factors for the lack of NO. This may explain the inverse correlation between peak reactive hyperemia before exercise and the percentage increase in peak reactive hyperemia after exercise.
ADMA inhibits endothelium-dependent vasodilatation and causes increased arterial tone. ADMA is a marker of cardiovascular risk and mortality both in patients with cardiovascular disease and in healthy populations (Citation2). In the present study, ADMA correlated negatively with peak reactive hyperemia before exercise, but the association between ADMA and the percent change in peak reactive hyperemia was even stronger. Additionally, unlike peak reactive hyperemia before exercise, the percentage increase in peak reactive hyperemia after exercise related to blood pressure, glucose, and IL-10. Thus, even if the measurement of peak reactive hyperemia before exercise may be sufficient to reveal a reduced endothelial function, strain-gauge plethysmography in combination with exercise seems to be a better method to identify patients with a reduced endothelial function. ADMA is an inhibitor of eNOS and it is not unexpected that a higher level of ADMA in part explains the low peak reactive hyperemia measured in some healthy males before exercise. This is in accordance with previous studies where intravenous administration of ADMA decreased forearm blood flow (Citation25).
Hyperglycemia decreases endothelium-derived NO availability by mechanisms involving phosphokinase C activity and reactive oxygen species (Citation26). Most of our patients had normal values for glucose and HbA1c. One might speculate if there is linearity between glucose and its effect on NO release also in the normal range of glucose levels since the percent change in peak reactive hyperemia was associated with both non-fasting glucose and HbA1c.
High blood pressure may disfavor an additional effect of exercise because further mobilization of NO through increased shear stress is impaired. This mechanism may be important for men with blood pressure within the normal range. In our study, there was a strong correlation between systolic and diastolic blood pressure and the percentage increase post exercise in peak reactive hyperemia.
OPG and sTNFRI are pro-inflammatory markers and members of the TNF-family elevated in patients with heart failure and regarded as a risk factor for cardiovascular disease (Citation8). OPG has been coupled to diabetes and endothelial dysfunction (Citation27). This is in accordance with the present study showing a correlation between OPG and the percent change in peak reactive hyperemia. IL-10 is an anti-inflammatory marker and is considered to have a protective effect. Those males with a high percentage change in peak reactive hyperemia and a high ADMA were more likely to have increased levels of IL-10. This may be due to general upregulation of the immune system in patients with endothelial dysfunction including protective mechanisms.
The sample size is small, which made it difficult to analyze the impact of possible confounders. Another limitation is that we did not compare strain-gauge plethysmography and PAT with FMD measured with brachial ultrasound. Furthermore, there is no established pathological value for strain-gauge plethysmography and PAT hyperemia ratio, which makes it difficult to decide whether some of the males truly have endothelial dysfunction. However, it is likely that males with a high increase in peak reactive hyperemia after exercise have endothelial dysfunction. A maximal exercise test would probably have caused increased forearm blood flow with more shear stress and a higher release of NO resulting in more uniform testing conditions among our participants. Nevertheless, a sub-maximal exercise effort was sufficient to reveal a significant association between ADMA and the percentage change in peak reactive hyperemia, but the association with OPG and sTNFRI may have been stronger with a maximal exercise test. A submaximal exercise test was easy to conduct and differentiated well between those who had a high percentage increase in peak reactive hyperemia from those with minimal or no increase.
The PAT signal amplitude during exercise has been shown to decrease in both patients with coronary artery disease and endothelial dysfunction (Citation15,Citation16). It might be that the use of PAT during rather than after exercise would have revealed an association with the endothelial and inflammatory markers, especially ADMA, and a stronger association with strain-gauge plethysmography.
