Abstract
Objective: To clarify the efficacy and safety of abatacept for secondary Sjögren’s syndrome (SS) associated with rheumatoid arthritis (RA).
Methods: The primary endpoint of this open-labeled, prospective, observational multicenter study for secondary SS with RA was the remission rate of Simplified Disease Activity Index (SDAI) at 52 weeks after initiation of abatacept. The secondary endpoints included Saxon’s test and Schirmer’s test. Adverse events and adherence rate during the study period were also analyzed.
Results: Thirty-six patients (all females) were enrolled in this study. The mean SDAI decreased significantly from 20.6 ± 11.2 (±SD) at baseline to 10.0 ± 10.5 at 52 weeks (p < 0.05). Patients with SDAI remission increased from 0 (0 week) to 12 patients (33.3%) at 52 weeks. Saliva volume assessed by Saxon’s test increased significantly from 2136 ± 1809 (0 week) to 2397 ± 1878 (24 weeks) mg/2 min (n = 34, p < 0.05). Saliva volume increased significantly from 2945 ± 2090 (0 week) to 3419 ± 2121 (24 weeks) mg/2 min in 11 patients with Greenspan grade 1 or 2 of labial salivary gland biopsy (p < 0.05), but no change was noted in 18 patients with Greenspan grade 3 or 4. Tear volume by Schirmer’s test increased significantly from 4.2 ± 4.8 (0 week) to 6.4 ± 7.8 (24 weeks) mm/5 min (n = 30, p < 0.05). The adherence rate to abatacept was 80.6% (29/36) over the 52-week period. Twelve adverse events occurred in 10 of the 36 patients, and 7 of these events were infections.
Conclusion: Abatacept seems to be effective for both RA and SS related manifestations.
Introduction
Sjögren’s syndrome (SS) is an autoimmune disease characterized pathologically by lymphocytic infiltration into the exocrine glands (including salivary and lacrimal glands) and clinically by dry mouth and dry eyes. SS is classified into primary SS, which is not associated with any other well-defined connective tissue disease (CTD), and secondary SS, which is associated with other well-defined CTD [Citation1]. With regard to secondary SS, all-Japan survey on the epidemiology of SS showed previously that secondary SS was associated with rheumatoid arthritis (RA) in 38.7% of the cases, and systemic lupus erythematosus (SLE) in 22.2% [Citation2]. These data highlight the clinical importance of secondary SS associated with RA.
Various types of biologics that target immune cells, cytokines, and cytokine receptors have been introduced for the treatment of diverse autoimmune diseases such as RA, SLE, inflammatory bowel disease, psoriasis, and systemic vasculitis [Citation3–7]. Although the usefulness of biologics for SS has not been established yet, the most encouraging results were obtained from randomized controlled trials (RCTs) of rituximab, a B cell targeting biologic, including its efficacy in the control of the disease especially for extra-glandular manifestations [Citation8–11]. However, a more recent large RCT of rituximab (TEARS trial) demonstrated that rituximab failed to improve glandular and extra-glandular manifestations of SS [Citation12]. Thus, while the efficacy of rituximab against extra-glandular involvements of SS has been suggested, its usefulness for glandular involvements is controversial at present.
With regard to other biologics, TNF inhibitors (infliximab and etanercept) are reported to have no significant effects on glandular or extra-glandular manifestations of SS [Citation13,Citation14]. Although B cell activating factor (BAFF) is a promising biologic for SS, belimumab, a monoclonal anti-BAFF antibody, did not improve salivary flow or lacrimation, although it significantly decreased a composite of disease activity measures, including the European League Against Rheumatism (EULAR) Sjögren’s Syndrome Disease Activity Index (ESSDAI) and EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI) [Citation15]. Another phase III RCT is currently being conducted to examine the effects of tocilizumab, a monoclonal anti-IL-6 receptor antibody, versus placebo, in SS patients [Citation8].
