Abstract
Objectives. A post-hoc analysis was performed to determine the relationship between the timing and magnitude of DAS28(ESR) response and long term outcomes in Japanese patients after 1 year of CZP treatment.
Methods. Our analysis included 82 J-RAPID trial patients treated with CZP 200 mg and methotrexate, and 116 HIKARI trial patients treated with CZP 200 mg alone or with disease-modifying agents other than methotrexate. Remission rates and changes in mTSS at year 1 were compared to the DAS28(ESR) response at week 12 of CZP treatment.
Results. After 1 year of treatment, remission was achieved in 41.3% of the J-RAPID and 34.9% of the HIKARI patients with a week 12 DAS28(ESR) response of ≥ 1.2. In comparison, patients with a DAS28(ESR) response of < 1.2 at week 12 only had a < 7% probability of achieving remission and displayed higher change in mTSS after 1-year treatment.
Conclusions. The likelihood of remission and extent of radiographic progression after 1 year was associated with the week 12 DAS28(ESR) response. The DAS28(ESR) response at 12 weeks could be beneficial for identifying patients that are unlikely to respond to prolonged CZP treatment.
Introduction
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease characterized by persistent and chronic joint inflammation. Joint inflammation causes tenderness and pain, which markedly reduces the quality of life of patients. If left untreated, the inflammation eventually leads to joint destruction and irreversible deformities that further impair the daily activity of these patients. The joint inflammation is perpetuated by the continuous vicious cycle of pro-inflammatory cytokine production by a variety of immune cells. Among these cytokines, tumor necrosis factor α (TNF-α) appears to play a pivotal role in maintaining the ongoing inflammation. Biological agents that target TNF-α including certolizumab pegol (CZP) have been found to exhibit tremendous therapeutic efficacy in patients with RA. However, long-term clinical strategies in utilizing these drugs have been in evolution over the last decade.
Two randomized controlled trials (RCTs), J-RAPID (NCT00791999) [Citation1], and HIKARI (NCT00791921) [Citation2], and following open-label extension studies (NCT00851318, NCT00850343) [Citation3,Citation4] demonstrated rapid and sustained improvements in disease activity and quality of life in Japanese RA patients. Although the majority of patients favorably respond to CZP therapy, some patients fail to receive long-term benefit from continued treatment. Earlier identification of patients who are unlikely to respond to long-term CZP therapy would be beneficial, as one could avoid unnecessary exposure to CZP and could devise alternative treatment plans for such patients. Thus, it would be useful for the clinician to be able to predict long-term CZP outcomes as early as possible. Recent studies suggested that the early assessment of Disease Activity Score in 28 joints (DAS28(ESR)) could predict long-term outcomes to CZP [Citation5]. It was found that the majority of patients with active RA from the RAPID1 (RA PreventIon of structural Damage 1) trial had a rapid response to CZP and that a lack of improvement in DAS28(ESR) by week 12 predicted failure to achieve low disease activity (LDA) at later time point [Citation6].
Following this study, we hypothesized that the DAS28(ESR) response could be also used to identify patients who are unlikely to obtain long-term benefits from CZP therapy in the Japanese population. In the present manuscript, we performed a post-hoc analysis of the J-RAPID and HIKARI clinical trials to determine the relationship between the timing and magnitude of DAS28(ESR) response and the likelihood of achieving disease remission (DAS28(ESR)< 2.6), and the extent of radiographic progression in Japanese patients after 1 year of CZP treatment. The proportion of patients exhibiting low disease activity (LDA, DAS28(ESR)≤ 3.2) was also included as a long-term outcome. Relationship between other efficacy criteria and long-term outcomes, and influence of baseline disease characteristics or concomitant medication on relationship were also evaluated.
Materials and methods
Patients
Patients who received CZP 400 mg on week 0, 2, 4, and 200 mg every other week (Q2W) thereafter in RCT phase of J-RAPID (n = 82) and HIKARI (n = 116) were included in this analysis.
