Abstract
Objectives. Epicardial adipose tissue (EAT) is the ectopic fat surrounding the heart, and it may contribute to coronary collateral vessel (CCV) formation. We aimed to evaluate the association of EAT with the presence of CCV in patients with acute coronary syndrome (ACS). Design. A total of 230 patients with ACS were included. The CCVs were graded according to the Rentrop scoring system, and a Rentrop grade 0–1 was accepted as low-grade CCV group, Rentrop grade 2–3 was accepted as high-grade CCV group. Results. According to angiography, 70 (30%) patients constituted the high-grade CCV group and 160 (70%) constituted the low-grade CCV group. The high-grade CCV group had thicker EAT than the low-grade CCV group (6.1 ± 1.4 vs. 5.3 ± 1.4 mm; p = 0.001). Multivariate logistic regression analysis showed that presence of CCV was independently associated with EAT thickness, ejection fraction, presenting with ST-segment elevation myocardial infarction, and presence of angina on admission. EAT thickness of > 5.7 mm can independently predict high-grade CCV with 73% sensitivity and 69% specificity (area under the curve or AUC: 0.65; 95% confidence interval or CI: 0.57–0.72). Conclusions. EAT thickness on admission was associated with the presence of CCVs in patients with ACS.
Introduction
Coronary collateral vessels (CCVs) can provide a perfusion reserve in case of increased myocardial oxygen demand. CCV can limit the myocardial ischemia and can protect the viable myocardium in patients with acute coronary syndrome (ACS) (Citation1–3). A well-developed CCV is associated with reduced infarction area and better ventricular function (Citation4,Citation5). The complex mechanisms that mediate the development of CCV in the heart are not well-understood. Some mediators such as C-reactive protein, uric acid, and circulating endothelial progenitor cells have been investigated, but they cannot fully explain this new vessel formation (Citation6,Citation7).
Epicardial adipose tissue (EAT) is the ectopic fat surrounding the heart, within the boundary of the pericardium. EAT is in direct contact with the major branches of the coronary vessels, and secretes numerous inflammatory cytokines (Citation8–10). Therefore, EAT is presumed to play an important role in the development of coronary artery disease (CAD) (Citation8,Citation11,Citation12). Moreover, as increased EAT thickness predicts the risk of CAD, it may also predict the presence of CCV, which is one of the major predictors of mortality in patients with stable CAD (Citation13).
In the present study, we hypothesized that EAT could be independently associated with the presence of CCV after adjustment for traditional cardiovascular risk factors in patients with ACS.
Material and methods
In the present study, between July 2013 and February 2014, 510 consecutive patients admitted to our emergency department with the diagnosis of ST segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) were included in this study. STEMI was defined by characteristic symptoms of myocardial ischemia in association with persistent electrocardiographic ST elevation and subsequent release of biomarkers of myocardial necrosis. Diagnostic ST elevation in the absence of left ventricular (LV) hypertrophy or left bundle branch block is defined by the European Society of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction as new ST elevation at the J point in at least 2 contiguous leads of ≥ 2 mm (0.2 mV) in men or ≥ 1.5 mm (0.15 mV) in women in leads V2–V3 and/or of ≥ 1 mm (0.1 mV) in other contiguous chest leads or the limb leads (Citation14). In the spectrum of ACS, UA/NSTEMI was defined by electrocardiographic ST-segment depression or prominent T-wave inversion and/or positive biomarkers of necrosis (e.g., troponin) in the absence of ST-segment elevation and in an appropriate clinical setting (chest discomfort or anginal equivalent) (Citation15). NSTEMI was diagnosed by the presence of positive cardiac enzymes, whereas UA was not associated with elevated cardiac biomarkers.
Patients with a history of renal disease, a history of coronary intervention or coronary artery bypass grafting, a history of inflammatory rheumatic disease, or a history of chronic obstructive pulmonary disease were excluded from study. Patients with less than 80% coronary artery stenosis were also excluded. Finally, 230 patients with ACS (88 STEMI, 110 NSTEMI, and 32 UA) were included in our study. Baseline characteristics, including age, sex, body mass index, smoking status, hyperlipidemia, hypertension, diabetes mellitus (DM), family history and previous medications, systolic blood pressure, diastolic blood pressure, heart rate (beats/min), and the presence of chest pain were recorded in all patients. Hypertension was defined as systolic blood pressure > 140 mmHg and/or a diastolic pressure > 90 mmHg at least two times, or if the individual was taking antihypertensive medications. The diagnosis of DM was based on the history of diabetes treated with or without drug therapies. Hyperlipidemia was defined as total cholesterol level > 5.2 mmol/l or treatment with a lipid-lowering agent. Furthermore, on admission, each patient was evaluated for serum creatinine, glucose, lipid profile, creatine kinase MB (CK-MB), troponin, and hematological indices. The extend of myocardial infarction was determined by the peak blood levels of troponin and CK-MB, which show amount of myocardial injury. This study was approved by the local ethics committee and informed consent was obtained from each patient.