In conclusion, strain-gauge plethysmography performed before and after a sub-maximal exercise effort is able to identify those with a decreased endothelial function, who may be at a higher risk for developing cardiovascular disease. Prospective studies are needed in order to confirm these results and show that the identification of high-risk individuals by this method is followed by cardiovascular disease.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- McLenachan JM, Williams JK, Fish RD, Ganz P, Selwyn AP. Loss of flow-mediated endothelium-dependent dilation occurs early in the development of atherosclerosis. Circulation. 1991;84:1273–8.
- Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–36.
- Schulze F, Lenzen H, Hanefeld C, Bartling A, Osterziel KJ, Goudeva L, et al. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am Heart J. 2006;152:493–8.
- Bongers TN, de Maat MP, van Goor ML, Bhaqwanbali V, van Vlief HH, Gomez Garcia EB, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37:2672–7.
- Yarnell J, McCrum E, Rumley A, Patterson C, Salomaa V, Lowe G. Association of European population levels of thrombotic and inflammatory factors with risk of coronary heart disease: the MONICA Optional Haemostasis Study. Eur Heart J. 2005;26:332–42.
- Anwaruddin S, Askari AT, Topol EJ. Redefining risk in acute coronary syndromes using molecular medicine. J Am Coll Cardiol. 2007;49:279–89.
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97.
- Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004; 109:2175–80.
- Patrianakos AP, Parthenakis FI, Papadimitriou EA, Diakakis GF, Tzerakis PG, Nikitovic D, et al. Restrictive filling pattern is associated with increased humoral activation and impaired exercise capacity in dilated cardiomyopathy. Eur J Heart Fail. 2004;6:735–43.
- Remick DG. Interleukin-8. Crit Care Med. 2005;33: S466–S467.
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, et al. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33: 1379–85.
- Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, et al. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–7.
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Harmann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15.
- Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockroft J, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–52.
- Chouraqui P, Schnall RP, Dvir I, Rozanski A, Quereshi E, Arditti A, et al. Assessment of peripheral artery tonometry in the detection of treadmill exercise-induced myocardial ischemia. J Am Coll Cardiol. 2002;40:2195–200.
- Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, et al. Peripheral endothelial function and cardiovascular events in high-risk patients. J Am Heart Assoc. 2013; 2:e000426. doi: 10.1161/JAHA.113.000426.
- De Filippis E, Cusi K, Ocampo G, Barria R, Buck S, Consoli A, Mandarino LJ. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91:4903–10.
- Ceravolo R, Maio R, Pujia A, Sciacqua A, Ventura G, Costa MC, et al. Pulse pressure and endothelial dysfunction in never-treated hypertensive patients. J Am Coll Cardiol. 2003;41:1753–8.
- Heitzer T, Yla-Herttuala S, Luoma J, Kurz S, Münzel T, Just H, et al. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–53.
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–8.
- Sarabi M, Vessby B, Millgård J, Lind L. Endothelium- dependent vasodilation is related to the fatty acid composition of serum lipids in healthy subjects. Atherosclerosis. 2001;156:349–55.
- Higashi Y, Yoshizumi M. New methods to evaluate endothelial function: method for assessing endothelial function in humans using a strain-gauge plethysmography: nitric oxide-dependent and -independent vasodilation. J Pharmacol Sci. 2003;93:399–404.
- Green DJ, Dawson EA, Groenewoud HJ, Jones H, Thijssen DH. Is flow-mediated dilatation nitric oxide mediated? A meta-analysis. Hypertension. 2014;63:376–82.
- Marshall JM, Ray CJ. Contribution of non-endothelium-dependent substances to exercise hyperemia: are they O2 dependent? J Physiol. 2012;590:6307–20.
- Calver A, Collier J, Leone A, Moncada S, Vallance P. Effect of local intra-arterial asymmetric dimethylarginine (ADMA) on the forearm arteriolar bed of healthy volunteers. J Hum Hypertens. 1993;7:193–4.
- Ryden L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007;28:88–136.
- Secchiero P, Corallini F, Pandolfi A, Consoli A, Candido R, Fabris B, et al. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol. 2006;169:2236–44.