J-RAPID and HIKARI study
Study design and primary results of the J-RAPID, HIKARI, and open-label extension studies have been presented previously [Citation1–4]. Briefly, patients in the J-RAPID and HIKARI clinical trials were aged ≥ 20 years to ≤ 75 years and had a diagnosis of adult-onset RA of 0.5–15 years’ duration as defined by the criteria of the American College of Rheumatology (ACR). All patients recruited to the J-RAPID trial had previously received methotrexate (MTX) treatment for ≥ 6 months with the MTX dose fixed for ≥ 2 months beforehand and within the range of 6–8 mg/week and they had an inadequate response to MTX. All patients recruited to the HIKARI trial must have previously failed treatment with or been resistant to ≥ 1 disease-modifying anti-rheumatic drug (DMARD) and were unable to receive MTX because of safety concerns or insufficient efficacy. Patients were excluded if they had a form of inflammatory arthritis other than RA, received any biologic treatment for RA in the 6 months preceding the study (3 months for etanercept), treated with any investigational drug in the preceding 3 months, previously treated with ≥ 2 TNF inhibitors, or had previously initially failed to respond to TNF inhibitor therapy. In the J-RAPID trial, 316 patients were randomized 1:1:1:1 to subcutaneous (1) placebo (saline), or (2) CZP 200 mg at weeks 0, 2, and 4, then 100 mg Q2W, (3) CZP 400 mg at weeks 0, 2, and 4, then 200 mg Q2W, or (4) CZP 400 mg at weeks 0, 2, and 4, then 400 mg Q2W for 24 weeks with MTX. Patients who completed the study were eligible to enter the open label extension (OLE) trial (J-RAPID OLE), where patients received CZP 200 mg Q2W or CZP 400 mg every 4 weeks (Q4W), in combination with MTX. Patients who failed to achieve an ACR 20% criteria for improvement (ACR20) response at both weeks 12 and 14 were withdrawn from the J-RAPID study at week 16 and were also eligible to enter the J-RAPID OLE (). In the HIKARI trial, 230 patients were randomized 1:1 to subcutaneous (1) placebo or (2) CZP 400 mg at weeks 0, 2, and 4, then 200 mg Q2W for 24 weeks. Patients who completed the study were eligible to enter the OLE trial (HIKARI OLE), where patients received CZP 200 mg Q2W or CZP 400 mg Q4W for 52 weeks. Patients who failed to achieve an ACR20 response at both weeks 12 and 14 were withdrawn from the HIKARI study at week 16 and were also eligible to enter the HIKARI OLE ().
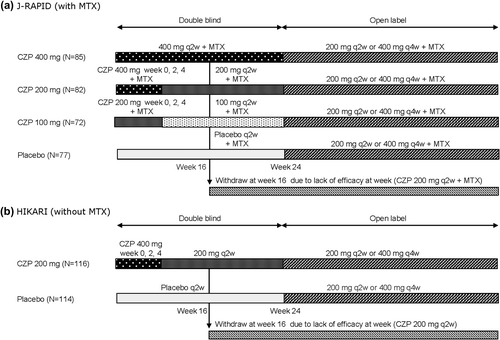
The mean baseline DAS28(ESR) of patients randomized to the CZP (200 mg Q2W) treatment groups in J-RAPID and HIKARI were 6.2 and 6.1, respectively. In J-RAPID, the ACR20 response was significantly higher in patients treated with CZP (200 mg) + MTX compared to placebo + MTX at week 12 (primary end point) (76.8% vs. 28.6%; p < 0.001, logistic regression analysis) [Citation1]. Similarly, in HIKARI, the ACR20 response was significantly higher in the CZP (200 mg Q2W) with or without DMARDs other than MTX group than the placebo group at week 12 (primary end point) (67.2% vs. 14.9%; p < 0.001, logistic regression analysis) [Citation2].
Predictability analysis
In the first analysis, the relationship between change in DAS28(ESR) from baseline at various time points and the proportion of patients who achieved remission (DAS28(ESR)< 2.6) at 1 year was examined by logistic regression analysis. The area under the ROC was also calculated to examine whether change in DAS28(ESR) predicts the probability of remission at 1 year.
In the second analysis, the proportion of CZP-treated patients who achieved remission (DAS28(ESR)< 2.6), and the mean change in van der Heijde modified Total Sharp Score (mTSS) at 1 year (during the OLE) was compared to the level of DAS28(ESR) response and non-response (i.e., DAS28(ESR) change from baseline of ≥ 1.2 or 0.6) at various time points. In some analyses, patients from both studies were stratified by other week 12 criteria of response including DAS28(ESR)≤ 5.1 (moderate disease activity), DAS28(ESR)≤ 3.2 (low disease activity), DAS28(ESR)< 2.6 (remission), The European League Against Rheumatism (EULAR) Good response, EULAR Good or Moderate response, and a decrease in CRP from baseline > 50%. The proportion of patients exhibiting low disease activity (LDA) was also included as a long-term outcome.