The effects of T cell-targeting biologic, abatacept (CTLA4-Ig), on SS were examined recently. Abatacept was designed for the treatment of SS manifestations based on the pathogenic role of CD4+ T cells in SS [Citation16,Citation17]. Actually, two open label pilot studies have so far examined the effectiveness of abatacept in primary SS [Citation18,Citation19]. Adler et al. [Citation18] showed that abatacept significantly reduced salivary gland inflammation, and increased saliva production when the dose was adjusted for disease duration. Meiners et al. [Citation19] reported that while abatacept improved ESSDAI, ESSPRI, and laboratory tests in patients with early and active primary SS, it had a minor beneficial effect on preservation of salivary and lacrimal gland functions. Furthermore, we designed an open-labeled, 1-year, prospective, observational, and multicenter study (ROSE trial; Rheumatoid Arthritis with Orencia Trial toward Sjögren’s syndrome Endocrinopathy) to establish the efficacy and safety of abatacept in secondary SS patients associated with RA [Citation20]. Interim analysis of 32 patients from the ROSE trial after 24 weeks of treatment demonstrated that abatacept improved both SS and RA manifestations of secondary SS with RA. With accumulation of more data, we present in this report the results of 36 patients after 52 weeks of ROSE trial.
Patients and methods
Patients
Patients aged more 20 years diagnosed with RA according to the American College of Rheumatology (ACR) 1987 [Citation21] or ACR/EULAR 2010 criteria [Citation22], and SS according to the 1999 Japanese Ministry of Health criteria for the diagnosis of SS [Citation23], who presented with sicca symptoms were eligible for this study. The 1999 Japanese Ministry of Health criteria for the diagnosis of SS include the presence of two or more of the following four clinicopathological findings: (1) lymphocytic infiltration of the salivary or lacrimal glands, (2) dysfunction of salivary glands, (3) keratoconjunctivitis sicca (KCS), and (4) presence of anti-SS-A or SS-B antibodies [Citation23]. The patients were followed up at the Departments of Rheumatology of three hospitals in Japan (University of Tsukuba hospital, University of Occupational and Environmental Health hospital, and Nagasaki University hospital). Approval for this study was obtained from the local ethics committees of each study site and a signed informed consent was obtained from each subject (approval number of University of Tsukuba: H23-29, certification date of approval: July 28, 2011). This study was registered at the University Hospital Medical Information Network (UMIN)-Clinical Trials Registry (CTR) (UMIN-ID: UMIN000005724).
Patients with contraindications for abatacept (e.g. hypersensitivity, severe infection, and hepatitis B virus infection), aged more than 75 or less than 20 years, leukopenia (leukocyte count ≤3000/mm2), severe liver or kidney diseases, severe hematological disorders, negative for both anti-SS-A and SS-B antibody and positive for anti-centromere antibody were excluded from this study. Patients who were pregnant, nursing, wanted to get pregnant, and those treated with palliative therapies for dryness including cevimeline, anetholtritione, and pilocarpine within the last 4 weeks were also excluded. We also excluded patients who were considered not suitable for this study by the attending physician.
Medications
The dosing regimen approved for the treatment of RA was used in this study. The weight-adapted dose of abatacept (500 mg for patients weighing less than 60 kg, and 750 mg for those weighing ≥60 kg) was administrated intravenously at weeks 0, 2, and 4, every 4 weeks, over a period of 1 year. Other disease-modifying anti-rheumatic drugs (DMARDs), corticosteroids, and non-steroidal anti-inflammatory drugs were allowed to be used during the 1-year treatment period based on the clinical judgment of the attending physician.
Analysis of efficacy of abatacept
ROSE trial was designed as an open-labeled, 1-year, prospective, and observational study. For RA manifestations, the number of tender and swollen joints among 28 joints, physicians’ global visual analog scale (VAS), patients’ global VAS, Simplified Disease Activity Index (SDAI), disease activity score (DAS) 28-erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), serum CRP, ESR, and rheumatoid factor (RF) were assessed at weeks 0 (baseline), 4, 12, 24, and 52. For SS manifestations, patients’ VAS for dry mouth, dry eye, and parotid pain, physicians’ VAS for dry mouth, KCS, and general condition, saliva volume by Saxon’s test, tear volume by Schirmer’s test, anti-SS-A/SS-B antibody, and serum IgG level were examined at weeks 0, 12, and 24. Because assessment for SS manifestations requires the examinations by ophthalmologists and has some limitations of availability in observational study, we excluded the assessment for SS manifestations at week 52 in this study. When available, magnetic resonance imaging (MRI) of parotid glands was also performed at weeks 0 and 52. Findings of parotid MRI by T1-weighted and short inversion time inversion recovery (STIR) sequence were visually evaluated and classified into five grades according to the methods reported previously by our group [Citation24]. Briefly, we assessed the degree of high-intensity areas on T1-weighted image (T1WI) and STIR image; grade 0: homogenous signal intensity on T1WI and no focal high intensity spots on STIR images; grade 1: sparse high signal intensity areas on T1WI and no focal high intensity spots on STIR images; grade 2: sparse high signal intensity areas on T1WI and presence of focal high intensity spots on STIR images; grade 3: moderate high signal intensity areas on T1WI images and presence of focal high intensity spots on STIR images; and grade 4: diffuse high signal intensity area on T1WI and no focal high intensity spots on STIR images [Citation24].
