Abstract
Aims. Complex fractionated electrogram (CFE) ablation in addition to pulmonary vein isolation is an accepted strategy for the treatment of non-paroxysmal atrial fibrillation (AF). We sought to determine the effect of flecainide on the distribution and extension of CFE areas. Methods. Twenty-three non-paroxysmal AF patients were enrolled in this prospective study. A first CFE map was obtained under baseline conditions by sampling 5 s of continuous recording from the distal electrodes of the ablation catheter. Intravenous flecainide (1 mg/kg) was administered over 10 min and followed by 30-min observation time. A second CFE map was obtained with the same modalities. CFE-mean values, CFE areas, and atrial electrogram amplitude were retrieved from the electro-anatomical mapping system (Ensite NavX). Results. After flecainide administration, CFE-mean values increased (111.5 ± 55.3 vs. 132.3 ± 65.0 ms, p < 0.001) with a decrease of CFE area (32.9%) in all patients. Atrial electrogram amplitude decreased significantly (0.30 ± 0.31 vs. 0.25 ± 0.20 mV, p < 0.001). We observed 80.9% preservation of CFE areas. A CFE mean of 78 ms was the best cutoff for predicting stable CFE areas. Conclusions. Flecainide reduces the extension of CFE areas while preserving their spatial localization. A CFE-mean value <80 ms may be crucial to define and locate stable CFE areas.
Introduction
Complex fractionated electrogram (CFE) ablation in addition to pulmonary vein (PV) isolation is an accepted strategy for the treatment of non-paroxysmal atrial fibrillation (AF) (Citation1–3). CFE refers to fractionated electrograms that display a continuous perturbation of the baseline and/or a short cycle length (<120 ms) (Citation1). Such characteristics are believed to be linked to areas of diseased myocardium and involved in the maintenance of AF (Citation1,Citation4–6). Although the mechanisms of CFE are not fully understood, it has been reported that a significant proportion of CFE areas may be functional in nature, reflecting passive atrial activation or the collision of different wavefronts (Citation4,Citation7–9). Furthermore, recent studies have demonstrated that CFE can be located in areas of normal voltage, which therefore makes them non-specific ablation targets (Citation7,Citation10).
In the human heart, particularly among non-paroxysmal AF patients, CFEs are often widespread and cover large portions of the left atrium (LA) (Citation7,Citation11). This may lead to time-consuming ablation procedures that potentially expose the patient to large-scale destruction of the atrial myocardium and to pro-arrhythmic hazards.
Flecainide, a class-IC anti-arrhythmic drug (AAD) is routinely used to either terminate or regularize AF. Previous human studies have reported a decrease in the level of fractionation following intravenous AAD administration, but none of them have specifically referred to flecainide (Citation9,Citation12–13). We sought to evaluate the effects of flecainide on the localization and extension of CFE areas in the LA of non-paroxysmal AF patients.
Methods
Twenty-three consecutive patients (21 males; mean age: 59 ± 7 years) were enrolled in this prospective single-group study. All patients were referred to catheter ablation for symptomatic, drug-refractory, non-paroxysmal AF (9 persistent and 14 long-standing persistent). Four patients had undergone a previous ablation procedure in which only PV isolation was performed. Hypertension was present in 11 (47.8%) patients. Structural heart disease was present in four (17.4%) (one with a mechanical aortic valve and three with coronary heart disease). The mean history of uninterrupted AF was 19 ± 21 (range: 6–108) months and the mean left atrial diameter was 4.5 ± 0.5 cm. The left ventricular ejection fraction was conserved in all the patients (mean: 55.7 ± 6.9%) and none presented absolute contraindications to the use of flecainide. All patients had tried beta-blockers unsuccessfully; additionally, four patients were on dronedarone, two on flecainide, one on amiodarone, and one on verapamil.
All patients presented with AF at the beginning of the procedure. The procedure, as performed at our institution, has been previously described (Citation3). Of particular relevance for this study was that all AADs were continued until 1 day before the procedure.