DAS28(ESR) at year 1 was evaluated at 52–56 weeks after the initiation of treatment with CZP. Last observation carried forward (LOCF) imputation was used for the data in patients who did not re-consent to enter the OLE, received rescue medication, or withdrew from the trials. For patients without mTSS data at week 52, linear extrapolation of mTSS taken at an earlier time point or at the withdrawal visit was used to estimate the mTSS at week 52. Week 40 data of mTSS was used for extrapolation in 11 and 24 patients of J-RAPID and HIKARI studies, respectively. In four patients of J-RAPID and eight patients of HIKARI, mTSS obtained at other time points were used. Remaining 66 and 82 patients of J-RAPID and HIKARI studies had mTSS data at week 52. Radiographic remission and rapid radiographic progression (RRP) were defined as a change in mTSS score of ≤ 0.5 and > 3, respectively, from baseline to week 52. Positive predictive values (PPVs) (number of patients who response at 1 year/number of responders at week 12), negative predictive values (NPVs) (number of patients who did not respond at 1 year/number of non-responders at week 12), and odds ratios with 95% confidence intervals have been determined. The influence of baseline disease characteristics on probability of LDA or remission was also investigated. For these analyses, patients were stratified by baseline CRP (higher or lower than the median), baseline DAS28(ESR) (higher or lower than the median), disease duration (> or ≤ 5 years), or rheumatoid factor (RF) positivity. Interaction between these baseline characteristics and week 12 response was determined by the logistic regression analysis. The patients were also stratified based on their concomitant treatment (none, with or without MTX, with disease-modifying anti-rheumatic drugs [DMARDs] other than MTX, without any DMARDs). Interaction between concomitant DMARDs use and week 12 response was also determined by logistic regression analysis in HIKARI.
Results
Patient characteristics and relevant clinical efficacy of CZP in J-RAPID and HIKARI
In J-RAPID, 87.8 and 76.8% of the CZP (200 mg)-treated patients had ≥ 0.6 and ≥ 1.2 DAS28(ESR) responses at week 12, respectively (). In HIKARI, 88.8% and 74.1% of CZP (200 mg)-treated patients had ≥ 0.6 and ≥ 1.2 DAS28(ESR) responses at week 12, respectively (). One year after treatment, DAS28(ESR) remission was achieved by 27/82 (32.9%) of patients in J-RAPID and by 32/116 (27.6%) of patients in HIKARI treated with CZP (200 mg).
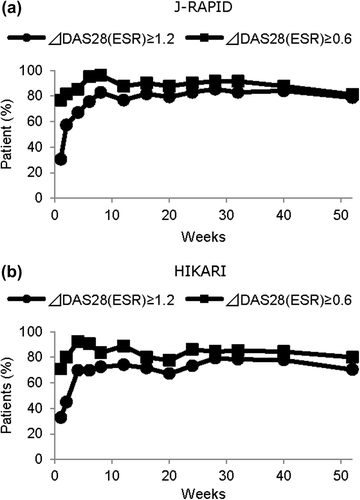
The overall relationship between change in DAS28(ESR) and long-term outcome of CZP-treated patients
Logistic regression of remission at 1 year for change in DAS28(ESR) from baseline at week 1 through week 40 in J-RAPID and at week 4 through week 40 in HIKARI revealed p values < 0.05. The coefficients of determination at week 4 in J-RAPID and HIKARI were 0.04 and 0.05, respectively, and gradually increased to 0.10 and 0.14 at week 12, 0.19 and 0.19 at week 24, respectively. The AUCs under the ROCs, in J-RAPID and HIKARI were 0.65 and 0.65 at week 4, 0.70 and 0.74 at week 12, 0.79 and 0.78 at week 24, respectively.
Prediction of long-term outcome of CZP-treated patients by week 12 DAS28(ESR) response
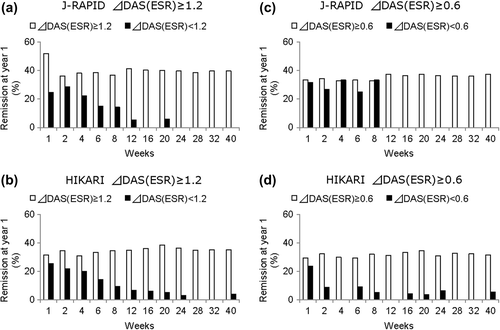
Using a cut-off of a DAS28(ESR) change of ≥ 1.2, the remission rates of each group at 1-year post CZP treatment were determined. Although almost 25% of patients in both the J-RAPID and HIKARI of the week 1 non-responders achieved remission at 1 year (), their remission rate dropped off when the DAS28(ESR) responsiveness was determined at later time points. In J-RAPID and HIKARI, 41.3% (26/63) and 34.9% (30/86) of week 12 responders achieved remission at 1 year, respectively, compared to a < 7% probability of the non-responders achieving remission at 1 year (). When a cut-off of a DAS28(ESR) change of ≥ 0.6 was used to determine response at week 12, a similar trend was observed. Using a cut-off score of 0.6 was better at identifying patients with low probability to achieve remission at 1 year, as none of the patients with a DAS28(ESR) response of < 0.6 at week 12 achieved remission at 1 year in both J-RAPID and HIKARI. However, patients with a DAS28(ESR) response of ≥ 0.6 at week 12 had a slightly lower rate of remission at 1 year compared to patients with a DAS28(ESR) response of ≥ 1.2 (). PPVs, NPVs, and odds ratios of each time points are provided in supplement 1.
