Abstract>
Objectives: To study the relationship between obesity and heart rate (HR) in women and men. Design: We studied 241 overweight and obese subjects without known heart disease. All subjects underwent ergospirometry during maximal exercise testing on treadmill and recording of body composition, electrocardiogram and clinic and ambulatory blood pressure. Results: Women (n = 132) were slightly older and had higher fat mass, but lower weight, blood pressure and prevalence of metabolic syndrome (MetS) than men (n = 109) (all p < 0.05), while prevalences of obesity and hypertension did not differ. A significant interaction between sex and HR was demonstrated (p < 0.05). In multivariate analysis, female sex (β = 0.99, p < 0.01) predicted higher resting HR independent of confounders. Higher resting HR was particularly associated with presence of MetS, hypertension, higher insulin resistance and lower relative muscle mass in men (all p < 0.05). Female sex also predicted higher peak exercise HR (β = 0.48, p < 0.01) independent of confounders. Higher peak exercise HR was particularly associated with higher exercise capacity and lower age and self-reported physical activity in men, while lower HbA1c and absence of obesity were the main covariates in women in multivariate analyses (all p < 0.05). Conclusions: In our study population, obesity and obesity-associated metabolic changes influenced both resting and peak exercise HR.
Key words::
Introduction
Resting heart rate (HR) is a well-known predictor of cardiovascular morbidity and mortality both in women and men (Citation1–3). Obesity may increase HR through sympathetic nervous system disturbances and insulin resistance (Citation4,Citation5). In addition, clustering of cardiometabolic risk factors, often referred to as metabolic syndrome (MetS), has been shown to be associated with higher resting HR (Citation6). However, in clinical studies, some studies have documented an association between increasing body mass index (BMI) and higher resting HR (Citation7,Citation8), while others have not (Citation9). Similar discrepancy has been reported for peak exercise HR. Some studies have found that overweight and obesity are associated with lower peak exercise HR (Citation9,Citation10), possibly related to poorer sympathetic response to exercise (Citation9) or lower level of physical fitness, while others found no association between peak exercise HR and BMI (Citation11). Finally, several studies in general population have documented that women have higher resting HR than men (Citation11,Citation12). The aim of this study was to add to current knowledge about the association of overweight and obesity with HR at rest and during peak exercise using sex-specific analyses.
Materials and Methods
Study population
Women and men aged 30–65 years with BMI > 27 kg/m2 were included in the FAT associated CardiOvasculaR dysfunction (FATCOR) study at Haukeland University Hospital in Bergen, Norway. Subjects were included at the collaborating general practice centre (Alfahelse AS), which prospectively invited subjects from the practice that fulfilled the inclusion criteria. Exclusion criteria were gastrointestinal disorder, severe psychiatric disorder, previous myocardial infarction and inability to understand Norwegian. The present analysis includes the 241 participants with completed examinations and results registered in the database recruited from October 2009 to November 2012. The FATCOR study was performed in accordance with the declaration of Helsinki from 1983, and approved by the Regional Ethics Committee. All participants signed written informed consent.
Measurements
A standardised clinical examination was performed by the general practitioner, including measurement of clinic blood pressure and resting HR following guideline recommendations (Citation13) as well as body composition analysis by bioimpedance (Tanita TBF-300A, Tanita Corporation of America, Arlington Heights, USA). All participants filled in a standardised questionnaire for self-reported health, use of any medication and level of physical activity (hours/week).
All participants underwent a maximal exercise capacity test on treadmill (Schiller CS-200, Schiller AG, Baar, Switzerland) using the standardised Chronotropic Assessment Exercise Protocol (CAEP). Peak exercise HR was measured from the electrocardiogram (ECG) at maximal exercise. Peak oxygen uptake (VO2max) and the respiratory exchange ratio (RER) between CO2 and O2 were measured with ergospirometry. Metabolic equivalents (METs), a measure of physical capacity independent of body weight, were calculated (Citation14). The test was observed by qualified personnel to detect critical arrhythmias or signs of ischaemia on the ECG. A peripheral venous cannula was introduced beforehand for safety reasons. The participants were instructed to continue until exhaustion or presentation of any discomfort or symptoms like chest pain, strong dyspnoea or fatigue.