The primary endpoint was the percentage of patients who achieved clinical remission by SDAI at 52 weeks. Secondary endpoints included CRP, ESR, RF, Saxon’s test, Schirmer’s test, anti-SS-A/SS-B antibody, IgG, patients’ VAS, physicians’ VAS, and parotid MRI.
Analysis for safety of abatacept
Adverse events (AEs) during the 52-week study period were analyzed at each visit. The type of AEs, onset, use of corticosteroids, treatment for AEs, hospital admission, cessation of use of abatacept, association with abatacept, and outcome were recorded. We also recorded the adherence rate to abatacept for 52 weeks and causes of discontinuation of abatacept.
Statistical analysis
Data are expressed as mean ± SD. Differences between baseline and after treatment with abatacept were examined for statistical significance using the Wilcoxon signed-rank test. Differences between groups were examined using the Mann–Whitney U test for continuous variables, and Cochran-Armitage Test for disease activity as assessed by SDAI. Correlation between variables was assessed by Spearman's rank correlation. A p value less than 0.05 denoted the presence of a statistically significant difference. The adherence rate to abatacept was analyzed by Kaplan–Meier method. The deficit of data was compensated by the last observation carried forward (LOCF) method.
Results
Clinicopathological features at baseline
Thirty-six patients (all females) were enrolled in this study. The baseline clinicopathological features of the 36 patients are summarized in . The mean age was 54.9 ± 14.0 years and RA disease duration was 116.5 ± 137.5 months. More than half of the patients were assessed as Stage I or II, and Class 1 or 2 (Steinbrocker classification). The RA disease activity assessed by DAS28 and SDAI was moderate. For SS manifestations, salivary volume assessed by Saxon’s test was 2136 ± 1809 mg/2 min (n = 34), tear volume by Schirmer’s test was 4.2 ± 4.8 mm/5 min (n = 30), Greenspan grading of labial salivary gland (LSG) biopsy was grade 1 or 2 in 12 patients, and grade 3 or 4 in 19 patients. MRI of the parotid glands was performed in 12 patients at baseline, showing grade 1 in 3 patients, grade 2 in 5 patients, grade 3 in 2 patients, and grade 4 in 2 patients. Corticosteroids were used in 17 of 36 (47.2%) patients, and the mean equivalent dose of prednisolone (PSL) was 5.1 ± 2.6 mg/day. Methotrexate (MTX) was administered in 75% of patients (27/36 patients), at a mean dose of 9.3 ± 3.9 mg/week. Furthermore, 19.4% of the patients (7/36 patients) were treated previously with biologics other than abatacept, while 29 patients were biologics-naïve. Collectively, the enrolled patients had secondary SS with moderate dryness, in addition to moderately active established RA.
Table 1. Baseline clinicopathological features of the 36 enrolled patients.
Effectiveness of abatacept against RA
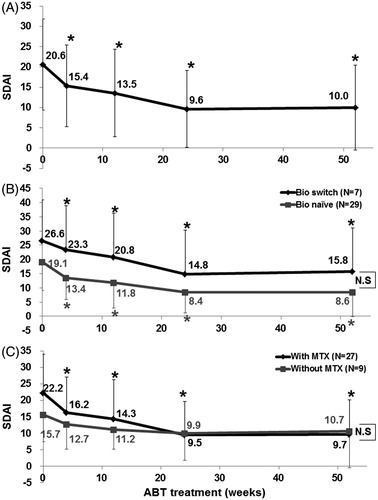
Disease activity of RA assessed by SDAI significantly decreased after treatment with abatacept from 20.6 ± 11.2 (0 week, baseline) to 10.0 ± 10.5 (52 weeks) (p < 0.05). Significant reduction of SDAI relative to baseline (p < 0.05) was noted at 4 weeks, and maintained over the 52-week period (). Comparison of bio-switch patients with bio-naïve patients showed significant improvements in SDAI after initiation of abatacept in both groups (p < 0.05) (). Although the disease activity by SDAI was higher in bio-switch patients than in bio-naïve patients, there was no significant difference between the two groups at each time point (). We also examined the effect of concomitant use of MTX. SDAI decreased significantly in patients treated with the combination of abatacept and MTX (p < 0.05) (). In comparison, only a moderate decrease was noted in SDAI of patients who were not treated with MTX ().
