Abstract
Objectives. It remains unknown whether non-electrocardiogram-gated coronary artery calcium (CAC) score in lung cancer screening provides incremental prognostic value. The aim of this study was to evaluate the prognostic value of CAC in the Danish Lung Cancer Screening Trial (DLCST), in addition to conducting a systematic review and meta-analysis including previously published studies regarding CAC in lung cancer screening. Design. In DLCST, we measured Agatston CAC scores in 1,945 current and former smokers. Causes of death were extracted from the Danish National Death Registry. We used Cox proportional hazards model to determine hazard ratios (HRs) of CAC scores. A weighted fixed-effects model was used for the meta-analysis. Results. Median follow-up in DLCST was 7.1 years, and 55% were men. Overall survival rates associated with CAC scores of 0, 1–400, and > 400 were 98%, 96%, and 92% (p < 0.001), respectively. Adjusted HR of cardiovascular death associated with CAC >400 was 3.8 (1.0–15) (p < 0.05). The meta-analysis included 28,045 asymptomatic participants. A high non-gated CAC score was associated with fatal or non-fatal cardiovascular events (p < 0.0001). Conclusion. Assessment of non-electrocardiogram-gated CAC in lung cancer screening programs is a robust prognostic measure of fatal or non-fatal cardiovascular events in current and former smokers independent of traditional cardiovascular risk factors.
Trial registration: ClinicalTrials.gov identifier: NCT00496977.
| Abbreviations | ||
| ACCF | = | The American College of Cardiology Foundation |
| AHA | = | The American Heart Association |
| BMI | = | Body mass index |
| CABG | = | Coronary artery bypass graft |
| CAC | = | Coronary artery calcium |
| CAD | = | Coronary artery disease |
| COPD | = | Chronic obstructive pulmonary disease |
| DLCST | = | The Danish Lung Cancer Screening Trial |
| ECG | = | Electrocardiogram |
| FEV1 | = | Forced expiratory volume in first second |
| FVC | = | Forced vital capacity |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| ICD | = | International Classification of Disease |
| MDCT | = | Multidetector computed tomography |
| MI | = | Myocardial infarction |
| NCBI | = | National Center for Biotechnology Information |
| PCI | = | Percutaneous coronary intervention |
Introduction
Smoking is associated with the development of coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), and lung cancer. The Danish Lung Cancer Screening Trial (DLCST) (Citation1), to which this study is a part of, is one of the lung cancer multidetector computed tomography (MDCT) screening trials among smokers that are currently aiming to reduce lung-cancer-related mortality and morbidity. While atherosclerotic disease accounts for substantial amounts of deaths and disability in these high-risk patient populations, it is of substantial clinical interest that these lung cancer screening trials might permit the concomitant evaluation of both lung disease and atherosclerotic disease (Citation2).
The extent of coronary artery calcium (CAC) deposit measured by MDCT and the Agatston scoring method (Citation3) is a marker of total atherosclerotic plaque burden (Citation4) in the coronary arteries and an independent predictor of future cardiovascular events (Citation5–7). The assessment of CAC with MDCT is ideally performed with electrocardiogram (ECG) synchronization and under beta-blocker administration to reduce heart rate and thus motion artifacts. Yet, this approach is time consuming and more complex than the type of chest CT used in lung cancer screening. The evaluation of subclinical atherosclerosis in the settings of lung cancer screening trials (i.e., non-ECG-gated and without beta-blocker administration) is still in the early phase and it remains unknown to what extent this method provides incremental prognostic value.
The aim of this study was to evaluate the prognostic value of non-ECG-gated MDCT CAC measurements in the DLCST in addition to conducting a systematic review and meta-analysis including DLCST data and previously published studies regarding the prognostic value of CAC measured in MDCT lung cancer screening programs.
Methods
Danish Lung Cancer Screening Trial
Study population and design
Participants for the CAC outcome study were recruited from DLCST (www.ClinicalTrials.gov, registration number: NCT 00496977), a randomized controlled trial conducted between 2004 and 2010 (Citation1). Participants in the DLCST volunteered in response to local media advertisements. Current and former smokers aged 50–70 years with at least 20 pack-years and forced expiratory volume in first second (FEV1) of more than 30% of predicted value were included. Participants with body weight above 130 kg, previous treatment for any kind of cancer within 5 years, tuberculosis within 2 years, and any serious illness with life expectancy less than 10 years were excluded. The study was approved by the National Ethics Committee of Denmark (identification no. H-KA-02045, supplementary protocol 20148) and all participants gave written informed consent. Overall, 4104 long-term smokers were randomized to either 5 annual MDCT screenings or no screening.
