Abstract
Objectives. We examined whether diastolic left ventricular function in young and senior lifelong endurance runners was significantly different from that in sedentary age-matched controls, and whether lifelong endurance running appears to modify the age-related decline in diastolic left ventricular function. Design. The study comprised 17 senior athletes (age: 59–75 years, running distance: 30–70 km/week), 10 young athletes (age: 20–36 years, matched for running distance), and 11 senior and 12 young weight-matched sedentary controls. Peak early (E) and late (A) mitral inflow and early (e’) and late (a’) diastolic and systolic (s’) annular longitudinal tissue Doppler velocities were measured by echocardiography during four stages (rest, supine bike exercise at 30% and 60% of maximal workload, and recovery). Results. The athletes had marked cardiac remodeling, while overall differences in mitral inflow and annular tissue Doppler velocities during rest and exercise were more associated with age than with training status. The senior participants had lower E/A at rest, overall lower E, e’ and s’, and greater E/e’ compared to the young participants (all values of P < 0.05). The athletes had greater E/A (P = 0.004), but tissue Doppler velocities were not different from those of the controls. Conclusions. Lifelong endurance running was not found to be associated with major attenuation of the age-related decline in diastolic function at rest or during exercise.
Introduction
The effects of aging on the human heart include a decline in diastolic function, which is characterized by reduced peak early mitral inflow velocity (E) and peak early mitral annular tissue Doppler (TD) velocity (e’), with a compensatory increase in late (atrial phase) peak inflow velocity (A) and peak TD velocity (a’) (Citation1).
The decline in cardiac function with advancing age is typically seen in parallel to reduced physical activity, and it has been proposed that lifelong exercise training might attenuate the effects of aging on the heart (Citation2,Citation3). A cross-sectional study of master athletes has indicated that regular endurance training may partly offset the age-related increase in LV stiffness (Citation4), and some exercise training intervention studies have shown improved diastolic function in terms of LV peak diastolic filling rate, increased resting E/A, and increased E and e’ at rest and during exercise (Citation5–9).
The aim of this study was to examine whether lifelong training in endurance running can counteract the age-related decline in cardiac diastolic function assessed during rest and supine exercise. We hypothesized that lifelong endurance running in the young would result in [1] greater peak early mitral inflow velocity (E) at rest and during exercise, [2] greater peak early mitral annular TD velocities (e’) at rest and during exercise, and [3] larger ratio between resting peak early and atrial phase mitral inflow velocities (E/A), and that this pattern of the “athlete's heart filling” would still be discernible in elderly athletes in spite of the expected age-related decline in diastolic LV function.
Materials and methods
Study design and participants
Fifty healthy males participated, and the group comprised 17 senior athletes (endurance runners); 10 young athletes (endurance runners matched for current running distance); and 11 senior and 12 young sedentary controls, matched with no difference in age, height and weight (). Overall, the trained participants were well-trained endurance middle-distance runners and marathon runners, as illustrated by the VO2peak () (Citation10). The running was largely of moderate to high intensity, performed 4–6 days/week. Senior athletes had been training regularly for a median of 27 years (range 15–45) with a mean weekly running distance of 49 km (SD 12), whereas the young athletes had been training for the last 6 (Citation4–10) years with a weekly running distance of 43 (Citation17) km (P = 0.29 for weekly running distance); the running distances during the last week before the study were 43 (Citation5) and 48 (Citation7) km respectively (between the groups, P = 0.61). To ensure that the effect of exercise training was uniform, only runners were recruited as athletes and the participants had no physically demanding occupation, thus their physical activity level was primarily governed by their leisure activities. The untrained subjects had been sedentary for at least 5 years.
Table I. Anthropometric characteristics of participants.
Table II. VO2peak, maximal workload, resting blood pressure, and heart rate.