Two CFE maps were created prior to ablation. The first (mappre) was obtained under basal conditions, sampling 5 s of bipolar recording from the distal electrodes of the ablation catheter for each point. Two types of ablation catheters, CoolPath™ (4-mm tip, electrode spacing: 1.5–5-2 mm, St. Jude Medical, Minnetonka, MN, USA) or CoolFlex™ (4-mm tip, electrode spacing: 0.5–5-2 mm, St. Jude Medical, Minnetonka, MN, USA) were used. Catheter stability was ascertained by the operator's judgement, with fluoroscopy upon collection of each point. Points were sampled exclusively in the LA, with the aim of uniformly covering the whole LA surface, including the left atrial appendage (LAA). The PVs and the mitral valve ring were excluded from CFE mapping, as were the coronary sinus (CS) and the right atrium (RA).
After mappre had been obtained, intravenous flecainide was administered at a dose of 1 mg/kg (maximum dose administered: 120 mg) over a period of 10 min, followed by an observation time of 30 min. A second CFE map (mappost) was then obtained using the same modalities. A three-dimensional navigation system (EnSite NavXTM, St. Jude Medical, Minnetonka, MN, USA) was employed in all the procedures for the acquisition of anatomical geometry and creation of CFE maps. The CFE-mean value, which is inversely correlated with the degree of fractionation, was calculated by the system software by measuring the interval between consecutive deflections during the sampling period (). Detection was based on –dV/dt with a deflection duration and refractory threshold of 20 and 40 ms, respectively (to avoid double counting). This technique has been validated and employed in previous studies (Citation2,Citation14).
Figure 1. Posterolateral view of the left atrium showing CFE distribution before (A) and after (B) flecainide administration. At baseline, CFE areas cover large portions of the lateral and floor segment (A); these are consistently reduced after flecainide administration (B) but substantially preserved in their original localizations. The color scale for different CFE-mean values is displayed on the left (white: < 80 ms and violet: >120 ms). The tracings in the lower panel demonstrate electrograms from the coronary sinus (HALO-5-6) and lateral wall (ROV Abl-D-2) that were collected in the same position (stars) before (A) and after (B) flecainide administration. A significant decrease in deflections detected (yellow spikes) together with a reduction in electrogram amplitude was observed after flecainide administration. CFE, complex fractionated electrogram; LAA, left atrial appendage; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MV, mitral valve; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
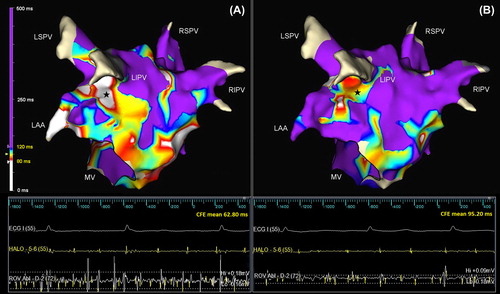
Electrograms with a CFE-mean value <400 ms were included in the final analysis but only those with a CFE-mean value <120 ms were considered as potentially fractionated. Average atrial electrogram amplitude was also measured by the system and retrieved for each mapping point.
The same system configuration was employed consistently for all the patients. In order to locate scar tissue, the threshold level (low V-id) was set at 0.03 mV. The “Field Scaling” tool was utilized and the LA, CFE and scar regions were delineated manually based on the CFE maps and areas measured automatically by the system. For the purposes of analysis, the LA was arbitrarily divided into five segments using consistent anatomical landmarks: roof, lateral, posterior, floor, and septum. The reduction in CFE area was expressed as a percentage and was defined by the proportion between the difference in CFE areas in the two maps and the total CFE area in mappre. The preservation of CFE area was expressed as a percentage and was defined by the proportion of CFE area in mappost that was preserved within the CFE area of mappre. Atrial fibrillation cycle length (AFCL) was measured in ms by placing the ablation catheter in the LAA and by averaging the number of cycles over a 10-s recording period. LAA electrogram amplitude was measured in mV by averaging the amplitude of five consecutive electrograms from the distal electrode of the ablation catheter being placed in the LAA.
Following completion of mappost, PV isolation was achieved by ablating circumferentially at the PV antrum (cutoff of temperature 50°C and output 35 W) and confirmed with a circular multi-electrode catheter. CFE ablation was performed in all the patients exclusively targeting areas with a CFE mean <80 ms as displayed in mappost (Citation15). Selected patients underwent additional CFE ablation in the CS and RA at operators’ discretion. No linear ablation was performed in the LA. Cavotricuspid isthmus ablation was performed if typical atrial flutter presented. Follow-up was performed on an ambulatory basis with clinical evaluation, electrocardiogram, and 24-h Holter recordings performed at 3, 6, and 12 months after the procedure. The frequency of further follow-up depended on the clinical situation.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Regional Ethics Committee of Western Norway.