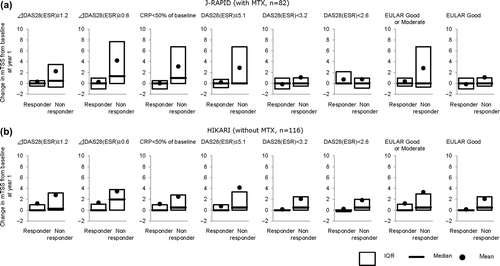
We next evaluated whether the DAS28(ESR) response measured on either week 1, 6, or 12 could predict the degree of radiographic progression of disease after 1 year of continued CZP treatment. When DAS28(ESR) response was determined at week 1, no differences in radiographic progression at 1 year were observed in responders vs. non-responders in both studies. However, when response was determined at later time points, differences in radiographic disease progression between the responding and non-responding group became apparent. In both J-RAPID and HIKARI, patients with a response in DAS28(ESR) of ≥ 1.2 at week 12 exhibited lower radiographic progression than those patients who did not respond. In J-RAPID, the mean change in mTSS at 1 year was 0.29 in responder patients (DAS28(ESR) change ≥ 1.2 at week 12) compared to 2.23 in non-responders (). Similarly, in HIKARI, the mean change in mTSS at 1 year was 1.23 in responder patients (DAS28(ESR) change ≥ 1.2 at week 12) compared to 2.79 in non-responders (). In both studies, proportion of radiographic non-progression was higher and proportion of RRP was lower in responders compared to non-responders at week 6 and 12 ().
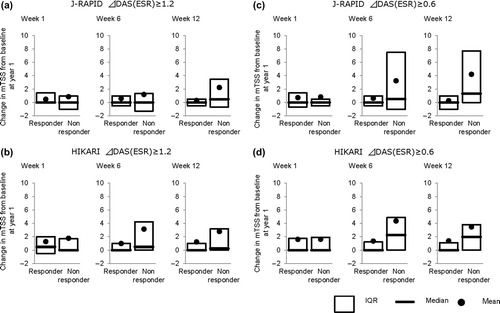
Table 1. Proportions of radiographic non-progression and rapid-radiographic progression after 1 year treatment by response at week 1, 6, and 12 in patients with active rheumatoid arthritis treated with certolizumab pegol with or without MTX.
When a cut-off of DAS28(ESR) response of ≥ 0.6 was used instead of ≥ 1.2, a similar trend was observed although patients with a DAS28(ESR) response of < 0.6 at week 12 in J-RAPID had greater radiographic progression at 1 year compared to patients with a DAS28(ESR) response of < 1.2. In J-RAPID, the patients with a DAS28(ESR) response of < 0.6 at week 12 had a mean change in mTSS of 4.20 at 1 year (). In HIKARI, the patients with a DAS28(ESR) response of < 0.6 at week 12 had a mean change in mTSS of 3.46 at 1 year (). Higher proportions of radiographic non-progression and lower proportions of RRP in responders compared to non-responders were also seen at week 6 and 12 (). Thus, the DAS28(ESR) response at week 12 seems to be predictive of the degree of radiographic progression after 1 year of CZP treatment.
Prediction of long-term CZP-treatment outcome by week 12 response as defined by several week 12 response criteria
Next, we assessed how week 12 DAS28(ESR) response would compare to the other criteria of response in predicting the long-term treatment outcomes. These criteria of responsiveness at week 12 included absolute week 12 DAS28(ESR) scores (DAS28(ESR) ≤ 5.1, DAS28(ESR) ≤ 3.2, or DAS28(ESR) < 2.6), EULAR responses (Good, Good/Moderate), or a > 50% decrease in CRP from baseline. For the long term outcomes, in addition to remission, LDA was also included in this analysis. The odds ratios of the prediction of LDA or remission were statistically higher than 1 based on 95% confidence intervals in most of all response cut-off criteria tested (DAS28 changes, absolute DAS28 scores, EULAR responses, CRP value). The NPV of both LDA and remission increased when higher cut-offs for absolute DAS28(ESR) scores were used (5.1 > 3.2 > 2.6; ). Likewise, the NPV for LDA and remission increased when responders included Good/ Moderate EULAR responses compared to Good EULAR responses alone (). Although some of these criteria (DAS ≤ 5.1, Good/Moderate EULAR, CRP < 50% of baseline) displayed similar NPVs compared to DAS28(ESR) change ≥ 1.2, none of them were as good at predicting long-term non-response of non-responders at achieving LDA or remission than DAS change ≥ 0.6. PPVs were similar for all week 12 criteria except for low DAS scores (DAS28(ESR) ≤ 3.2, or DAS28(ESR) < 2.6) and Good EULAR response (), which showed a higher PPV than DAS28(ESR) change ≥ 1.2.
Table 2. Probability of low disease activity or remission after 1 year treatment by response at week 12 in patients with active rheumatoid arthritis treated with certolizumab pegol with or without MTX.