Fifty-one males were recruited through advertising. To minimize the possible effects of parameters other than running and age, the exclusion criteria for this investigation were obesity (body mass index [BMI] > 28 kg∙m− 2), use of medication, smoking, or diabetes. An oral glucose tolerance test was performed to determine prediabetes. Data on anthropometry and peak oxygen consumption (VO2peak) have previously been published (Citation11), an additional two senior athletes who had impaired glucose tolerance but normal mean glucose levels (HbA1c) were included, whereas one senior sedentary participant was excluded because of significant aortic regurgitation.
The study was in compliance with the Declaration of Helsinki and was approved by the Scientific Ethics Committee of the Capital Region of Denmark (ref. 25543). Informed written consent was given by all participants.
Cardiopulmonary exercise test
To ensure training status and provide data on maximal workload for calculation of workload applied during echocardiography, VO2peak was measured during an incremental exhaustive protocol on an electronically braked upright ergometer bike (Monark 839 Ergomedic, ProTerapi, Brøndby, Denmark) as previously described (Citation11). The protocol started at 50 W (senior sedentary group) or 75 W (all other groups), with a 25 W increase in load every minute until volitional exhaustion, which was reached at a point between 4 and 12 min for all subjects. VO2, CO2 excretion, respiratory exchange ratio (RER), heart rate (HR), minute ventilation (VE), and cadence in revolutions per minute (RPM) were continuously recorded. All subjects reached RER values above 1.09 (range 1.09–1.34). The VO2peak was determined as the highest value reached during a 15-sec period and reported as absolute values (mL∙min− 1) relative to body weight (mL∙kg− 1∙min− 1). shows the results of the cardiopulmonary exercise test (CPET) together with resting blood pressure and HR.
Echocardiography
Transthoracic echocardiography was performed using a Philips iE33 (Philips Medical Systems, Andover, MA, USA) with a standard S5-1 probe. Loops of three to six cycles were stored for post hoc analysis by an expert cardiologist blinded for age and training status of the participants. Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) dimensions, posterior wall thickness (PWT), and septum wall thickness (SWT) in end-diastole were measured from the parasternal long-axis view (PLAX). Relative wall thickness (RWT) was calculated as 2∙PWT∙LVEDD− 1. The LV ejection fraction (LVEF) was estimated by the biplane method of disks (modified Simpson's rule). Mean LV wall thickness (LVWT) was calculated as mean of PWT and SWT, left ventricular mass (LVM) was calculated as 0.8∙(1.04∙(LVEDD +PWT + SWT)3 ‐(LVEDD)3) + 0.6 g, left atrium volume (LAV) was estimated from the apical 4-chamber view, and indexed (LVWTI, LVMI and LAVI) for body surface area (BSA) calculated using the Du Bois formula (0.007184∙[weight in kg]0.425∙[height in cm0.725] m− 2), and in addition, LVM was allometrically adjusted as LVM∙height− 2.7. LVEDD (LVEDDI) and LVESD (LVESDI) were also indexed for BSA. LV mitral inflow pattern with peak early (E) and atrial-phase (A) diastolic inflow velocities were measured by pulsed wave Doppler (PW) in apical 4-chamber view, and the ratio of these (E/A) was calculated. PW tissue Doppler (TD) was used to measure peak early (e’) and atrial-phase (a’) diastolic and systolic (s’) LV longitudinal velocities in the septal, lateral, anterolateral, and inferior positions of the mitral annulus from apical 4-chamber and 2-chamber views, and the averages of these values were used.
We have previously estimated the inter-rater variability in our laboratory to be between 4% and 8% for E and e’, and between 16% and 26% for A during rest, exercise, and recovery (Citation12). The agreement was similar between the different conditions and similar to those reported by others (Citation13).