In this DLCST substudy, participants who underwent MDCT at DLCST study inclusion (n = 2052) were included. Participants with implanted cardiac pacemaker or identified with clinically manifested CAD defined as “previous coronary artery bypass graft (CABG) surgery, percutaneous coronary intervention (PCI), or myocardial infarction (MI)” at entry were excluded (n = 41). Participants with angina pectoris by Rose Angina Questionnaire (Citation8) (n = 46), poor MDCT image quality, or missing image slices (n = 20) were excluded. Overall, 1945 participants were included.
Data acquisition
All CT scans were performed in a single institution with a 16-row Philips Mx8000 MDCT scanner, Philips Medical Systems, Eindhoven, the Netherlands. A detailed description of the scan technique has previously been published (Citation9). In brief, scans were performed in supine position after full inspiration in caudocranial scan direction including the entire rib cage and upper abdomen with a low-dose technique, at 120 kV and 40 mAs. Scans were performed with spiral data acquisition with the following parameters: section collimation, 16 × 0.75 mm; pitch, 1.5; and rotation time, 0.5 s. The obtained data were reconstructed with a section width of 3 mm and a soft kernel algorithm. All image data were stored in DICOM format.
CAC was assessed using Vitrea V6.0 (Vital Images Inc., Minnesota, USA) and the Agatston scoring method (Citation3). The method has previously been described in detail (Citation9). CAC scores at study inclusion were categorized into very-low-, low–moderate-, and high-risk groups according to CAC score (0, 1–400, and > 400, respectively) as suggested by American College of Cardiology Foundation/American Heart Association or ACCF/AHA (Citation10). For the meta-analysis, low and high CAC scores in DLCST were defined as “CAC scores ≤ 400 and > 400,” respectively.
Clinical characteristics regarding smoking status, family history of premature coronary heart disease, and medical treatment for diabetes, hypertension, or hypercholesterolemia were collected at inclusion as previously defined and described (Citation9).
Furthermore, spirometry was performed according to recommendations by the European Respiratory Society (Citation11), as previously described (Citation9). Measurements included FEV1, forced vital capacity (FVC), and the ratio of FEV1/FVC; severity of COPD was defined and classified according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (Citation12).
Mortality data
All participants were followed up as part of the lung cancer screening protocol and mortality data were extracted from the Danish National Death Registry. Deaths were categorized into all-cause mortality and cardiovascular mortality. Cardiovascular mortality was defined as “International Classification of Disease or ICD-10 codes I00-99 as the underlying or contributing cause of death.” Median follow-up was 7.1 years.
Systematic literature review and meta-analysis
We conducted a systematic literature search of published studies regarding CAC outcome in lung cancer screening. We searched the National Center for Biotechnology Information (NCBI) website (pubmed.gov) using the keywords “coronary artery calcium,” “low-dose CT,” and “lung cancer.” The search was ended in February 2014. To be included in the meta-analysis, studies should provide extractable outcome frequencies in high versus low CAC score groups. Authors were contacted in case of insufficiently published data. Event data were not extractable or suitable for inclusion in two studies (Citation13,Citation14). In two other studies (Citation15,Citation16), event data in CAC groups were not directly extractable and were presented as percentages in a case cohort and in a subcohort, where we were able to extrapolate frequencies to represent the full event-free cohort and the case cohort.
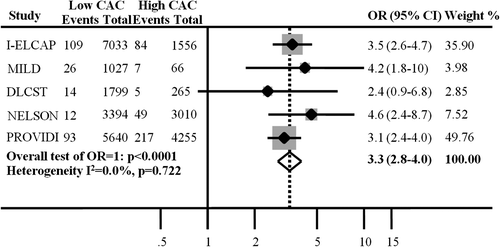
Among previous and ongoing lung cancer screening trials (Citation17–26), we found six studies regarding non-gated MDCT CAC and clinical outcome. Additionally, we found one study in a clinical care population that was comparable to the above-mentioned lung cancer screening trials regarding non-gated CAC outcome. There were three studies from the NEederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) study (Citation14,Citation16,Citation27), in which only one could be included in the meta-analysis while two were excluded—one in which CAC was classified in interquartile ranges and one with mainly male participants. Furthermore, one study focusing on the prognostic value of detectable versus non-detectable CAC was excluded. Consequently, including DLCST, five studies were included in the meta-analysis () (Citation1,Citation15,Citation16,Citation28,Citation29). The total number of participants in the meta-analysis was 28.045 with a mean follow-up period ranging from 18 to 85 months. In total, there were 616 fatal or non-fatal cardiovascular events. No participants in the included studies had a history or symptoms of cardiovascular disease before CT examination.