Similar to the analysis performed with LDA and remission rates, we compared the 1 year change in mTSS scores of week 12 DAS28(ESR) responders (DAS28(ESR) change ≥ 1.2 or ≥ 0.6) to other responder criteria. All responder criteria including absolute DAS28(ESR) scores, EULAR responses, and > 50% CRP reduction from baseline resulted in higher mTSS scores in the non-responder compared to the responder population (). Tendency of higher proportion of radiographic non-progression and lower proportion of RRP was also demonstrated for all week 12 criteria (data not shown).
Prediction of long-term CZP treatment outcome, regardless of disease severity by week 12 DAS28(ESR) responses
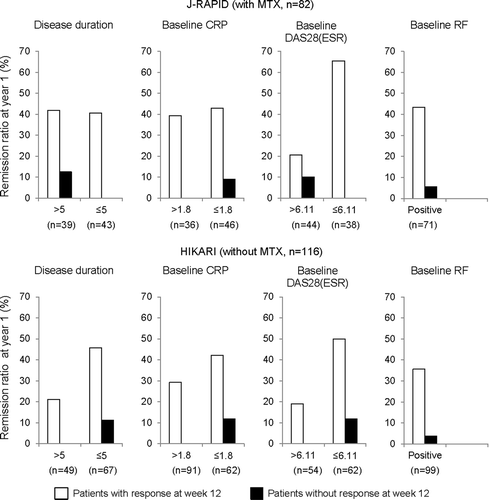
To determine whether the baseline characteristics of disease affected the predictability of long-term treatment outcome, we stratified the responder and non-responder populations based on disease characteristics at baseline. In this analysis, responders were defined by a week 12 DAS28(ESR) change ≥ 1.2. Four different parameters (baseline CRP > 1.8, disease duration > 5 years, baseline DAS28(ESR) > 6.11, baseline RF ≥ 14) were used to breakdown the population by disease characteristics.
Lower remission rates was observed in patients with baseline CRP > 1.8 compared to patients with baseline CRP ≤ 1.8 (30.6% vs. 34.8% in J-RAPID and 23.0% vs. 32.7% in HIKARI). Same tendency was observed regarding baseline DAS28(ESR) (18.2% vs. 50.0 in J-RAPID and 14.5% vs. 39.3% in HIKARI). Lower remission rate in patients with longer disease duration was shown in HIKARI (16.0% vs. 36.4%), whereas no such tendency was observed in J-RAPID (35.9% vs. 30.2%).
The week 12 responder population showed increased rates of remission in both studies irrespective of baseline disease characteristics (). Interaction between week 12 DAS28(ESR) change and baseline CRP, disease duration, and baseline DAS28(ESR) were not significant by logistic regression analysis, except for week 12 DAS28(ESR) change ≥ 1.2 and baseline DAS28(ESR) in J-RAPID study. Although there was no statistically significant difference between responder and non-responder patients with higher baseline DAS28(ESR) in J-RAPID study, the patient in remission at year 1 was one out of 10 non-responders, and the remission rate at year 1 in non-responders was numerically lower compared to that in responders. PPVs, NPVs, odds ratios, and p values for interactions are provided in supplement 2.
Prediction of long-term outcomes of CZP-treated patients by week 12 DAS28(ESR) response not affected by concomitant therapy
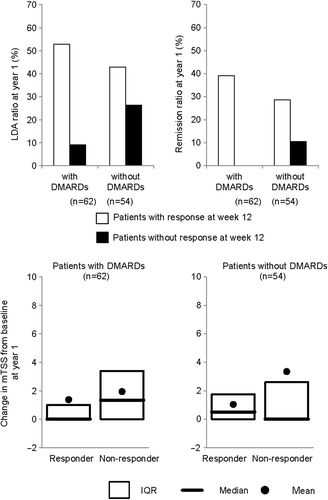
We next tested whether differences in concomitant treatment impacted the predictability of long-term outcomes based on DAS28(ESR) response to CZP at week 12. We stratified the population based on whether the patients were treated with or without DMARDs other than MTX in HIKARI patients. When limiting the analysis to the population treated without any additional DMARDs, the favorable long-term outcomes of the responder population were decreased but the outcomes of the non-responder population showed low probability of remission (). No significant interaction between DAMRDs use and week 12 DAS28(ESR) response was observed by logistic regression analysis. Smaller progression of joint destruction was also shown in both patients with and without DMARDs. Concomitant DMARDs appeared to have minimal impact on predictability of long-term outcomes based on week 12 DAS28(ESR) responses (). PPVs, NPVs, odds ratios, and p value for interaction are provided in supplement 3.