Figure 1. Effects of abatacept on RA. (A) Effects of abatacept treatment on SDAI in 36 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. ABT, abatacept. (B) Effects of abatacept treatment on SDAI in 7 bio-switch patients and 29 bio-naïve patients. Data deficit compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. The difference between two groups was examined by Mann–Whitney U test. ABT, abatacept, NS, not significant. (C) Effects of abatacept and MTX combination treatment on SDAI in 27 patients and of abatacept alone in 9 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. The difference between two groups was examined by Mann–Whitney U test. ABT, abatacept, NS, not significant.
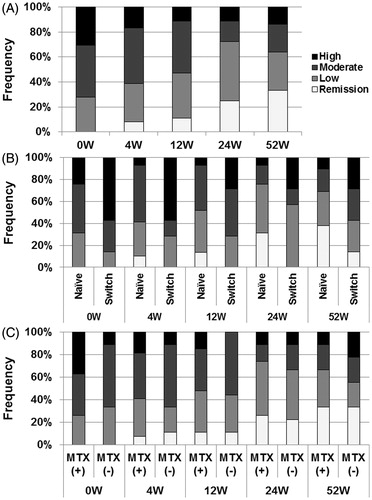
Patients with clinical remission, as assessed by SDAI, increased from 0 patient at 0 week to 12 patients (33.3%) at 52 weeks (). In contrast, the number of patients with moderate or high disease activity, as assessed by SDAI, decreased from 26 patients (72.2%) at 0 week to 13 patients (36.1%) at 52 weeks (). More bio-naïve patients achieved clinical remission by SDAI at 52 weeks than bio-switch patients, though the difference between the groups was not statistically significant (). Comparison between patients treated with and without MTX showed no statistically significant difference in disease activity as assessed by SDAI over the 52-week period (). These findings confirmed the effectiveness of abatacept against RA manifestations in patients with secondary SS with RA.
Figure 2. Effects of abatacept treatment on RA disease activity. (A) Effects of abatacept treatment on disease activity as assessed by SADI in 36 patients over the 52-week period. Data deficit was compensated by the LOCF method. (B) Effects of abatacept treatment on disease activity as assessed by SDAI in 7 bio-switch patients and 29 bio-naïve patients. Data deficit was compensated by the LOCF method. Difference between two groups was examined by Cochran-Armitage Test. There was no significant difference between the groups. (C) Effects of abatacept and MTX combination treatment on disease activity as assessed by SDAI in 27 patients and of abatacept alone in 9 patients. Data deficit was compensated by the LOCF method. Difference between two groups was examined by Cochran-Armitage Test. There was no significant difference.
Effectiveness of abatacept against SS
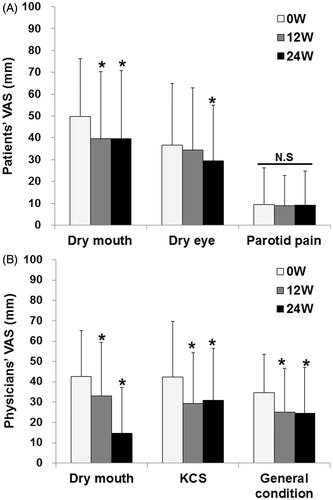
Abatacept significantly reduced the patients’ VAS values for dry mouth and dry eye from 49.8 ± 26.5 and 36.5 ± 28.3 mm at 0 week to 39.5 ± 31.3 and 29.5 ± 25.5 mm at 24 weeks (p < 0.05, n = 33), respectively (). However, abatacept did not improve the VAS values for parotid pain (). Abatacept significantly decreased physicians’ VAS values for dry mouth, KCS, and general condition from 42.7 ± 22.5, 42.3 ± 27.3, and 34.6 ± 19.0 mm at 0 week to 14.7 ± 22.6, 31.0 ± 25.5, and 24.7 ± 22.5 mm at 24 weeks (p < 0.05, n = 32), respectively ().
Figure 3. Effects of abatacept on VAS in SS. (A) Effects of abatacept treatment on patients’ VAS for dry mouth, dry eye, and parotid pain in 33 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. VAS, visual analog scale, NS, not significant. (B) Effects of abatacept treatment on physicians’ VAS for dry mouth, KCS, and general condition in 32 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. VAS, visual analog scale; KCS, keratoconjunctivitis sicca.