Table I. Summary of published studies regarding non-gated CAC score outcome in asymptomatic persons included in the meta-analysis.
Statistical analysis
Categorical variables were expressed as percentages, and continuous variables were reported as medians and interquartile ranges. Differences in baseline characteristics between groups were assessed with the chi-square test for discrete variables and Kruskal–Wallis test for continuous variables. All-cause survival was illustrated with Kaplan–Meier survival curves. Curves were compared for differences with the log-rank test. Cox proportional hazard test was used to compute unadjusted and adjusted CAC score group hazard ratios (HRs). Besides CAC score groups, the adjusted Cox regression model included gender, age, current smoking, hypertension, hypercholesterolemia, and diabetes. To secure that the Cox hazards assumptions were satisfied, the hazard function was tested to secure proportionality over time. Additionally, variables in the Cox regression model were tested to secure linearity and no interactions were found. A two-tailed p value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS® for Windows, version 9.1 (SAS institute, Cary, North Carolina).
Regarding the meta-analysis, the reported numbers of events in high versus low CAC score groups were pooled together providing the odds ratio (OR) with 95% confidence interval (CI). We used a weighted fixed effects model for data combination when the data were homogeneous. Heterogeneity was tested using Chi2 method and I2 statistic. The I2 (measured as 0–100%) indicates the percentage of variation in the study results attributed to between-study heterogeneity rather than sampling error. A value of I2 of >20% was considered significant. Meta-analysis package of the statistic software program STATA version 12 (STATA Corporation, Lakeway Drive, College Station, TX, USA) was used for meta-analysis.
Results
Danish Lung Cancer Screening Trial
Characteristics of participants
Baseline characteristics of the included population are given in . Men were slightly older than women and had slightly higher body mass index (BMI) and more pack-years than women. Additionally, men had a little higher frequency of diabetes. Furthermore, men had higher CAC scores.
Table II. Summary of clinical characteristics in the DLCST.
CAC score and outcome
Characteristics of participants who died from cardiovascular causes are given in . Participants who died from cardiovascular causes had higher number of pack-years and higher frequencies of diabetes and hypercholesterolemia compared with participants who survived or died from non-cardiovascular causes. Additionally, they had significantly higher CAC scores at baseline. Furthermore, both participants who died from cardiovascular and non-cardiovascular causes had higher frequency of hypertension.
Table III. Clinical characteristics of participants in the DLCST, who were alive or died from cardiovascular and non-cardiovascular causes.
All-cause mortality according to CAC group (0, 1–400, and > 400) is illustrated in . There were a total of 72 deaths. Participants with CAC scores of 1–400 and >400 had significantly higher all-cause mortality rates than participants with a CAC score of 0 (log-rank < 0.001). Cox HRs associated with CAC scores of 1–400 and > 400 are given in . CAC scores of 1–400 and > 400 were associated with unadjusted HRs of 2.1 (1.3–3.6) and 3.4 (1.6–7.2), respectively, compared with a CAC score of 0, while adjusted for gender, age, smoking, hypertension, hypercholesterolemia, and diabetes HRs were 1.7 (1.0–2.9) and 2.1 (1.0–4.8) (p = 0.06 for both), respectively.
Figure 1. Kaplan–Meier survival curves in the DLCST for all-cause mortality according to CAC category (log-rank < 0.001).
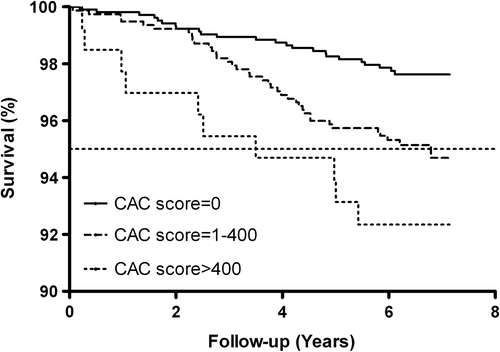
Table IV. Mortality rate in addition to unadjusted and adjusted Cox regression HRs according to CAC score category in the DLCST.