Supine bicycle echocardiography
Participants exercised on a supine ergometer bike (Lode, Groningen, Netherlands) with a back angle support of approximately 20° and left lateral tilt between approximately 20° and 30° while performing 55–65 RPM at 30% and 60% of the maximum workload performed during the CPET. Two minutes into each stage of the examination, measures of LV inflow and mitral annulus TD were obtained as described above. Measures were repeated after 2 min of passive recovery to ensure return to baseline and to strengthen the statistical analyses, by including 4 measurements per parameter for each individual. Blood pressure was measured using an oscillometric blood pressure monitor at the end of each stage. The stages lasted between 4 and 8 min, depending on image acquisition. Increase in HR will eventually (typically when HR exceeds 100–120 beats per minute) cause merging of E with A, and e’ with a’. In case of fully merged curves, the velocity was classified as E and e’ respectively.
Data analysis
The characteristics based on which participants were selected, that is, height, weight, and age, were analyzed using one-way analysis of variance (ANOVA). If significant differences were present, the Holm multiple comparison post hoc test was used to correct for multiple testing when comparing group differences within the senior (senior sedentary vs senior athletes) and the young (young sedentary vs young athletes) groups, as well as group differences within the trained (young athletes vs senior athletes) and the untrained (young sedentary vs senior sedentary) groups. Two-way ANOVA was used to test independent and interacting effects of age and training status on resting HR and blood pressure, peak exercise variables, and resting echocardiographic measures. Significant interactions were explored by the Holm multiple post hoc comparison test, as stated above. Additionally, multiple linear regressions of resting Doppler and TD parameters as outcomes of age and training status as categorical covariates and resting HR as a continuous covariate were performed.
Mixed-model repeated measures of ANOVA were used to test for effects of age, training status (athlete), stage (rest, 30%, 60% and recovery), and interactions between these for each outcome assessed during exercise echocardiography. The mixed-model ensures that individuals with missing data are not excluded from the statistical model. The likelihood ratio test of nested models (models including and excluding the term to be tested) was used to test interaction terms and stage. In case of a significant interaction term, the Wald test with Holm correction for multiple comparisons was used to test the significance of each level of the interaction term. Non-significant interactions were excluded while the main effects remained in the models. Due to the non-normally distributed residuals, LAVI and E/e’ were tested after logarithmic transformation. Pearson correlation coefficients (r) of VO2peak with LVEDDI, LVEF, Doppler, and TD measures were estimated. Further, stepwise multiple linear regression of VO2peak on LVEDDI, LVEF, Doppler, and TD measures were performed using both backward elimination and forward selection. Backward elimination included covariates that were significantly correlated with VO2peak, the covariate with the greatest P-value was eliminated in each step, and only significant covariates remained in the final model. Forward selection included the covariate with the most significant correlation to VO2peak, and in each step, the covariate that correlated most significantly with the residuals of the model was included, and the process was terminated when no covariates correlated with the residuals.
Results are given as mean (SD) in text and tables, and as mean (SEM) in figures, unless stated otherwise. For brevity, only P-values less than 0.1 are given in the text and figures. A value of P < 0.05 was considered statistically significant. All statistical analysis was conducted using STATA 11.2 (StataCorp, College Station, TX, USA).
Results
Mitral inflow and TD parameters
Overall, the echocardiographic parameters of mitral peak inflow velocities and annular TD velocities during rest and exercise were more influenced by age than by training status (, ). Athletes had higher E than the sedentary participants at rest, though the difference was small compared to the overall effect of age (P < 0.001, ). Full merging of E and A occurred in 16 (14 young and 2 senior) and 24 (18 young and 6 senior) participants at exercise levels of 30% and 60%, respectively, leaving only 8 and 4 young participants without full merging of E and A at 30% and 60%, respectively. If fully merged E waves were excluded, the athletes were found to have significantly greater E than the sedentary participants at all stages (P = 0.012). The effect, however, was minor compared to the effect of age, and the athlete effect on resting E was eliminated if correction for resting HR was performed.
Figure 1. (A–H) Mitral inflow, tissue Doppler velocities, and heart rate during supine exercise echocardiography. Values are mean (SEM). P-values < 0.1 of mixed model repeated measures ANOVA are given. A, atrial-phase mitral inflow velocity; a’, atrial-phase mitral annular tissue Doppler velocity; E, early mitral inflow velocity; e’, early mitral annular tissue Doppler velocity; E/A, ratio of early and atrial-phase mitral inflow velocities; E/e’, ratio of early mitral inflow and early mitral annular tissue Doppler velocities; HR, heart rate; s’, systolic mitral annular tissue Doppler velocity.