Discussion
The treatment of RA has been revolutionized by the advent of biologics such as anti-TNF agents. These agents are recommended for patients who failed first DMARDs and have poor prognostic factors, such as very active disease or early structural damage, and patients who failed second conventional synthetic DMARDs strategy in EULAR recommendations [Citation7]. In ACR recommendations, patients with established RA who failed non-biologic DMARDs therapy and patients with early RA in high disease activity who have poor prognostic features [Citation8] were recommended to treat with anti-TNFs. Remission is recommended as primary target of treatment of rheumatoid arthritis [Citation9], and substantial part of patients can achieve remission with anti-TNF treatment. However, some patients still remain in active disease even after treatment with anti-TNFs. Currently we cannot predict clinical long-term outcomes in individual patients before starting treatment.
In the treat-to-target recommendations, it is recommended that drugs therapy should be adjusted at least every 3 month until the desired treatment target is reached. In patients in whom the disease activity does not show major improvement within 3 months, changing the drug regimen may have to be considered at that point in time [Citation9]. Aletaha et al. reported that the level of disease activity at baseline and during the first 3 months of treatment is significantly related to the level of disease activity at 1 year [Citation10]. Alternative treatment strategies for non-responding patients can be devised at an early time point which could limit further progression of disease caused by ineffective treatment. The post-hoc analyses of the RAPID1 study showed that patients with improvement of DAS28(ESR) less than 1.2 after 3 months have very little chance to be in low disease activity 1 or 2 years after starting treatment with CZP [Citation6].
In this analysis, change in DAS28(ESR) after starting of treatment with CZP seemed overall fairly associated with remission after 1 year. Although this association seemed apparent after 4 weeks treatment with CZP in both studies, the coefficients of determination and AUCs under the ROCs were too low to predict accurately whether a patient achieves the desired long-term outcome. Then we hypothesized that an early response to treatment could be used to identify patients who are unlikely to receive benefit from prolonged CZP treatment. We began our analyses by testing which early time points after treatment were best at predicting this outcome. In the EULAR response criteria [Citation11,Citation12], change of DAS28(ESR) 1.2 and 0.6 are the minimal changes to be classified as “good” or “moderate” response. So, we initially used the change in DAS28(ESR) score from baseline (≥ 1.2 or ≥ 0.6) to divide the patients into responders and non-responders. Most of patients showed response within 12 weeks and response was sustained during 1 year of treatment in both studies when response was defined as improvement of DAS28(ESR) 0.6 or 1.2. Although little difference was noted between the responders and non-responders at week 1, by week 12 there was a clear difference in remission rates at year 1 between the two groups. In addition, we observed that the magnitude of the DAS28(ESR) response at week 12 inversely correlated with long term radiographic disease progression. Thus, an early response to treatment measured at week 12 seemed best at predicting long-term outcomes compared to responses measured earlier than 12 weeks.
Having established week 12 as the best time point to determine early treatment responses, we next compared the ability of different criteria of response to predict long-term outcomes. The odds ratios of the prediction of LDA or remission were statistically higher than 1 based on 95% confidence intervals in most of all response cut-off criteria tested (on DAS28(ESR) changes, absolute DAS28(ESR) scores, EULAR responses, CRP value), indicating that many early response parameters at week 12 are predictive of long-term outcomes. However, among all the criteria tested, the DAS28(ESR) response of ≥ 0.6 exhibited the highest NPV. The higher the NPV, the higher the association between early non-response and long-term non-response. Using a stringent cut-off value such as a DAS28(ESR) response < 0.6 for non-responders increases the NPV and hence, decreases the chance of capturing patients that are false negatives (those who will eventually respond to CZP treatment despite being an early non-responder). However, at the same time, the number of non-responders that meet this criterion is decreased (12.2% in J-RAPID and 11.2% in HIKARI non-responders when cut-off is 0.6 vs. 23.2% in J-RAPID and 25.9% in HIKARI non-responders when cut-off is 1.2). Use of less stringent non-response criteria enable to identify nearly twice as many patients (22.0% (18/82) in J-RAPID and 24.1% (28/116) in HIKARI for a 1.2 cut-off vs. 12.2% (10/82) in J-RAPID and 11.2% (13/116) in HIKARI for a 0.6 cut-off) who are unlikely to respond to continued CZP treatment. It is difficult to determine whether it is beneficial to identify more non-responding patients at the expense of removing treatment from a fraction of patients who would eventually respond. Although this decision would ultimately lie on the physician and should be considered in relation to other clinical parameters and disease characteristics, cut-off value of DAS28(ESR) 1.2 is better than 0.6 to find-out patients with lower probability of remission after long-term treatment with CZP.
Our data suggest that the week 12 DAS28(ESR) 1.2 response predicts long-term outcomes regardless of baseline disease status or concomitant therapy. Obviously, patients with higher disease activity and CRP, or longer disease duration at baseline tended to have worse long-term outcomes. However, even in these patients, week 12 DAS28(ESR) responders showed an increased probability of remission. Similarly, concomitant treatment with DMARDs had minimal effect on the ability of week 12 DAS28(ESR) responses to predict long-term outcome. In general, there was always a lower probability of remission in non-responders compared to responders.