In total, there were 19 cardiovascular deaths. Unadjusted Cox HRs of cardiovascular mortality associated with CAC scores of 1–400 and > 400 were 2.4 (0.8–7.6) and 8.3 (2.4–29) compared with a CAC score of 0, while CAC scores of 1–400 and > 400 adjusted for gender, age, smoking, hypertension, hypercholesterolemia, and diabetes were associated with HRs of 1.6 (0.5–4.9) (p = 0.43) and 3.8 (1.0–15) (p < 0.05), respectively.
Meta-analysis
The meta-analysis of outcome of included studies is shown in . A high non-gated MDCT CAC score was significantly associated with fatal or non-fatal cardiovascular events (unstable angina pectoris, acute MI, CABG, and PCI) [OR (95% CI): 3.3 (2.8–4.0)].
Discussion
In the DLCST, an Agatston CAC score of >400 was associated with an adjusted HR of cardiovascular death of 3.8 (1.0–15) (p < 0.05) compared with a CAC score of 0. This finding corresponds well with the value of 5.8 (CAC = 400–699 vs. CAC = 0) reported by Budoff et al. using classical electron beam CT imaging (N = 25.253) (Citation6). The correspondence between ECG-gated and non-ECG-gated CAC score prediction of outcome is further supported by comparable CAC score values provided by “state-of-the-art” ECG-gated and non-gated CAC score measurements (Citation30–32).
The results of the DLCST study are in accordance with previous prognostic studies regarding non-gated CAC. In a large case–control study from the NELSON trial by Jacobs et al. () (Citation16,Citation27), there were a total of 56 all-cause deaths and 61 fatal and non-fatal cardiac events during the follow-up period. Adjusting for sex, age, smoking, hypertension, diabetes, and hypercholesterolemia, CAC was found to be significantly associated with all-cause mortality and fatal and non-fatal cardiac events. Thus, in spite of a relative short follow-up period of 21.5 months, the total number of fatal and non-fatal cardiac events was enough to show statistical significance. Mets et al. (Citation14) also studied participants, primarily men, from the NELSON study. CAC volume measured in mm3 was significantly associated with fatal or non-fatal cardiac events which corroborate results from DLCST, although differing in the inclusion of participants with known CAD. This inclusion poses a relative problem because the CAC measures in the presence of coronary artery stents or coronary artery bypass clips produce relatively higher CAC results than in their absence. While, in DLCST, the follow-up period was relatively long in comparison to the above studies, only mortality events were measured in a smaller population than in the NELSON study (Citation16,Citation27), thus counting fewer events. Nevertheless, the results corroborate those from DLCST.
In another large study, the PROgnostic Value of unrequested Information in Diagnostic Imaging (PROVIDI) study, Jacobs et al. (Citation15) () studied a clinical care population in a case-cohort study. Although representing a clinical care population, the study was comparable to the lung cancer screening trials regarding non-ECG-gated CAC score prediction of outcome. During the follow-up period there were 310 cardiac events and 128 non-cardiac events. Although CAC was only measured semiquantitatively rather than more precisely with the Agatston CAC score and the relatively short follow-up period of 17.8 months, CAC was predictive of both cardiac and non-cardiac events corresponding with the findings in DLCST.
Although limited by the use of a single-slice MDCT scanner with a slice thickness of 10 mm, Itani et al. (Citation13) studied a large lung cancer screening population. Due to the low scan quality, this study was limited only to examine the association between the detection versus no detection of CAC rather than a stratification of CAC scores in the prediction of outcome. During the follow-up period, there were 14 cardiac and 64 non-cardiac deaths. CAC was detected in 10 of 14 (71%) participants who died from cardiac causes and 31 of 64 (48%) participants who died from non-cardiac causes. Despite the above-mentioned limitations, the detection of CAC tended to predict cardiac mortality corresponding with the findings in DLCST.
In the large International Early Lung Cancer Action Program (I-ELCAP) study which also provided a long follow-up, Shemesh et al. (Citation28) studied the association between visually scored CAC (as in the PROVIDI study) and cardiovascular mortality (). During the follow-up period, there were 192 cardiovascular deaths and visual CAC was found to be an independent predictor of cardiovascular death.