Statistical analysis
Continuous variables were presented as means and standard deviations; categorical variables were presented in percentages. For comparison between continuous variables, un-paired and paired t-tests were used when appropriate. Chi-square test was employed for comparison between categorical variables. A receiver operating characteristic (ROC) curve was used for prediction analysis. SPSS statistical package version 19 was used for data analysis (IBM 2010). All tests were two-tailed; a p-value <0.05 was considered to be statistically significant.
Results
No conversion to sinus rhythm (SR) attributable to flecainide occurred before ablation and no flecainide-related adverse event was observed. The duration of the QRS complex was slightly but significantly prolonged after flecainide administration (104 ± 15 vs. 111 ± 18 ms, p < 0.001).
A total of 2835 and 2815 points were collected in mappre and mappost, respectively, similarly between each patient and each segment (). The proportions of points with a CFE mean <80 ms decreased and that with a CFE mean >120 ms increased significantly after flecainide administration. No difference was found in the CFE-mean range: 80–100 ms and 100–120 ms. These changes resulted in a right shift of the distribution curve shown in .
Figure 2. Distribution curves of mapped points sorted by CFE-mean values in mappre (before flecainide, stippled line) and mappost (after flecainide, solid line). Amount of mapped points with relative percentages and statistical differences are presented in the embedded table. CFE, complex fractionated electrogram.
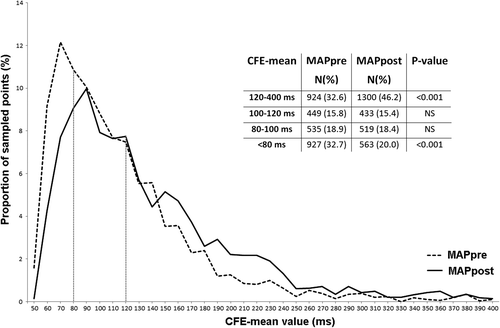
Table I. CFE-mean values in the LA before (mappre) and after (mappost) flecainide administration.
When a CFE-mean value cutoff <120 ms was employed, CFE areas covered an average of 52.6 ± 26.6% of the total LA surface before flecainide administration, although inter-patient variation was large, ranging from 2.8% to 92.6%. A lower CFE-mean cutoff resulted in a smaller proportion of the CFE area (). The only patients with a CFE area that covered less than 5% of the total LA surface were the two females enrolled in this study.
Table II. Distribution of CFE areas by different CFE-mean cutoffs (areas are presented as percentage of the total left atrial surface).
CFEs were found in all LA segments but were particularly marked in the roof, septum, and floor segments (). After flecainide administration, the CFE-mean value increased in all patients, from an average of 111.5 ± 55.3 ms to 132.3 ± 65.0 ms (p < 0.001). An analogous increase was found by analyzing each segment separately (). AFCL was similarly affected (155.1 ± 20.8 vs. 195.0 ± 27.1 ms, p < 0.001). Overall, average atrial electrogram amplitude decreased significantly after flecainide administration (0.30 ± 0.31 vs. 0.25 ± 0.20 mV, p < 0.001). The electrogram amplitude measured in the LAA also decreased in all patients but one (0.96 ± 0.68 vs. 0.64 ± 0.34 mV, p < 0.01). After flecainide administration, we observed a reduction of CFE area in all regions (average: 32.9%) in mappost, with the largest reduction of 40.1% in the posterior segment (, ). CFE areas after flecainide administration were reduced in all patients, but in three of them the reduction was only modest (<10%). Detected scar area increased (1.6 ± 4.2 to 4.2 ± 6.6 cm2) in all patients following flecainide administration (p < 0.01). We observed a large degree of preservation of CFE areas in mappost, with an average of 80.9%. The highest degree of preservation (88.8%) was observed in the posterior segment (). No differences were found in terms of extension of CFE areas and reduction after flecainide administration between patients with and without a previous PV isolation.
Table III. Size of CFE (CFE mean: < 120 ms) area before (mappre) and after (mappost) flecainide administration.