A 24-hour ambulatory blood pressure (ABP) recording was performed on the non-dominant arm (Diasys Integra II, Novacor, Cedex, France), with measurements every 20 minutes at daytime and every 30 minutes at night. The participants were instructed to have normal daily activity and to relax the arm during measurements (Citation13).
Fasting venous blood samples were drawn for measurement of serum lipid profile, fasting serum glucose and serum creatinine. All non-diabetic patients underwent a glucose tolerance test.
Classification of overweight and obesity follow the guidelines of the World Health Organization, where overweight is classified as BMI ≥ 25 kg/m2 and obesity as BMI ≥ 30 kg/m2 (Citation15).
We used the American Heart Association/National Heart, Lung, and Blood Institute criteria to identify presence of MetS (Citation16). Persons were considered to have MetS if at least three of the following five criteria at baseline were fulfilled: waist circumference ≥ 88 cm in women and ≥ 102 cm in men; blood pressure ≥ 130/85 mmHg; fasting serum glucose ≥ 100 mg/dl; serum triglycerides ≥ 1.7 mmol/l; and serum high-density-lipoprotein (HDL) cholesterol < 1.3 mmol/l in women and < 1.03 mmol/l in men (Citation16).
Diabetes mellitus was defined as previously known diabetes, use of anti-diabetic treatment, or fasting blood glucose ≥ 7 mmol/L, impaired glucose tolerance as blood glucose 7.8–11.0 mmol/L 2 hours after oral intake of 75 g of glucose, and insulin resistance from homeostatic model assessment (HOMA-IR), respectively (Citation17).
Statistical analyses
The SPSS statistical software (version 21) was used for all statistical analyses and data management. We performed analysis on the whole study population, and for women and men separately. Groups were compared by Student's t-test for continuous variables and by Chi-Square test for categorical variables and the results were presented as mean ± standard deviation and percentages, respectively. Univariate analyses were performed with Pearson's correlation and univariate linear regression analysis for the whole group and for women and men separately. Multivariate linear regression with collinearity tools were used to find independent variables of resting HR and peak exercise HR in the whole group, and separately for women and men, including variables significant in univariate analyses as well as known confounders from the literature. A sex–HR interaction term was included in the models assessing the total study population. Results presented are the standardised correlation coefficients (ß) and p values for the individual variables and multiple R2 for the overall models. Level of significance was set to p < 0.05 for all statistical analyses.
Results
Clinical characteristics
The study population included 132 women (54.8%) and 109 men, on average 49 ± 9 years old with an average BMI of 32.2 ± 4.3 kg/m2. Obesity was present in 65.1% and MetS in 40.1% of the study population (). Women were slightly older and had higher fat mass, despite lower body weight and waist circumference than men (all p < 0.05), while prevalences of obesity and hypertension did not differ between sexes (). In contrast, clinic blood pressure, muscle mass and prevalence of MetS were all higher in men (all p < 0.05) ().
Table I. Clinical characteristics of the study population and in women and men separately.
Correlates of resting HR in uni- and multivariate analyses
In the total study population, having diabetes (ß = 0.21), hypertension (ß = 0.23), MetS (ß = 0.26) and being treated for hypertension (ß = 0.21) were all significantly associated with higher resting HR in univariate analyses (all p < 0.01), while female sex was not (ß = 0.12, p = 0.07). Also higher fat mass and fasting serum glucose were associated with higher resting HR (both p < 0.05) (). Although mean resting HR did not differ between women and men, a significant interaction between sex and resting HR was demonstrated in multivariate analysis, and female sex was independently associated with higher resting HR (multiple R2 = 0.39, p < 0.01) after adjusting for important confounders ().