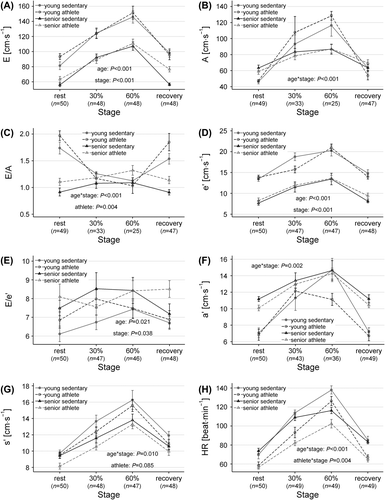
Table III. Resting mitral inflow and mitral annular tissue Doppler velocities.
Athletes had higher E/A than the sedentary participants at rest, but the difference was small compared to the impact of age, and the athlete effect was eliminated by adding resting HR to the model (). Due to the merging of the E and A waves and the resulting small number of young participants with measurable E/A during exercise, the power for detection of differences within the young participants (including age*athlete interaction) in the mixed-model was limited with regard to E/A. Though the E/A of the sedentary young does not seem different from that of the young athletes during exercise in , there were no significant age*athlete or athlete*stage interactions, and E/A was overall higher in the athletes compared to the sedentary participants. The value of e’ was significantly lower for the senior participants (P < 0.001), and increased generally during exercise (P < 0.001), with no influence of training status. These results were not altered by the exclusion of fully merged e’ ().
The ratio of E/e’ was generally higher for the senior participants (P =0.021), and increased slightly but significantly for the total study population from rest to exercise at 60% (rest vs 60%: P = 0.001, Holm-corrected) (). When fully merged waves were excluded, the age effect was still significant (P = 0.004), whereas the increase in E/e’ from rest to exercise was no longer significant.
All Doppler velocities rapidly returned toward resting values in the recovery phase.
Matching exercise intensity by relative workload led to greater increase in HR of the young (age*stage P < 0.001) and greater increase in HR of the sedentary group, compared to the athletes during each stage of the exercise echocardiography (athlete*stage P = 0.004) ().
Echocardiographic dimensions
LV volumes, diameters, and mass, both absolute and adjusted for BSA, were larger in the athletes, with no difference in LVEF (). LV diameters and mass were also greater in senior participants, without significant differences in LV volumes, regardless of indexation to BSA and allometric adjustment of LVM to height2.7. Consequently, RWT and mass/volume ratio only increased with age. As expected, LAV increased with age, but more so with training status. The effect of age was not modified by training status, and there were no significant age*athlete interactions for echocardiographic dimensions.
Table IV. Echocardiographic dimensions.
The VO2peak was significantly correlated with several Doppler and TD measures (). Stepwise multiple linear regression yielded the same model when backward elimination and forward selection were applied ().
Table V. Correlations between VO2peak [mL∙kg− 1∙min− 1] and selected echocardiographic parameters.
Table VI. Multiple linear regression with VO2peak as outcome.
Discussion
The main finding of the current study is that only age has a major impact on mitral peak inflow and TD velocities, whereas both age and training status have a compelling impact on exercise capacity and cardiac dimensions. We found minor differences between sedentary participants and athletes in terms of E, E/A and s’ during rest, but age-related decline in LV-diastolic function did not appear to be counteracted by training status, nor did exercise stress echocardiography reveal improved adaptation to exercise in the older athletes with regard to these parameters.
Age-related changes in cardiac function
Importantly, the effects of age were not found to be different between the athletes and the sedentary participants.