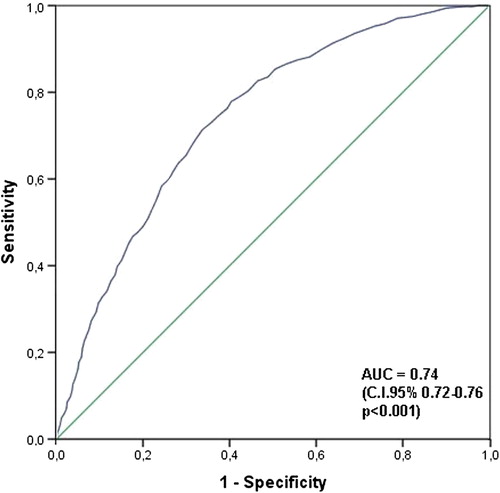
In order to identify the role of CFE-mean value and atrial electrogram amplitude in predicting the stability of CFE areas, we compared the portions of CFE areas in mappre that were preserved in mappost with those that had disappeared in mappost. A shorter CFE-mean value was found in the preserved portions compared with those that disappeared (73.8 ± 17.4 vs. 89.4 ± 17.1 ms; p < 0.001). ROC analysis showed an area under the curve of 0.74 (95% confidence interval: 0.72–0.76; p < 0.001; ). A CFE-mean value of 78 ms was the best cutoff point for predicting the disappearance of CFE areas from mappre to mappost (71% sensitivity and 66% specificity). On the other hand, a higher atrial electrogram amplitude was found in the preserved portions compared with those that disappeared (0.37 ± 0.28 vs. 0.28 ± 0.20 mV; p < 0.0001).
Figure 3. ROC curve: CFE mean in mappre. ROC curve exploring the predictive level of CFE-mean values before flecainide in assessing disappearance of CFE areas after flecainide. AUC, area under the curve; CFE, complex fractionated electrogram.
After PV isolation and CFE ablation, AF was terminated into atrial flutter in 4 patients. In 3 of them SR was restored by cavotricuspid isthmus block. In all the other patients SR was obtained by direct current cardioversion. Total duration of procedure, fluoroscopy and ablation were 356 ± 40, 47 ± 12 and 98 ± 22 min, respectively. After a mean follow-up of 22.1 ± 5.0 months from the index procedure, 15 patients (65.2%) maintained stable SR, 5 (21.7%) suffered from recurrences of AF, and 3 (13.1%) developed persistent atrial tachycardia. One patient died of cancer 15 months after the procedure. Among the patients in stable SR, 9 (60%) were still on beta-blocker.
Discussion
Main findings
In non-paroxysmal AF patients, intravenous flecainide administration results in an increase in global CFE-mean values, a prolongation of AFCL in the LAA and an abatement of atrial electrogram amplitude. These changes translate into a significant reduction of more than 30% in CFE areas. CFE areas localized after flecainide administration are preserved within the same regions as before, demonstrating a high degree of spatial and temporal stability. A CFE-mean value of 78 ms appears to be the best cutoff to identify stable CFE areas.
Interpretation of CFE
The pioneer observations by Konings et al., which associated fragmented potentials with areas of pivoting points or slow conduction in persistent AF, have raised interest in the understanding of the AF substrate (Citation6). The nature of CFE has been long debated without consensus being reached. Although CFEs were initially associated with areas of diseased myocardium, voltage mapping and imaging studies have demonstrated that CFE can in fact also be found in areas of otherwise normal tissue (Citation7,Citation10).
Theoretically, if all CFE areas were involved in the maintenance of AF, we would expect to find a similar effect in terms either of conversion to SR or prolongation of AFCL from every CFE-targeted ablation. On the contrary, the work of Hunter et al. showed that only ablation of CFE areas with a high degree of fractionation resulted in the prolongation of AFCL (Citation4). Ultimately, one of the main clinical challenges of CFE is their wide distribution; in our data, CFE areas (CFE mean: < 80 ms) covered an average of 20.7 ± 21.1% of the LA, consistent with previous findings (Citation7).
These characteristics have therefore cast doubt on the specificity of CFE as potential contributor to the AF substrate. Many factors may be involved in the fractionation of electrograms: remote activation, anisotropy, overlaying structures, alterations in tissue conduction velocity (wavefront curvature, tissue discontinuities, and fibrillatory conduction), and the autonomic tone (Citation16). The transient effect of these factors may reveal that a large degree of CFE is functional in nature. Previous studies acknowledged the possibility that functional characteristics may be implicated in sustained AF. This might be true especially in the early stages of AF when the presence of SR can promptly revert electrical remodeling. After longer time in persistent AF, structural remodeling may take place and provide directly for the maintenance of AF (Citation17). When CFEs are targeted for ablation, measures to reduce the amount of functional CFE should be implemented.