Figure 1. (upper panel) Univariate correlations of higher resting HR in the total population and in women and men separately. (lower panel) Univariate correlations of higher peak exercise HR in the total population and in women and men separately. HDL, High-density-lipoprotein; HbA1c, Haemoglobin A1c; HOMA-IR, insulin resistance from homeostatic model assessment; ABP, Ambulatory blood pressure; DBP, Diastolic blood pressure; SBP, Systolic blood pressure; BMI, Body mass index; LDL, Low-density-lipoprotein; RER, Respiratory exchange ratio; METs, Metabolic equivalents; VO2max, peak oxygen uptake
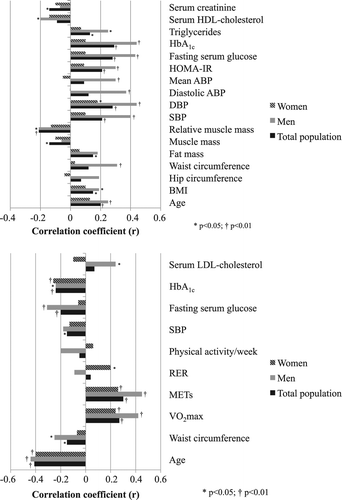
Table II. Multivariate regression analyses of resting HR in the total study population and in women and men separately.
Sex-specific analyses identified the same covariates of resting HR in men as described for the total study population above, while for women only diastolic blood pressure was identified as a significant covariate of resting HR (), revealing a pronounced sex difference. In multivariate analyses, presence of MetS and hypertension, in addition to lower relative muscle mass and higher HOMA-IR, were the key covariates of higher resting HR in men (multiple R2 = 0.46, p < 0.01), while no model could be constructed in women ().
Correlates of peak exercise HR in uni- and multivariate analyses
In the total study population, not having either diabetes (ß = −0.17), hypertension (ß = −0.24) or being treated for hypertension (ß = −0.29) were all associated with higher peak exercise HR (all p < 0.05). Although mean peak exercise HR during maximal treadmill testing did not differ between women and men (), a significant interaction between sex and peak exercise HR was demonstrated in multivariate analysis, and female sex was independently associated with higher peak exercise HR in multivariate regression analysis (multiple R2 = 0.23, p < 0.01) ().
Table III. Characteristics from exercise testing in the total study population and in women and men separately.
Table IV. Multivariate regression analyses of peak HR in the total study population and in women and men separately.
In sex-specific analyses, not having either diabetes (ß = −0.25), hypertension (ß = −0.31), MetS (ß = − 0.20) or being treated for hypertension (ß = −0.44) were all associated with higher peak exercise HR in men in univariate analyses (all p < 0.05), while not having hypertension was associated with higher peak exercise HR in women (ß = −0.20, p < 0.05). Also lower HbA1c was associated with higher peak exercise HR in women (p < 0.01) (). In multivariate regression analysis, higher peak exercise HR was independently associated with higher exercise capacity and lower age and self-reported physical activity in men (multiple R2 = 0.36, p < 0.01), while lower HbA1c and absence of obesity were independently associated with higher peak exercise HR in women (multiple R2 = 0.30, p < 0.01) ().
Discussion
This study demonstrates several interactions of obesity and obesity-associated metabolic changes with resting and peak exercise HR. Average values for resting and peak exercise HR were similar between women and men, but a significant interaction between sex and HR was demonstrated in multivariate analyses, and female sex was independently associated with both higher resting and peak exercise HR when adjusted for confounders.