Each patient underwent a transthoracic echocardiography using a 3.5-MHz transducer (Vivid 7, GE-Vingmed Ultrasound AS; Horten, Norway) within the first 24 h after hospital admission. Echocardiograms were obtained with subjects in the left lateral decubitus position. EAT thickness was measured by two cardiologists who were blinded to the patients’ data, as described by Iacobellis et al. (Citation16). EAT was defined as the echo-free space in front of the right ventriclular free wall on transthoracic parasternal long-axis images. The measurement of EAT thickness was made perpendicular to the aortic annulus at end-diastole. All measurements were performed for three consecutive cardiac cycles, and an average value was obtained. LV ejection fraction (EF) was determined by the Simpson method, according to the recommendations of the American Society of Echocardiography (Citation17).
Judkin's technique was used through a femoral approach to selective coronary angiography. Left anterior descending or LAD and left circumflex or LCx coronary arteries were evaluated in at least 4, and right coronary artery or RCA in at least two angiographic views. The coronary angiograms were examined by two specialists blinded to the clinical and laboratory findings of the cases. Coronary vessel disease was described when the percent diameter stenosis is 80% or more in at least one coronary artery. Thrombolysis in myocardial infarction (TIMI) flow grade was also determined during coronary angiography. Collateral circulation was graded according to the Rentrop classification. Accordingly, grade 0 was classified as no filling; grade 1 was classified as filling of side branches via collateral channels without visualization of the epicardial segment; grade 2 was classified as a partial filling of epicardial major coronary artery via collateral channels; and grade 3 was classified as complete filling of epicardial major coronary artery. Patients were divided into two groups based on the Rentrop classification; low-grade CCV group was defined as grade 0–1 and high-grade CCV was defined as grade 2–3.
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as percentages. The Kolmogorov–Smirnov test was used to verify the normality of distribution of continuous variables. The independent sample t-test or the Mann–Whitney U test was used for the continuous variables and the chi-square test for categorical variables. Multivariate, stepwise backward conditional logistic regression analysis was used to determine the independent predictors of presence of CCV and all significant parameters in the univariate analysis were selected in the multivariate model. A receiver-operating characteristic or ROC curve analysis was performed to identify the optimal cut-off point of EAT thickness to predict the presence of CCV in patients with ACS. The area under the curve (AUC) value was calculated as a measure of the accuracy of the test; a two-tailed p < 0.05 was considered to be statistically significant.
Results
The mean age of 230 patients was 57.4 ± 7.8 years and 74% of the study population were men. On the admission angiography, 70 (30%) patients had Rentrop grade 2–3 CCV and they constituted high-grade CCV group. The remaining 160 (70%) patients had grade 0–1 CCV and they constituted the low-grade CCV group. The baseline demographic, echocardiographic variables, and prehospital medications of the study patients are shown in . High-grade CCV group had a higher values of EAT thickness compared with low-grade CCV group (6.1 ± 1.4 vs. 5.3 ± 1.4 mm; p = 0.001) ().
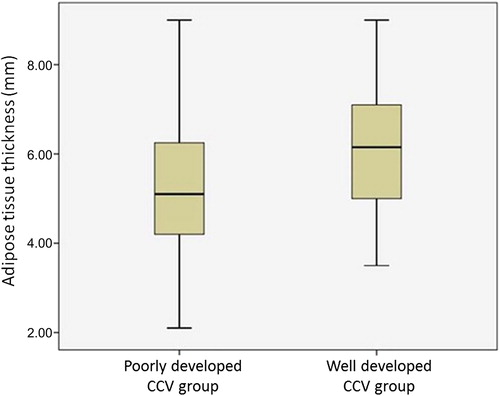
Table I. Baseline characteristics of study groups.
There were no significant differences between the study groups in terms of hemoglobin, white blood cell count, creatinine, low-density lipoprotein, and high-density lipoprotein cholesterol levels. The extent of myocardial infarction, as measured by peak CK-MB and troponin T levels, were significantly higher in the low-grade CCV group (). TIMI 0 flow grade was statisticaly significantly more prominent in both of the groups (). There were 32 patients with diagnosis of UA. Two of them had TIMI 3 flow, 22 of them had TIMI 2 flow and the remaining eight patients had TIMI 1 flow.