In one study, although limited by a rather small number of participants, Sverzellati et al. (Citation29) studied participants from the Multicentric Italian Lung Detection (MILD) study (). The Agatston CAC score was measured in non-standard 5-mm slices, referred to as modified CAC (mCAC). During the follow-up period, there were 33 cardiovascular events and 14 deaths of which 2 were related to cardiac causes. Despite the small number of participants and relative short follow-up, a mCAC of > 400 was an independent predictor of both cardiovascular events and all-cause mortality.
As it appears above and in , studies regarding prognostic performance were highly heterogeneous in MDCT scanners used, MDCT scan protocol, population composition, and method of measuring CAC. Nonetheless, despite these heterogeneities, the meta-analysis of the DLCST by Jacobs et al., (Citation15,Citation16) Shemesh et al., (Citation28) and Sverzellati et al. (Citation29) showed a significant association between a high non-ECG-gated CAC and fatal or non-fatal cardiovascular events in current and former smokers in the setting of lung cancer screening [OR: 3.3 (2.8–4.0)], which is furthermore in agreement with “gold standard” ECG-gated findings (Citation6).
The findings of this study highly support the usefulness of CAC detection and quantification in non-ECG-synchronized lung cancer MDCT screening to identify subjects in high risk of cardiovascular events. It is therefore highly recommended that CAC is evaluated in these high-risk populations screened for lung cancer to designate subjects who might benefit from preventive medical treatment to potentially decrease cardiovascular morbidity and mortality.
Limitations
The inclusion of participants in response to advertisements in the written media in the DLCST constituted a bias for selection that favored the more enlightened and possibly less sick smokers (Citation33). Our study is limited to be representative only for current or former long-term smokers, while never smokers and smokers with less than 20 pack-years were not included. Furthermore, blood pressure and blood cholesterols were not measured at baseline. Consequently, only people with known hypercholesterolemia and hypertension were selected to represent these two groups, rather than both participants with known and unknown diseases. Data about arrhythmias and heart rate during CT were not available. Additionally, our study was limited to report HRs of mortality rather than a composite of fatal and non-fatal events.
An Agatston CAC score threshold of 400 was considered a cutoff in DLCST to distinguish between severe and non-severe CAD (Citation34). While non-severe CAC was stratified into a no detectable CAC group and a detectable non-severe CAC group, we were not able to further stratify all-cause or cardiovascular mortality according to more CAC score categories because of the relatively small number of events.
Data from two case–control studies in the meta-analysis were extrapolated to represent the complete cohorts rather than representing exact data.
Conclusion
Assessment of non-ECG-gated CAC in lung cancer screening programs is a robust prognostic measure of fatal or non-fatal cardiovascular events in current and former smokers independent of traditional cardiovascular risk factors.
Acknowledgements
Dr. TR is responsible for making the statistical work and drafting of the manuscript, while LK, JA, JHP, MMW, AD, and KFK contributed substantially in the process of critical reviewing. The authors thank the investigators, staff, and participants of the Danish Lung Cancer Screening Trial for their valuable contributions.
Financial disclosures
Dr. TR was supported by an unrestricted grant from AstraZeneca AB and the Danish Heart Foundation. The DLCST trial was funded in full by a governmental grant by the Danish Ministry of Health and Prevention from 2004 to 2011. KFK and LK were supported by the Danish Agency of Science, Technology and Innovation and by the Danish Council for Strategic Research (grant 09-066994). LK reports personal fees from Speaker for Servier and personal fees from Honorarium from Novartis, outside the submitted work.
Declaration of interests: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pedersen JH, Ashraf H, Dirksen A, Bach K, Hansen H, Toennesen P, et al. The Danish randomized lung cancer CT screening trial—overall design and results of the prevalence round. J Thorac Oncol. 2009;4:608–14.
- Chiles C, Paul NS. Beyond lung cancer: A strategic approach to interpreting screening computed tomography scans on the basis of mortality data from the national lung screening trial. J Thorac Imaging. 2013;28:347–54.
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
- Rumberger JA. Using noncontrast cardiac CT and coronary artery calcification measurements for cardiovascular risk assessment and management in asymptomatic adults. Vasc Health Risk Manag. 2010;6:579–91.
- Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33.
- Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: Observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70.
- Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358: 1336–45.
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58.