Some studies in obesity have found resting HR to increase in parallel with BMI (Citation7), which may be secondary to obesity-associated autonomic disturbances like increased sympathetic activity, reduced vagal activity and increased insulin resistance (Citation4,Citation5). In our study we found a positive univariate association between BMI and resting HR in the total study population and in men, but this was not confirmed in multivariate models. In contrast, higher insulin resistance and presence of MetS were associated with higher resting HR, particularly in men, reflecting that subjects with the MetS, impaired glucose tolerance, insulin resistance and diabetes may also have enhanced sympathetic activity secondary to relative metabolic stress. This adds to previous findings from the large European Global Cardiometabolic Risk Profile in Patients with hypertension disease (GOOD) survey, documenting higher resting HR in hypertensive subjects with MetS (Citation6). Compared to the prevalence of MetS in the Health Study in North Trøndelag (HUNT3), where 12.8% of the general population had MetS, our finding of MetS in 32.5% of women and 49.1% of men was considerably higher, reflecting the close association between obesity and clustering of cardiovascular risk factors (Citation18). Others have found MetS to be associated with hypertension as well as left ventricle hypertrophy and systolic dysfunction in general population (Citation19), all known factors affecting exercise capacity (Citation20). However, echocardiography was not included in the present study.
We also discovered a pronounced difference in covariates of resting HR as well as peak exercise HR between women and men. Having MetS, hypertension and higher HOMA-IR were all associated with higher resting HR in men, but these associations were not found in women, although women had higher fat mass. Absence of obesity was particularly associated with higher peak exercise HR in women. This finding in women adds to the results in the study by Gondoni et al. (Citation9), demonstrating lower peak exercise HR in trained and unfit obese subjects without heart disease compared to normal and overweight subjects. In contrast, lower self-reported physical activity and higher exercise capacity were particularly associated with higher peak exercise HR in men.
It is well demonstrated by others that resting HR increases in parallel with age (Citation21). Gondoni et al. reported that age alone explained 36% of the variance in peak exercise HR (Citation9). Age was also the strongest covariate of peak exercise HR in the present study.
As demonstrated, average values of resting HR and peak exercise HR were comparable in women and men. However, in multivariate analysis, female sex independently predicted higher resting HR, in line with previous reports in healthy subjects (Citation11,Citation12). Ogawa et al. studied healthy sedentary and trained women and men of different age, and could not find differences in peak exercise HR between sexes, independent of training status and age (Citation22). In contrast, results from the HUNT Fitness Study, investigating a Norwegian healthy adult population, demonstrated a slightly lower peak HR in women when adjusted for age (Citation23).
In our study population, the peak oxygen uptake was 23% lower in women than men, a slightly larger difference than the results from the HUNT Fitness Study (Citation24), reflecting the lower muscle mass in women. Of note, the protocol used for exercise testing in our study, the CAEP protocol, is probably superior to the Bruce protocol in overweight and obese subjects with low expected physical capacity. The CAEP protocol has smaller increase in exercise load between stages, probably resulting in a more accurate test in sedate and obese subjects with lower exercise capacity, as pointed out by Bratberg et al. (Citation25).
Our study has several potential limitations. The FATCOR study prospectively included women and men aged 30–65 years with BMI > 27.0 kg/m2, thus age and BMI were truncated by design, and this may have influenced the prevalence of metabolic syndrome in the study population, which in particular was high among men. Projection of results to individuals with age and BMI values outside our range should be done with caution. Both health status and physical activity were self-reported in a standardised questionnaire. Although the information was quality assured by the general practitioner, we cannot exclude that health problems may have been underreported.
Conclusions
Among overweight and obese women and men without known cardiovascular disease participating in the FATCOR study, obesity and obesity-associated metabolic disturbances influenced both resting and peak exercise HR. In particular absence of obesity and lower HbA1c were associated with higher peak exercise HR in women and presence of MetS, hypertension and higher insulin resistance with higher resting HR in men in sex-specific multivariate analyses.
Acknowledgements
We thank Britt Gjellefall, research nurse (RN), Liv Himle, RN, and Synnøve Ygre Hauge, RN, for technical assistance and management of study participants in the FATCOR study. We thank Reinhard Seifert, MSc, Department of Heart Disease, Haukeland University Hospital, Bergen, Norway for statistical analysis support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J.Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–62.