Table II. Baseline laboratory findings of patients.
Table III. TIMI flow grades of patients.
Multivariate logistic regression analysis showed that the presence of CCV was independently associated with EAT thickness (odds ratio [OR]: 1.41; 95% confidence interval [CI]: 1.15–1.78; p = 0.001), EF (OR: 1.10; 95% CI: 1.04–1.16; p = 0.011), presenting with STEMI (OR: 0.75; 95% CI: 0.60–0.92; p = 0.044). and the presence of angina at admission (OR: 0.60; 95% CI: 0.38–0.97; p = 0.041) (). EAT thickness of > 5.7 mm can independently predict the presence of high-grade CCV with 73% sensitivity and 69% specificity (AUC: 0.65; 95% CI: 0.57–0.72) ().
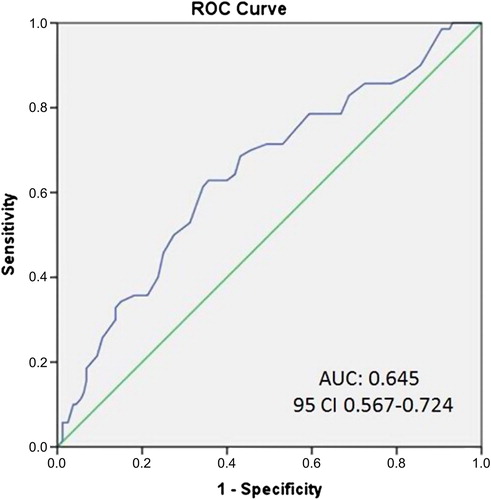
Table IV. Multivariate logistic regression analysis of variables related to CCV development.
Discussion
Our study results demonstrated that a thick EAT, a high EF, the presentation with STEMI, and the presence of angina were independent predictors of well-developed CCVs in patients with ACS.
CCVs are structures that are normally present in the human heart, though invisible by angiography. These are interconnecting branches between the main arteries, and serve as alternative conduits for blood flow in obstructive coronary heart disease. Many factors influence the development of the CCV, such as percent diameter of coronary artery stenosis, duration of angina, DM, hypertension, smoking status, hypoxia, endothelial functions, exercise, oxidative stress, genetic factors, and medications (Citation18). It has previously been shown that coronary stenosis with less than 80% luminal narrowing was rarely associated with angiographically demonstrable collateral circulation, whereas lesions of 95% or more narrowing were nearly always associated with good collateral formation (Citation19,Citation20).
The presence or absence of CCV is important during acute ischemia. Coronary collateral formation can limit myocardial ischemia, minimize infarct size, protect viable myocardium, and correlate with clinical outcomes in patients with ACS. Also, the presence of collaterals may contribute to myocardial viability for a prolonged period in patients with ACS. The CCV development in ACS has been associated with better myocardial perfusion and ventricular function as well as lower incidence of adverse cardiovascular events during long-term follow-up (Citation21). Our study population was composed of ACS patients with diagnosis of STEMI, NSTEMI, and UA. Their mean peak values of CK-MB and troponin T levels, which show the extent of myocardial damage, was found to be relatively low. This may be explained by inclusion of UA patients, which had normal levels of cardiac enzymes, early revascularization, and high percentage of patients with the presence of high-grade coronary collaterals.
In CCV development, many endogenous mediators such as growth factors, mainly vascular endothelial growth factor, nitric oxide, inflammatory markers, and neurohumoral markers are involved (Citation22). EAT plays an important role in inflammatory processes in the cardiovascular system because it releases several bioactive molecules including adiponectin and proinflammatory cytokines such as interleukin-1, interleukin-6, vasoactive peptides, and tumor necrosis factor (Citation23). All of these molecules can independently play a role in terms of CCV development. Although the causes of CCV development are unknown, one of the responsible mechanisms is thought to be the activation of the immune system with predominant involvement of cytokines and chemokines. Several methods and cardiac locations have been used for measurement of EAT. In comparison with computed tomography (CT)-based measurement, echocardiography is a non-invasive imaging modality that is cost-effective and time-saving. Echocardiographic measurement of the epicardial fat thickness has, however, several limitations for quantification of EAT, including the lack of an adequate window around all cardiac segments and a low reproducibility. CT provides a more accurate quantification of epicardial fat distribution, but multislice CT is a time-consuming process and involves considerable radiation exposure. Several locations have been used to measure EAT, like right ventricular free wall, left atrio-ventricular groove, and left main coronary artery region (Citation24). The association between location-specific EAT thickness at the right ventricular free wall and CAD is still controversial. Several studies using echocardiography have demonstrated a significant relationship between CAD and location-specific EAT thickness measured at right ventricular free wall (Citation25,Citation26), while other studies using echocardiography or CT failed to observe a significant association (Citation27,Citation28). In our study, EAT was measured from right ventricular free wall by standard echocardiography. Since our study population was composed of ACS patients, we preferred echocardiography to assess LVEF and recognize possible mechanic infarct complications.