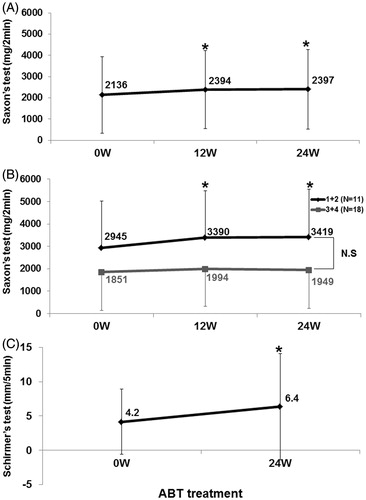
Saliva volume by Saxon’s test significantly increased from 2136 ± 1809 mg/2 min at 0 week to 2397 ± 1878 mg/2 min at 24 weeks (n = 34, p < 0.05) (). Interestingly, in 11 patients with Greenspan grade 1 or 2 of LSG biopsy, saliva volume was significantly higher at 24 weeks (3419 ± 2121 mg/2 min) compared with 2945 ± 2090 mg/2 min at 0 week (p < 0.05), whereas no change was noted in 18 patients with Greenspan grade 3 or 4 (). Tear volume by Schirmer’s test was significantly higher at 24 weeks (6.4 ± 7.8 mm/5 min), compared with 4.2 ± 4.8 mm/5 min at 0 week (n = 30, p < 0.05) ().
Figure 4. Effects of abatacept on secretory function in SS. (A) Effects of abatacept treatment on saliva volume assessed by Saxon’s test in 34 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. ABT, abatacept. (B) Effect of abatacept treatment on saliva volume assessed by Saxon’s test in 11 patients with Greenspan grading 1 or 2 of LSG biopsy and in 18 patients with grade 3 or 4. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. The difference between the two groups was examined by Mann–Whitney U test. ABT, abatacept; NS, not significant. (C) Effects of abatacept treatment on tear volume assessed by Schirmer’s test in 30 patients. Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline), Wilcoxon signed-rank test. ABT, abatacept.
Changes in parotid MRI from 0 to 52 weeks were investigated in six patients. In four of these patients, the MRI grade of the parotid gland did not change, though it deteriorated in one and improved in the other (). Importantly, the grade of parotid MRI at 0 week correlated significantly with the Greenspan grade of LSG biopsy at baseline (Spearman's rank correlation coefficient = 0.822, p < 0.05) (). However, changes in the grade of parotid MRI did not correlate with those in salivary secretion by Saxon’s test from 0 to 52 weeks (Spearman's rank correlation coefficient = 0.169, p = 0.749) (). These results suggest the effectiveness of abatacept for dryness, secretory dysfunction, and suppression of salivary gland destruction in patients with secondary SS with RA.
Table 2. Changes in parotid MRI and correlation with Greenspan grading of LSD biopsy and salivary secretion.
Effect of abatacept for immunological features
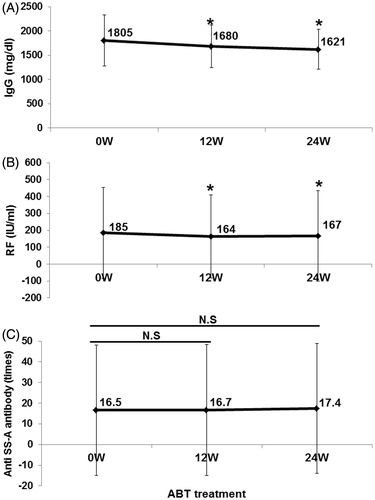
Treatment with abatacept resulted in significant reductions in serum IgG and RF levels from 1805 ± 525 mg/dl and 185 ± 270 IU/ml at 0 week to 1621 ± 415 mg/dl and 167 ± 268 IU/ml at 24 weeks, respectively (p < 0.05) (). None of the patients had hypogammaglobulinemia with IgG level <700 mg/dl during the 24 weeks. However, abatacept had no effect on anti-SS-A antibody titer (). These findings suggest that abatacept inhibits the production of some antibodies.
Figure 5. Effects of abatacept on IgG, RF, and anti-SS-A antibody. Effects of abatacept treatment on (A) serum IgG level (n = 36), (B) serum RF level (n = 35). (C) Titer of anti-SS-A antibody (n = 22). Data deficit was compensated by the LOCF method. *p < 0.05 versus 0 week (baseline); NS, not significant versus baseline, Wilcoxon signed-rank test. ABT, abatacept.