In our recent post-hoc analysis, CZP treatment without loading dose showed delayed initial kinetics of ACR20/50/70 responses and sustained lower response to week 24 compared to CZP treatment with loading dose. Similar safety profiles were reported between CZP treatment with and without loading dose [Citation13]. Rapid onset of response and sustained efficacy in patients treated by CZP with loading dose are thought to result in the predictability of outcomes after long-term treatment through its rapid and sustained efficacy of CZP without any additional safety concern.
Our data are in agreement with a recently reported post-hoc study performed on patients on the RAPID1 multicenter clinical trial [Citation5,Citation6]. Previous study compared the DAS28(ESR) change to long-term LDA scores using multiple cut-offs at different time points after treatment [Citation6]. Similar to our data, DAS28(ESR) responses measured at week 12 were best at predicting long-term outcome. Given that the ethnicity of the patient populations tested in these two studies were very different, these data suggest that the early DAS28(ESR) response could be generalizable to a broad population. In addition to LDA, our analyses revealed that DAS28(ESR) non-response had impact on remission rates and in radiographic disease progression. We also compared the DAS28(ESR) changes to other responder criteria and conclude that while many parameters indicated early response to treatment predicts long-term outcome, DAS28(ESR) responses exhibited the best NPVs in identifying long-term non-responders.
The limitations of this report include the post-hoc design of our analyses compared with prospective approaches. Relatively smaller numbers of subjects in sub-group analyses is also a limiting factor to obtain firm conclusions, but sub-group analyses in this report, stratifying patients with baseline disease characteristics or concomitant medications, showed consistent tendency of poor long-term outcome and higher progression of joint damage in patients without early response. Additionally, patients’ characteristics were limited compared to patients in real world clinical practice in Japan. Patients included in this analysis have had relatively higher baseline disease activity compared to patients seen in real world clinical practice in Japan. Proportion of patients with prior anti-TNF treatment was low because of inclusion criteria of J-RAPID and HIKARI studies. Although likelihood of achieving LDA at 28 weeks could be predicted based on the initial change in DAS28 in broad RA population, including patients with prior anti-TNF exposure, from the phase IIIb REALISTIC study [Citation14], further work in a broader population of patients is required to confirm the findings.
In conclusion, we propose that an early response to CZP at week 12 as measured by DAS28(ESR) changes predicts long-term probability of LDA and remission, and progression of joint destruction. This information could be useful as a prognostic indicator for responder patients and for identifying patients that are unlikely to respond to prolonged CZP treatment. Thus, assessment of an early response to CZP at week 12 is a valuable tool to make an informed decision on changes in CZP therapy.
Supplementary material available online
imor_a_904475_sm7284.pdf
Download PDF (29.1 KB)Acknowledgements
We thank the investigators who were part of J-RAPID and HIKARI studies. This study was funded by UCB. Ikuko Kambayashi provided editorial services for this manuscript. All costs associated with development of this manuscript were funded by UCB.
Conflict of interest
T. Takeuchi has received grants from Abbott Japan Co., Ltd., Astellas Pharma, Bristol–Myers K.K., Chugai Pharmaceutical Co, Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Sanofi–Aventis K.K., Santen Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., AbbiVie GK, Asahikasei Pharma Corp., and Taisho Toyama Pharmaceutical Co., Ltd., speaking fees from Abbott Japan Co., Ltd., Bristol–Myers K.K., Chugai Pharmaceutical Co,. Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Astellas Pharma, and Diaichi Sankyo Co., Ltd., and consultant fees from Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Co., Asahi Kasei Medical K.K., AbbiVie GK, and Daiichi Sankyo Co., Ltd.
K. Yamamoto has served as a consultant for UCB, Pfizer, Abbott, BMS, Roche, Chugai, Mitsubishi-Tanabe and Eisai and has received research funding from UCB, Pfizer, Abbott, Santen, Mitsubishi-Tanabe and Eisai.
H. Yamanaka have received honorarium for the lecture from AbbVie, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Pfizer, Takeda, Teijin Pharma. H Yamanaka have received research grant from AbbVie, Asahikasei Pharma, Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, MSD, Nippon Kayaku, Pfizer, Santen, Taishotoyama, Takeda, Teijin Pharma.
N. Ishiguro has received research funding from Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, Bristol-Myers Squibb, Eisai, Daiichi Sankyo Company Ltd,Janssen, Kaken and Pfizer and has served on speaker bureaus for Daiichi Sankyo Company Ltd, Takeda Pharmaceutical Co Ltd, Hisamitsu Pharmaceutical Co Inc, Otsuka Pharmaceutical Co Ltd, Taisho Toyama Pharmaceutical Co Ltd, Kaken Pharmaceutical Co Ltd, Eisai Co Ltd, Janssen Pharmaceutical K.K, Bristol-Myers Squibb, Abbott Japan, Chugai Pharmaceutical Co Ltd, Mitsubishi Tanabe Pharmaceutical, UCB Japan, Astellas Pharma Inc, and Pfizer Japan Inc.