The results of the present study support conclusions of previous larger studies that resting LV diastolic (E, E/A, e’ and E/e’) and systolic function (measured as s’) decline with age (Citation1). The effects of age and endurance training on mitral inflow peak velocities and mitral annular TD velocities during exercise have recently been examined in cross- sectional studies by Donal et al. (Citation13), Molmen et al. (Citation6), and Carrick-Ranson et al. (Citation14). They matched exercise intensity in different ways—absolute HR and relative HR (percentage of maximum HR), respectively. Both mitral peak inflow and TD velocities might correlate with HR; in spite of this, the overall conclusions are in line with the present study.
Consistently, we, Donal et al. (Citation13), and Carrick-Ranson et al. (Citation14) find that the ratio of E/A decreases during exercise in the young, reaching values comparable to those of the older participants who have merely unchanged E/A during exercise. This should be seen in context of the difficulties in interpretation of the A-wave during exercise in the present study, particularly in the young, because of higher HR. The A-wave amplitude may be regarded as artificially elevated during tachycardia, when partial merging with the E-wave is present (Citation15). Thus, the increased A-wave may be interpreted as an “actual” and smaller A-wave amplitude riding on the residual contribution from the E-wave (Citation15). This would cause us to overestimate the increase in A-wave amplitude in the presence of partially merged E- and A-waves, and overestimate the E-wave amplitude in the presence of fully merged waves. In the study by Carrick-Ranson et al. (Citation14), merging occurred predominantly in the senior participants at the highest workload. Thus, in spite of these challenging technical issues regarding the effects of HR and wave fusion, it seems consistent that young adults have less contribution to LV-filling from atrial systole at rest, with a relatively greater exercise-induced increase of LV-filling during atrial systole, compared to the elderly.
The value of e’ was lower both at rest and during exercise in the senior participants in the current study and in the studies by Donal et al. (Citation13) and Carrick-Ranson et al. (Citation14). The higher E/e’ during both rest and exercise in the older participants, indicative of higher filling pressures, was in line with a similar, albeit non-significant trend found by Carrick-Ranson et al. (Citation14). Though E/e’ can be used even in case of complete merging of E and A (Citation16), it should be noted that E/e’ does not reliably track changes in ventricular filling pressures during cardiac loading and unloading in healthy individuals (Citation17).
Effect of training status on resting cardiac function
As hypothesized, both young and senior athletes had greater resting E (8%) and E/A (7%), although the athlete effect was modest in comparison to the 30% higher resting E and 44% higher resting E/A of the young compared to the senior participants. This is in line with most of the previous studies that either found no difference or only modestly higher E and E/A among athletes (Citation6,Citation13,Citation14,Citation18–23). Experiments with increased HR by means of atrial pacing or exercise find A and E/A, and to a lesser extent E, to be affected by HR in young healthy subjects (Citation15,Citation24). The athlete effect was eliminated by correction for resting HR. Conclusions should, however, be drawn with caution, because athlete status and resting HR are closely related and HR as a continuous variable holds greater statistical power. Further, resting HR had significant effect on A but not on E. We found no effect of training status on A, and as such, our findings for E and E/A are probably not solely due to the lower HR of the athletes. Nor were there indications of differences in relaxation (e’) or filling pressures (E/e’). Thus, probable causes of higher E/A are also greater suction by the larger ventricles, greater LV compliance, and greater preload in athletes (Citation4,Citation14,Citation19,Citation25).
We did not find the hypothesized impact of training status on e’, which is in accordance with some of the previous studies (Citation13,Citation14,Citation21), whereas studies by Nottin et al. (Citation22) and Molmen et al. (Citation6), which did not include young athletes, reported higher e’ of senior athletes compared to sedentary seniors.
It cannot be excluded that the differences in findings could relate to intensity of the training. Generally, improvement in VO2peak is directly related to the “dose” of exercise, that is, intensity (how hard), duration (how long), and frequency (how often) of training. The intensity of the exercise seems to have a pivotal role in the training effects including VO2peak (Citation26). Some intervention studies in heart failure and arterial hypertension indicate that intensive interval training could have greater effect on diastolic parameters than moderate continuous exercise (Citation27,Citation28). However, none of the cross-sectional studies directly report the exact intensity of the exercise training that also will vary over season and years.