Shan et al. demonstrated in a goat model that administration of cibenzoline (a class-IA AAD) resulted in a decrease in electrogram fractionation (Citation18). The idea of using intravenous AAD in order to guide CFE ablation was first presented by Singh et al., who employed intravenous ibutilide to reduce the size of CFE areas (Citation9). In another study, nifekalant administration similarly reduced CFE areas, thus leading to a remarkable reduction in ablation and procedural times (Citation13). Finally, a reduction in fractionation has been demonstrated following autonomic blockade with intravenous atropine and beta-blocker (Citation12).
Rationale for flecainide and its effects on CFE areas
Wavefront collision, functional conduction block, wave break, and wave fusion may cause fractionated electrograms in AF (Citation16). Flecainide is a class-IC AAD with a use-dependent effect in blocking sodium channels. Its administration during sustained AF results in effective prolongation of phase 0 of the cardiac action potential, with no effect on repolarization. Flecainide is known to widen the excitable gap, thus homogeneously slowing the conduction of fibrillation waves in the atria and progressively prolonging AFCL which is itself strongly correlated with the degree of fractionation (Citation18). Our results confirm that this effect may be implicated in the reduction of CFE areas. However, following flecainide administration we could demonstrate that 20% of the electrograms were still highly fractionated (CFE mean <80 ms). This finding suggests that other mechanisms may be implicated and their effect may not be uniform on the LA surface. In regard to this issue it is important to emphasize that the CFE-mean parameter measures intervals between consecutive deflections of electrograms. Accordingly, an increase in CFE mean (as induced by flecainide) indicates not only a prolongation of AFCL, but also a reduction in electrogram complexity.
In a computer model, Jacquemet et al. were able to show that sodium channel blockade resulted in smaller electrogram amplitude (Citation5). Our measurements of scar area and electrogram amplitude in the LA could confirm this finding.
The stability of CFE areas is a crucial factor in support of the involvement of these targets in the maintenance of human AF. Since their discovery, conflicting results have been published about the temporal and spatial stability of CFE areas (Citation14,Citation19). In our study, we were able to demonstrate that after flecainide administration, at least 40 min from the initial map, there was a large degree of preservation of CFE.
Previous studies employed multi-electrode catheters for CFE mapping in the attempt to acquire a higher amount of data points. We chose to use the ablation catheter itself as a mapping device, since stability, tip–tissue contact, and signal detection are easier to control. Moreover, the ablation catheter may be able to access the complete anatomy of the LA.
Clinical implications
We have shown that intravenous flecainide produces a major reduction in CFE area and confirmed that the use of a stricter criterion of CFE based on a CFE-mean cutoff < 80 ms may be crucial to define and locate the most stable culprit CFE. After a single ablation procedure, long-term outcomes are comparable with previously published data, suggesting that the disappeared CFE areas after flecainide may be functional (Citation3,Citation20). The administration of flecainide may therefore be beneficial in CFE ablation by potentially reducing the duration of energy delivery, procedural time, and fluoroscopic exposure. The results of further investigations will help to assess the clinical validity of an integrated pharmaco-ablative strategy in the management of non-paroxysmal AF patients (Citation21).
Limitations
This was a small-sample study: in particular, only two females were included in the investigation. Two types of ablation catheters were employed. Although the difference in electrode spacing was only 1 mm, this might have affected CFE mapping. However, within each patient the same catheter was used for both maps and the conditions of measurement were identical. This study did not include a control group thus limiting the interpretation of our findings concerning CFE stability. Nevertheless, previous studies have demonstrated temporal and spatial stability of CFE areas (Citation14). Finally, in selected patients additional ablation was performed in the CS and RA at operators’ discretion, potentially influencing clinical outcomes.
Conclusions
Flecainide reduces the extension of CFE areas in non-paroxysmal AF patients by slowing atrial conduction velocity and diminishing complexity and amplitude of atrial electrograms. CFE areas appear to be spatially and temporally stable after flecainide administration. A CFE-mean value < 80 ms may be crucial to define and locate stable CFE areas.
Acknowledgement
We acknowledge the collaboration and technical support of Peter Schuster and Li-Zhi Sun.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by funding from Helse-Vest, Norway. The study has been presented in part at the Annual Meeting of the Heart Rhythm Society, Denver, CO, May 2013.