- Rasmussen T, Kober L, Pedersen JH, Dirksen A, Thomsen LH, Stender S, et al. Relationship between chronic obstructive pulmonary disease and subclinical coronary artery disease in long-term smokers. Eur Heart J Cardiovasc Imaging. 2013;14:1159–66.
- Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402.
- Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–61.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Available from: http://www.goldcopd.org/. Accessed at 23.09.2011.
- Itani Y, Sone S, Nakayama T, Suzuki T, Watanabe S, Ito K, et al. Coronary artery calcification detected by a mobile helical computed tomography unit and future cardiovascular death: 4-year follow-up of 6120 asymptomatic Japanese. Heart Vessels. 2004;19:161–3.
- Mets OM, Vliegenthart R, Gondrie MJ, Viergever MA, Oudkerk M, de Koning HJ, et al. Lung cancer screening CT-based prediction of cardiovascular events. JACC Cardiovasc Imaging. 2013;6:899–907.
- Jacobs PC, Gondrie MJ, Mali WP, Oen AL, Prokop M, Grobbee DE, et al. Unrequested information from routine diagnostic chest CT predicts future cardiovascular events. Eur Radiol. 2011;21:1577–85.
- Jacobs PC, Gondrie MJ, van der Graaf Y, de Koning HJ, Isgum I, van Ginneken B, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198:505–11.
- Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–71.
- Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: History, organization, and status. Control Clin Trials. 2000;21: 251S–72S.
- Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, et al. The National Lung Screening Trial: Overview and study design. Radiology. 2011;258:243–53.
- Pastorino U, Bellomi M, Landoni C, De Fiori E, Arnaldi P, Picchio M, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet. 2003;362:593–7.
- Infante M, Lutman FR, Cavuto S, Brambilla G, Chiesa G, Passera E, et al. Lung cancer screening with spiral CT: Baseline results of the randomized DANTE trial. Lung Cancer. 2008;59:355–63.
- Blanchon T, Brechot JM, Grenier PA, Ferretti GR, Lemarie E, Milleron B, et al. Baseline results of the Depiscan study: a French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer. 2007;58:50–8.
- Lopes Pegna A, Picozzi G, Mascalchi M, Maria Carozzi F, Carrozzi L, Comin C, et al. Design, recruitment and baseline results of the ITALUNG trial for lung cancer screening with low-dose CT. Lung Cancer. 2009;64:34–40.
- van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–9.
- Becker N, Motsch E, Gross ML, Eigentopf A, Heussel CP, Dienemann H, et al. Randomized study on early detection of lung cancer with MSCT in Germany: Study design and results of the first screening round. J Cancer Res Clin Oncol. 2012;138:1475–86.
- Baldwin DR, Duffy SW, Wald NJ, Page R, Hansell DM, Field JK. UK Lung Screen (UKLS) nodule management protocol: Modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax. 2011;66:308–13.
- Jacobs PC, Prokop M, van der Graaf Y, Gondrie MJ, Janssen KJ, de Koning HJ, et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209:455–62.
- Shemesh J, Henschke CI, Shaham D, Yip R, Farooqi AO, Cham MD, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology. 2010;257:541–8.
- Sverzellati N, Cademartiri F, Bravi F, Martini C, Gira FA, Maffei E, et al. Relationship and prognostic value of modified coronary artery calcium score, FEV1, and emphysema in lung cancer screening population: the MILD trial. Radiology. 2012;262:460–7.
- Budoff MJ, Nasir K, Kinney GL, Hokanson JE, Barr RG, Steiner R, et al. Coronary artery and thoracic calcium on noncontrast thoracic CT scans: Comparison of ungated and gated examinations in patients from the COPD Gene cohort. J Cardiovasc Comput Tomogr. 2011;5:113–8.
- Wu MT, Yang P, Huang YL, Chen JS, Chuo CC, Yeh C, et al. Coronary arterial calcification on low-dose ungated MDCT for lung cancer screening: Concordance study with dedicated cardiac CT. AJR Am J Roentgenol. 2008;190:923–8.
- Xie X, Zhao Y, de Bock GH, de Jong PA, Mali WP, Oudkerk M, Vliegenthart R. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: systematic review and meta-analysis. Circ Cardiovasc Imaging. 2013;6:514–21.
- Hestbech MS, Siersma V, Dirksen A, Pedersen JH, Brodersen J. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer. 2011;73:325–31.
- Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: A review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–52.