Y. Tanaka, has received consulting fees, speaking fees, and/or honoraria from Mitsubishi-Tanabe, Eisai, Chugai, Abbott Japan, Astellas, Daiichi-Sankyo, AbbVie, Janssen, Pfizer, Takeda, Astra-Zeneca, Eli Lilly Japan, GlaxoSmithKline, Quintiles, MSD, Asahi-Kasei and has received research grants from Bristol-Myers, Mitsubishi-Tanabe, AbbVie, MSD, Chugai, Astellas, Daiichi-Sankyo.
K. Eguchi has served as a consultant for UCB.
A. Watanabe has received research support from Daiichi-Sankyo, Kyorin, Shionogi, Taisho, Dainippon-Sumitomo, Taiho, Toyama Chemical and Meiji Seika and has served on speaker bureaus for MSD, GSK, Shionogi, Daiichi-Sankyo, Taisho-Toyama, Dainippon-Sumitomo, Mitsubishi-Tanabe and Pfizer.
H. Origasa has served as a consultant for UCB and Astellas.
T. Shoji is an employee of UCB.
N. Miyasaka has received research support from Pfizer, Takeda, Mitsubishi-Tanabe, Chugai, Abbott, Eisai and Astellas.
T. Koike has served on speaker bureaus for UCB, Pfizer, Chugai, Abbott, Mitsubishi-Tanabe, Takeda, Eisai, Santen, Astellas, Taisho-Toyama, BMS, Teijin and Daiichi-Sankyo.
References
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol plus methotrexate in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: The J-RAPID randomized, placebo-controlled trial. Mod Rheumatol. 2013; (epub ahead of print) doi:10.3109/14397595.2013.864224.
- Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Efficacy and safety of certolizumab pegol without methotrexate co-administration in Japanese patients with active rheumatoid arthritis: The HIKARI randomized, placebo-controlled trial. Mod Rheumatol. 2013; (epub ahead of print) doi:10.3109/14397595.2013.843764.
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients with an inadequate response to methotrexate: 52-week results from an open-label extension of the J-RAPID study. Mod Rheumatol. 2014; (epub ahead of print) doi: 10.3109/14397595.2014.881709.
- Tanaka Y, Yamamoto K, Takeuchi T, Yamanaka H, Ishiguro N, Eguchi K, et al. Long-term efficacy and safety of certolizumab pegol in Japanese rheumatoid arthritis patients who could not receive methotrexate: 52-week results from an open-label extension of the HIKARI study. Mod Rheumatol. 2013; (epub ahead of print) doi: 10.3109/14397595.2013.865822.
- Keystone EC, Curtis JR, Fleischmann RM, Furst DE, Khann D, Smolen JS, et al. Rapid improvement in the signs and symptoms of rheumatoid arthritis following certolizumab pegol treatment predicts better longterm outcomes: post-hoc analysis of a randomized controlled trial. J Rheumatol. 2011;38(6):990–6; doi:10.3899/jrheum.100935
- van del Heijde D, Keystone EC, Curtis JR, Landewé RB, Schiff MH, Khanna D, et al. Timing and magnitude of initial change in disease activity score 28 predicts the likelihood of achieving low disease activity at 1 year in rheumatoid arthritis patients treated with certolizumab pegol: A post-hoc analysis of the RAPID 1 trial. Rheumatol.2012;39(7):1326–33; doi:10.3899/jrheum.111171
- Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. published online Octorber25, 2013. doi: 10.1136/annrheumdis-2013-204573.
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–39. doi:10.1002/acr.21641.
- Smolen JS, Aletaha D, Fijlsma JWJ, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7; doi:10.1136/ard.2009.123919
- Aletaha D, Funovits J, Keystone EC, Smolen JS. Disease activity early in the course of treatment predicts response to therapy after one year in rheumatoid arthritis patients. Arthritis Rheum. 2007; 56(10):3226–35.
- van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European league against rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism Criteria. Arthritis Rheum. 1996;39(1):34–40.
- Welsing PMA, van Riel PLCM. The Nijmegen inception cohort of early rheumatoid arthritis. J Rheumatol. 2004;69:14–21.
- Takeuchi T, Yamamoto K, Yamanaka H, Ishiguro N, Tanaka Y, Eguchi K, et al. Post-hoc analysis showing better clinical response with the loading dose of certolizumab pegol in Japanese patients with active rheumatoid arthritis. Arthritis Rheum. 2013;65(Suppl.10): S989–S989.
- Weinblatt M, Fleishmann R, Davis O, Luijtens K, van del Heijde D. Timing and magnitude of initial response to certolizumab pegol in a broad population of patients with active rheumatoid arthritis predicts likelihood of LDA at week 28. Ann Rheum Dis. 2012;71(suppl3):520.