Correlation between improvement of SS and RA manifestations
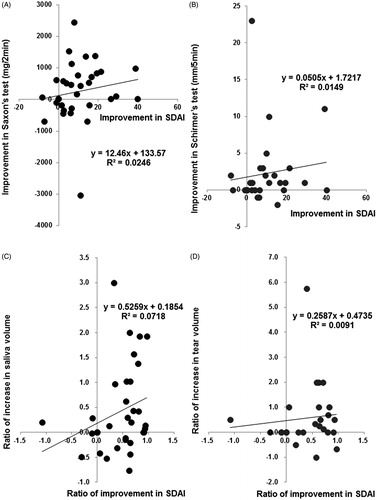
There was no significant correlation between the increase in saliva volume by Saxon’s test and the improvement in SDAI from baseline to 24 weeks (Spearman's rank correlation coefficient = 0.300, p = 0.085, n = 34) (), nor between the increase in tear volume by Schirmer’s test and improvement in SDAI from baseline to 24 weeks (Spearman's rank correlation coefficient = 0.287, p = 0.124, n = 30) (). Interestingly, the ratio of increase in saliva volume to baseline value (the increase in saliva volume from baseline to 24 weeks/saliva volume at baseline) significantly correlated with the ratio of improvement in SDAI to baseline value (the improvement in SDAI from baseline to 24 weeks/SDAI at baseline) (Spearman's rank correlation coefficient = 0.347, p = 0.044, n = 34) (). However, there was no significant correlation between the ratio of increase in tear volume to baseline value (the increase in tear volume from baseline to 24 weeks/tear volume at baseline) and the ratio of improvement in SDAI to baseline value (Spearman's rank correlation coefficient = 0.220, p = 0.270, n = 27) (). Saliva volume and SDAI improved simultaneously in 17 of 34 (50.0%) patients, and tear volume and SDAI in 15 of 30 (50.0%) patients from baseline to 24 weeks.
Figure 6. Correlation between improvement of SS and RA manifestations. Correlation between (A) increase in saliva volume (assessed by Saxon’s test) and improvement in SDAI (n = 34) from baseline to 24 weeks. Spearman's rank correlation coefficient = 0.300, p = 0.085, and (B) between increase in tear volume (assessed by Schirmer’s test) and improvement in SDAI (n = 30) from baseline to 24 weeks. Spearman's rank correlation coefficient = 0.287, p = 0.124. Correlation between (C) the ratio of increase in saliva volume to baseline value (the increase in saliva volume from baseline to 24 weeks/saliva volume at baseline) and the ratio of improvement in SDAI to baseline value (the improvement in SDAI from baseline to 24 weeks/SDAI at baseline) (n = 34). Spearman's rank correlation coefficient = 0.347, p = 0.044, and (D) between the ratio of increase in tear volume to baseline value (the increase in tear volume from baseline to 24 weeks/tear volume at baseline) and the ratio of improvement in SDAI to baseline value (n = 27). Spearman's rank correlation coefficient = 0.220, p = 0.270. Data deficit was compensated by the LOCF method.
Adherence and safety of abatacept
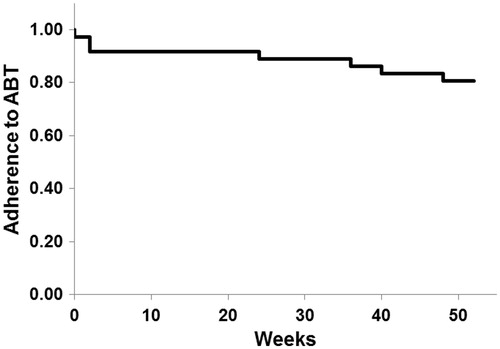
The adherence rate to abatacept was 80.6% (29/36) for 52 weeks (). Seven patients dropped out before completion of the 52-week study. The reasons for dropout were adverse events in two patients (at 0 and 36 weeks), inadequate effects in two patients (at 24 and 48 weeks), economic reasons in one patient (at 40 weeks), and hospital transfer in two patients (both at 2 weeks).
Figure 7. Adherence to abatacept. The adherence rate to abatacept analyzed by Kaplan–Meier method was 80.6% (29/36) for 52 week.