References
- Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53.
- Verma A, Sanders P, Macle L, Deisenhofer I, Morillo CA, Chen J, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation Trial-Part II (STAR AF II): Design and rationale. Am Heart J. 2012;164:1–6.e6.
- De Bortoli A, Ohm OJ, Hoff PI, Sun LZ, Schuster P, Solheim E, Chen J. Long-term outcomes of adjunctive complex fractionated electrogram ablation to pulmonary vein isolation as treatment for non-paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2013;38:19–26.
- Hunter RJ, Diab I, Tayebjee M, Richmond L, Sporton S, Earley MJ, Schilling RJ. Characterization of fractionated atrial electrograms critical for maintenance of atrial fibrillation: A randomized, controlled trial of ablation strategies (the CFAE AF trial). Circ Arrhythm Electrophysiol. 2011;4:622–9.
- Jacquemet V, Henriquez CS. Genesis of complex fractionated atrial electrograms in zones of slow conduction: A computer model of microfibrosis. Heart Rhythm. 2009;6:803–10.
- Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–80.
- Jadidi AS, Duncan E, Miyazaki S, Lellouche N, Shah AJ, Forclaz A, et al. Functional nature of electrogram fractionation demonstrated by left atrial high-density mapping. Circ Arrhythm Electrophysiol. 2012;5:32–42.
- Oral H, Chugh A, Yoshida K, Sarrazin JF, Kuhne M, Crawford T, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol. 2009;53:782–9.
- Singh SM, D’Avila A, Kim SJ, Houghtaling C, Dukkipati SR, Reddy VY. Intraprocedural use of ibutilide to organize and guide ablation of complex fractionated atrial electrograms: Preliminary assessment of a modified step-wise approach to ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:608–16.
- Viles-Gonzalez JF, Gomes JA, Miller MA, Dukkipati SR, Koruth JS, Eggert C, et al. Areas with complex fractionated atrial electrograms recorded after pulmonary vein isolation represent normal voltage and conduction velocity in sinus rhythm. Europace. 2013;15:339–46.
- Roux JF, Gojraty S, Bala R, Liu CF, Dixit S, Hutchinson MD, et al. Effect of pulmonary vein isolation on the distribution of complex fractionated electrograms in humans. Heart Rhythm. 2009;6:156–60.
- Knecht S, Wright M, Matsuo S, Nault I, Lellouche N, Sacher F, et al. Impact of pharmacological autonomic blockade on complex fractionated atrial electrograms. J Cardiovasc Electrophysiol. 2010;21:766–72.
- Kumagai K, Toyama H. Usefulness of ablation of complex fractionated atrial electrograms using nifekalant in persistent atrial fibrillation. J Cardiol. 2013;61:44–8.
- Verma A, Wulffhart Z, Beardsall M, Whaley B, Hill C, Khaykin Y. Spatial and temporal stability of complex fractionated electrograms in patients with persistent atrial fibrillation over longer time periods: Relationship to local electrogram cycle length. Heart Rhythm. 2008;5: 1127–33.
- Solheim E, Off MK, Hoff PI, Schuster P, Ohm OJ, Chen J. Characteristics and distribution of complex fractionated atrial electrograms in patients with paroxysmal and persistent atrial fibrillation. J Interv Card Electrophysiol. 2010;28: 87–93.
- de Bakker JM, Wittkampf FH. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol. 2013;3:204–13.
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: Mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73.
- Shan Z, Van Der Voort PH, Blaauw Y, Duytschaever M, Allessie MA. Fractionation of electrograms and linking of activation during pharmacologic cardioversion of persistent atrial fibrillation in the goat. J Cardiovasc Electrophysiol. 2004;15:572–80.
- Lau DH, Maesen B, Zeemering S, Verheule S, Crijns HJ, Schotten U. Stability of complex fractionated atrial electrograms: A systematic review. J Cardiovasc Electrophysiol. 2012;23:980–7.
- Hayward RM, Upadhyay GA, Mela T, Ellinor PT, Barrett CD, Heist EK, et al. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm. 2011;8:994–1000.
- Singh SM, D’Avila A, Kim YH, Aryana A, Mangrum JM, Michaud GF, et al. The Modified Ablation Guided by Ibutilide Use in Chronic Atrial Fibrillation (MAGIC-AF) Study: Clinical background and study design. J Cardiovasc Electrophysiol. 2012;23:352–8.