Our overall findings in runners are not different from those of the previous studies which also included athletes from sports that imply static components or upper body work (Citation6,Citation13,Citation14,Citation19–22,Citation29). Though long-term longitudinal studies are lacking, it seems from the consistency of findings that long-term endurance training, compared to aging, does not have a marked impact on resting velocities of mitral peak inflow velocities or diastolic and systolic annular TD (Citation6,Citation13,Citation14,Citation19–22,Citation29).
Effect of training status on cardiac function during exercise
Training status did not impact E and e’ during exercise as hypothesized, though there was a minor effect on E when fully merged waves were excluded. E/A was higher in the athletes compared to the sedentary participants, both during rest and during exercise. This is in agreement with previous cross-sectional studies (Citation6,Citation13,Citation14), although Donal et al. (Citation13) report that E/A was only higher for young athletes. This difference might be explained by the senior athletes of Donal et al. (Citation13) having trained for less than 10 years, in contrast to our senior athletes who had been training consistently for up to 45 years.
Stepwise regression showed supplemental predictive power of LV volume, and diastolic and systolic functional measures on VO2peak.
Importantly, the remodeling of the athletes’ heart was distinctly different from that of disease-related remodeling, for example, as in hypertrophic cardiomyopathy. The proportions between the cardiac wall and chamber dimension were preserved in the athletes, and systolic and diastolic TD velocities increased appropriately during exercise and returned rapidly toward resting values in recovery.
Limitations
Longitudinal LV velocities do not fully describe the movements involved in LV contraction and recoil. Differences between aging with and without endurance training might exist in the twist movement, as indicated by a recent study showing shorter time from mitral valve opening to peak E and peak untwisting velocity, for endurance-trained compared to untrained middle-aged men at HR-matched exercise (Citation30).
The supine exercise was unusual for the participants, whereas they were all familiar with upright cycling. We applied a workload in the supine position equal to 60% of the maximal workload obtained in the upright position, which corresponds to a considerably greater percentage of the maximal supine workload; however, it does not correspond to exercise at maximal HR. Further, the relative work load of the supine exercise was derived from the maximal work load of the upright CPET, and we cannot exclude that the transfer from upright to supine could have a differential effect depending on age and training status.
Based on interviews and exercise capacity, we assumed that running was largely of moderate to high intensity. However, intensity was not directly measured in the present study.
The selection of senior participants with a sedentary lifestyle but without overt lifestyle-related disease, that is, diabetes or CVD, could lead to selection bias and thus underestimation of the effect of exercise training.
Clearly, the study is cross-sectional, allowing no conclusions of causality. Further, cross-sectional studies are likely to overlook minor improvements in TD velocities at rest and during exercise, which has been attained with well-controlled exercise intervention in young and senior individuals (Citation6–9).
Perspectives
We found that despite marked cardiac remodeling, life-long endurance running was not associated with major attenuation of the age-related decline in diastolic function. The improvements in diastolic function attained in some interventional studies with exercise training for up to 1 year are minor compared to the apparently inherent effect of age found in the cross-sectional studies.
The effect of endurance training on exercise capacity and LV dimensions is well known. The greater LV chamber dimension of endurance athletes implies greater stroke volume and lower resting HR, and thereby greater HR reserve, all of which together contribute to greater functional reserve, maximal cardiac output and VO2peak of exercise-trained individuals.
In conclusion, young and elderly well-trained endurance runners had only moderately higher resting E and E/A, with little or no further adaptations during exercise. Conversely, the effects of age were marked, both during rest and exercise.
Declaration of interest: The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:614–22.
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: Molecular mechanisms. Circulation. 2010; 122:1221–38.
- Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail Rev. 2012;17:545–54.
- Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–805.
- Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116–26.
- Molmen HE, Wisloff U, Aamot IL, Stoylen A, Ingul CB. Aerobic interval training compensates age related decline in cardiac function. Scand Cardiovasc J. 2012;46:163–71.