Twelve AEs were recorded in 10 of 36 patients (27.8%) over the period of 52 weeks, and 7 of these events (58.3%) were infections. AEs occurred between 0 and 48 weeks, and all 12 required treatment, including admission to the hospital in 3 patients. Importantly, 7 of 12 AEs (58.3%) and 4 of 7 infections (57.1%) occurred under corticosteroids use. During these complications, abatacept was withheld in four patients, and discontinued in two patients ().
Table 3. Adverse events in 36 cases during 52 weeks.
Discussion
In the present open-labeled, prospective, 1-year, and observational multicenter study (ROSE trial), we confirmed the effectiveness of abatacept for both SS and RA manifestations of secondary SS with RA, as previously demonstrated by the interim analysis of 32 patients for 24 weeks of ROSE trial [Citation20]. The study established the clinical usefulness of abatacept with regard to the following five aspects.
First, we proposed that abatacept is a promising therapeutic option for RA manifestations in patients with secondary SS with RA. In the present study, we demonstrated that 33.3% of patients with established long-standing moderately active RA associated with secondary SS, the majority (75%) of whom showed inadequate response to MTX, were able to achieve remission (by SDAI) for 52 weeks after the commencement of treatment with abatacept. Especially, in 29 bio-naïve patients, the remission rate was 37.9% (11/29 patients) at 52 weeks, compared with 14.3% in 7 bio-switch patients. A previous meta-analysis study that examined the effectiveness of abatacept combined with MTX against active RA, reported that the remission rate, defined by DAS28-ESR < 2.6, was 40.2% (95% confidence interval: 10.4–80.3%) [Citation25]. Thus, the remission rate in our study seems to be comparable with that of previous studies on similar RA patients. Interestingly, a recent study showed that RA patients with secondary SS developed more severe arthritis and higher RA disease activity than those without secondary SS [Citation26]. Another study showed that the presence of anti-SS-A antibody and secondary SS in patients with RA could lower clinical response to TNF inhibitors [Citation27]. The latter study reported that the EULAR responder (good or moderate) rate in RA patients with secondary SS or anti-SS-A antibody was significantly lower (63% in secondary SS and 58% in anti-SS-A antibody-positive patients) than in those without secondary SS or anti-SS-A antibody (82% and 83%, respectively) [Citation27]. In the present study, we demonstrated that abatacept was effective even for RA involvement in most (82.9%) patients with RA and secondary SS who were positive for anti-SS-A antibody with a high adherence rate for 52 weeks, though such patients are considered to have severe disease and are often resistant to TNF inhibitors. Our previous interim report [Citation20] and this extended report of ROSE trial are the first to investigate the effectiveness of biologics in RA with secondary SS.
Second, our results demonstrated that abatacept improved salivary and lacrimal secretory dysfunction in SS in a large number of patients (34 patients for saliva volume and 30 patients for tear volume) compared with previous studies [Citation18–20]. To date, only two open-labeled prospective single-arm studies using abatacept for treatment of SS patients have been published, excluding our ROSE trial, and no RCT has been reported. The first study by Adler et al. [Citation18] showed that abatacept significantly reduced salivary gland inflammation and serum gamma globulin levels, and increased saliva production when adjusted for disease duration in 11 primary SS patients for 24 weeks. The next study by Meiners et al. [Citation19] (Active Sjögren Abatacept Pilot study; ASAP study) reported that ESSDAI, ESSPRI, RF, and IgG levels decreased significantly during 24-week abatacept treatment and re-increased in post-treatment phase in 15 patients with early and active primary SS. With regard to glandular involvement, abatacept treatment did not change salivary and lacrimal gland functions, though a significant decrease in salivary flow was noted after the completion of treatment [Citation19]. Our study is unique relative to the above two studies in that we assessed a larger number of patients with secondary SS and RA, and our results showed that abatacept improved both salivary and lacrimal functions as well as subjective sicca symptoms. Importantly, saliva volume was significantly increased by abatacept but this was only noted in patients with Greenspan grade 1 or 2 of LSG biopsy but not in those with grade 3 or 4. These observations indicate that early intervention with abatacept against glandular manifestations is important for the recovery of the secretory function in SS patients, adding support to the report of Adler et al. [Citation18].
Third, the ratio of increase in saliva volume to baseline value significantly correlated with the ratio of improvement in SDAI to baseline value. Moreover, both secretory function and SDAI improved simultaneously in 50% of our patients. These findings suggest that T cells play a common pathogenic role in the development of SS and RA, indicating that abatacept is an ideal therapeutic strategy for secondary SS with RA, ala “kill two birds with one stone”.