Cytokines, which are one of the important sources of inflammatory mediators, have emerged as key cellular determinants for development of the CCV. EAT also contributes to development of CCV via its secretory properties. We hypothesize that EAT may play a role in the development of CCVs, since an increased thickness of EAT was significantly associated with the presence of CCV in patients with ACS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986;74:469–76.
- Fukai M, Ii M, Nakakoji T, Kawakatsu M, Nariyama J, Yokota N, et al. Angiographically demonstrated coronary collaterals predict residual viable myocardium in patients with chronic myocardial infarction: a regional metabolic study. J Cardiol. 2000;35:103–11.
- Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–31.
- Hirai T, Fujita M, Nakajima H, Asanoi H, Yamanishi K, Ohno A, Sasayama S. Importance of collateral circulation for prevention of left ventricular aneurysm formation in acute myocardial infarction. Circulation. 1989;79:791–6.
- Kodama K, Kusuoka H, Sakai A, Adachi T, Hasegawa S, Ueda Y, et al. Collateral channels that develop after an acute myocardial infarction prevent subsequent left ventricular dilation. J Am Coll Cardiol. 1996;27:1133–9.
- Duran M, Ornek E, Murat SN, Turfan M, Vatankulu MS, Ocak A, et al. High levels of serum uric acid impair development of coronary collaterals in patients with acute coronary syndrome. Angiology. 2012;63:472–5.
- Kadi H, Ceyhan K, Karayakali M, Koc F, Celik A, Onalan O.[The relationship between coronary collateral circulation and blood high-sensitivity C-reactive protein levels]. Turk Kardiyol Dern Ars. 2011;39:23–8.
- Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–17.
- Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6.
- Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1.
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13.
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9.
- Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33:614–21.
- de Lemos JA, Ettinger SM. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J Am Coll Cardiol. 2013;61:78–140.
- Amsterdam EA, Holmes DR Jr, Mukherjee D, Zieman SJ, Bozkurt B, Kovacs FRJ, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. J Am Coll Cardiol. 2014;64:139–228.
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2: 358–67.
- Celik T, Celik M, Iyisoy A.[Coronary collateral circulation]. Turk Kardiyol Dern Ars. 2010;38:505–14.
- Cohen M, Sherman W, Rentrop KP, Gorlin R. Determinants of collateral filling observed during sudden controlled coronary artery occlusion in human subjects. J Am Coll Cardiol. 1989;13:297–303.
- Fujita M, Tambara K. Recent insights into human coronary collateral development. Heart. 2004;90:246–50.
- Nathoe HM, Koerselman J, Buskens E, van Dijk D, Stella PR, Plokker TH, et al. Determinants and prognostic significance of collaterals in patients undergoing coronary revascularization. Am J Cardiol. 2006;98:31–5.
- Meier P, Schirmer SH, Lansky AJ, Timmis A, Pitt B, Seiler C. The collateral circulation of the heart. BMC Med. 2013; 11:143.
- Iacobellis G, Sharma AM. Epicardial adipose tissue as new cardio-metabolic risk marker and potential therapeutic target in the metabolic syndrome. Curr Pharm Des. 2007; 13:2180–4.
- Wu FZ, Chou KJ, Huang YL, Wu MT. The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: systemic review and meta-analysis of observational studies. BMC Cardiovas Disord. 2014;14:62.
- Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovas Dis. 2009;19:211–7.
- Yun KH, Rhee SJ, Yoo NJ, Oh SK, Kim NH, Jeong JW, et al. Relationship between the echocardiographic epicardial adipose tissue thickness and serum adiponectin in patients with angina. J Cardiovasc Ultrasound. 2009;17:121–6.
- Wu FZ, Huang YL, Wang YC, Lin HS, Chen CS, Ju YJ, et al. Impact of location of epicardial adipose tissue, measured by coronary artery calcium-scoring computed tomography on obstructive coronary artery disease. Am J Cardiol. 2013; 112:943–9.
- Bastarrika G, Broncano J, Schoepf UJ, Schwarz F, Lee YS, Abro JA, et al. Relationship between coronary artery disease and epicardial adipose tissue quantification at cardiac CT: comparison between automatic volumetric measurement and manual bidimensional estimation. Acad Radiol. 2010;17:727–34.