- Esfandiari S, Sasson Z, Goodman JM. Short-term high- intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur J Appl Physiol. 2014;114:331–43.
- Rodrigues ACT, de Melo Costa J, Alves GB, Ferreira da Silva D, Picard MH, Andrade JL, et al. Left ventricular function after exercise training in young men. Am J Cardiol. 2006;97:1089–92.
- Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, et al. Training-specific changes in cardiac structure and function: A prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008; 104:1121–8.
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38:1104–15.
- Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, et al. Life-long endurance exercise in humans: Circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134:531–40.
- Monk-Hansen T, Dall CH, Christensen SB, Snoer M, Gustafsson F, Rasmusen H, Prescott E. Interval training does not modulate diastolic function in heart transplant recipients. Scand Cardiovasc J. 2014;48:91–8.
- Donal E, Rozoy T, Kervio G, Schnell F, Mabo P, Carré F. Comparison of the heart function adaptation in trained and sedentary men after 50 and before 35 years of age. Am J Cardiol. 2011;108:1029–37.
- Carrick-Ranson G, Doughty RN, Whalley GA, Walsh HJ, Gamble GD, Baldi JC. The larger exercise stroke volume in endurance-trained men does not result from increased left ventricular early or late inflow or tissue velocities. Acta Physiol (Oxf). 2012;205:520–31.
- Chung CS, Kovács SJ. Consequences of increasing heart rate on deceleration time, the velocity-time integral, and E/A. Am J Cardiol. 2006;97:130–6.
- Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quinones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia: A new application of tissue Doppler imaging. Circulation. 1998;98:1644–50.
- Bhella PS, Pacini EL, Prasad A, Hastings JL, Adams-Huet B, Thomas JD, et al. Echocardiographic indices do not reliably track changes in left-sided filling pressure in healthy subjects or patients with heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2011;4:482–9.
- Gates PE, Tanaka H, Graves J, Seals DS. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24:2213–20.
- Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart: A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–44.
- George KP, Naylor LH, Whyte GP, Shave RE, Oxborough D, Green DJ. Diastolic function in healthy humans: Non-invasive assessment and the impact of acute and chronic exercise. Eur J Appl Physiol. 2010;108:1–14.
- Baldi JC, Mcfarlane K, Oxenham HC, Whalley GA, Walsh HJ, Doughty RN. Left ventricular diastolic filling and systolic function of young and older trained and untrained men. J Appl Physiol. 2003;95:2570–5.
- Nottin S, Nguyen L-D, Terbah M, Obert P. Long-term endurance training does not prevent the age-related decrease in left ventricular relaxation properties. Acta Physiol Scand. 2004;181:209–15.
- Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, et al. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol. 2014;116:736–45.
- Harrison MR, Clifton GD, Pennell AT, DeMaria AN. Effect of heart rate on left ventricular diastolic transmitral flow velocity patterns assessed by Doppler echocardiography in normal subjects. Am J Cardiol. 1991;67:622–7.
- Hagberg JM, Goldberg AP, Lakatta L, O’Connor FC, Becker LC, Lakatta EG, Fleg JL. Expanded blood volumes contribute to the increased cardiovascular performance of endurance-trained older men. J Appl Physiol. 1998; 85:484–9.
- Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007; 39:665–71.
- Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–94.
- Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, et al. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19: 151–60.
- Teske AJ, Prakken NH, De Boeck BWL, Velthuis BK, Doevendans PA, Cramer MJM. Effect of long term and intensive endurance training in athletes on the age related decline in left and right ventricular diastolic function as assessed by Doppler echocardiography. Am J Cardiol. 2009;104:1145–51.
- Lee LS, Mariani JA, Sasson Z, Goodman JM. Exercise with a twist: Left ventricular twist and recoil in healthy young and middle-aged men, and middle-aged endurance-trained men. J Am Soc Echocardiogr. 2012;25:986–93.