- Hsia J, Larson JC, Ockene JK, Sarto GE, Allison MA, Hendrix SL, et al. Resting heart rate as a low tech predictor of coronary events in women: prospective cohort study. BMJ. [serial online] 2009 Feb 04 [cited 2015 May 7]; 338:b219. Available from: URL: http://dx.doi.org/10.1136/bmj.b219.
- Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–30.
- Hillebrand S, Swenne CA, Gast KB, Maan AC, le Cessie S, Jukema JW, et al. The role of insulin resistance in the association between body fat and autonomic function. Nutr Metab Cardiovasc Dis. 2015;25:93–9.
- Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–40.
- Perlini S, Naditch-Brule L, Farsang C, Zidek W, Kjeldsen SE. Pulse pressure and heart rate in patients with metabolic syndrome across Europe: insights from the GOOD survey. J Hum Hypertens. 2013;27:412–6.
- Vergnaud AC, Protogerou AD, Li Y, Czernichow S, Vesin C, Blacher J, et al. Pulse pressure amplification, adiposity and metabolic syndrome in subjects under chronic antihypertensive therapy: the role of heart rate. Atherosclerosis. 2008;199:222–9.
- Lund BP, Gohlke-Barwolf C, Cramariuc D, Rossebo AB, Rieck AE, Gerdts E. Effect of obesity on left ventricular mass and systolic function in patients with asymptomatic aortic stenosis (a Simvastatin Ezetimibe in Aortic Stenosis [SEAS] substudy). Am J Cardiol. 2010;105:1456–60.
- Gondoni LA, Titon AM, Nibbio F, Augello G, Caetani G, Liuzzi A. Heart rate behavior during an exercise stress test in obese patients. Nutr Metab Cardiovasc Dis. 2009; 19:170–6.
- Zhu N, Suarez-Lopez JR, Sidney S, Sternfeld B, Schreiner PJ, Carnethon MR, et al. Longitudinal examination of age-predicted symptom-limited exercise maximum HR. Med Sci Sports Exerc. 2010;42:1519–27.
- Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39:822–9.
- Jaquet F, Goldstein IB, Shapiro D. Effects of age and gender on ambulatory blood pressure and heart rate. J Hum Hypertens. 1998;12:253–7.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood press. 2014;23:3–16.
- Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol. 1990;13:555–65.
- World Health Organization [Internet]. Obesity and overweight, Fact sheet N° 311 2014 [updated 2015 Jan; cited 2015 May 7]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52.
- Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes care. 2003;26:3160–7.
- Brumpton BM, Camargo CA, Jr., Romundstad PR, Langhammer A, Chen Y, Mai XM. Metabolic syndrome and incidence of asthma in adults: the HUNT study. Eur Respir J. 2013;42:1495–502.
- Chinali M, Devereux RB, Howard BV, Roman MJ, Bella JN, Liu JE, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). Am J Cardiol. 2004;93:40–4.
- Gerdts E, Bjornstad H, Toft S, Devereux RB, Omvik P. Impact of diastolic Doppler indices on exercise capacity in hypertensive patients with electrocardiographic left ventricular hypertrophy (a LIFE substudy). J Hypertens. 2002;20:1223–9.
- Molmen HE, Wisloff U, Aamot IL, Stoylen A, Ingul CB. Aerobic interval training compensates age related decline in cardiac function. Scand Cardiovasc J. 2012;46:163–71.
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503.
- Nes BM, Janszky I, Wisloff U, Stoylen A, Karlsen T. Age-predicted maximal heart rate in healthy subjects: The HUNT Fitness Study . Scand J Med Sci Sports. 2013; 23:697–704.
- Aspenes ST, Nilsen TI, Skaug EA, Bertheussen GF, Ellingsen O, Vatten L, Wisløff U. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43:1465–73.
- Bratberg JA, Bulut E, Rieck AE, Lonnebakken MT, Hetland T, Gerdts E. Determinants of systolic blood pressure response during exercise in overweight subjects. Blood Press. 2014;23:200–5.