Fourth, abatacept suppressed antibody production, such as IgG and RF in secondary SS with RA, in agreement with the above two studies [Citation18,Citation19]. Moreover, a recent study showed that abatacept reduced signs of polyclonal B cell activation in RA patients, inducing a trend toward normalization of serum levels of different classes of immunoglobulins and free light chains, decreasing titers of anti-citrullinated protein antibodies and RF, and percentages of post-switch memory B cells [Citation28]. Thus, abatacept seems a logical treatment for SS, in which activated B cell as well as T cells play important pathogenic roles [Citation16].
Fifth, no unexpected serious AEs were encountered in the present study, and abatacept seems to be well tolerated in patients with secondary SS with RA. Notably, infections should be regarded among the AEs, similar to RA patients. More than half of AEs and infections occurred under corticosteroids use in this study, thus we should pay enough attention to these patients.
Moreover, we suggest that abatacept might suppress salivary gland destruction in patients with SS. However, we assessed only six cases by parotid MRI (four cases with no change, one case with deterioration, and the other with improvement) without control group in this study. There has been no report showing the progression of salivary destruction in SS using MRI in the longitudinal studies to the best of our knowledge. Thus, we need to compare the progression of salivary destruction by MRI between the abatacept treated group and the control group in the future study, to confirm the effectiveness of abatacept on inhibition of salivary destruction.
In conclusion, the present study highlighted the potential therapeutic usefulness of abatacept for both RA and SS manifestations of secondary SS with RA. Importantly, early intervention against glandular involvement might be necessary for proper recovery of secretory function in SS patients. The results need to be confirmed in a larger cohort study and in RCTs that include placebo groups using newly proposed composite disease activity measures such as ESSDAI and ESSPRI.
Conflict of interest
This work was supported by Health and Labour Sciences Research Grants for research on intractable diseases (The Research Team for Autoimmune Diseases) from the Ministry of Health, Labour and Welfare of Japan. This study was supported in part by Bristol-Myers Squibb (BMS). H.T. received lecture fees and/or honoraria from BMS. Y.T. has received consulting fees, speaking fees, and/or honoraria from Abbvie, Daiichi-Sankyo, Chugai, Takeda, Mitsubishi-Tanabe, BMS, Astellas, Eisai, Janssen, Pfizer, Asahi-kasei, Eli Lilly, GlaxoSmithKline, UCB, Teijin, MSD, Santen and has received research grants from Mitsubishi-Tanabe, Takeda, Chugai, Astellas, Eisai, Taisho-Toyama, Kyowa-Kirin, Abbvie, BMS. I.M., Y.H., H.N., A.K., and T.S. received research grants, lecture fees and/or honoraria from BMS.
Acknowledgments
We thank Dr. F. G. Issa for the critical reading of the manuscript.
References
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8.
- Tsuboi H, Asashima H, Takai C, Hagiwara S, Hagiya C, Yokosawa M, et al. Primary and secondary surveys on epidemiology of Sjogren's syndrome in Japan. Mod Rheumatol. 2014;24:464–70.
- Wang D, Li Y, Liu Y, Shi G, et al. The use of biologic therapies in the treatment of rheumatoid arthritis. Curr Pharm Biotechnol. 2014;15:542–8.
- Mok CC. Emerging biological therapies for systemic lupus erythematosus. Expert Opin Emerg Drugs. 2014;19:303–22.
- Mozaffari S, Nikfar S, Abdolghaffari AH, Abdollahi M, et al. New biologic therapeutics for ulcerative colitis and Crohn's disease. Expert Opin Biol Ther. 2014;14:583–600.
- Lynch M, Kirby B, Warren RB. Treating moderate to severe psoriasis—best use of biologics. Expert Rev Clin Immunol. 2014;10:269–79.
- Vishwanath S, Relan M, Shen L, Ambrus JL Jr, et al. Update on the use of biologics in vasculitides. Curr Pharm Biotechnol. 2014;15:558–62.
- Sada PR, Isenberg D, Ciurtin C. Biologic treatment in Sjögren's syndrome. Rheumatology (Oxford). 2015;54:219–30.
- Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG, et al. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol. 2012;8:399–411.
- Dass S, Bowman SJ, Vital EM, Ikeda K, Pease CT, Hamburger J, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67:1541–4.
- Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:960–8.
- Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, Berthelot JM, Perdriger A, Puéchal X, et al. Treatment of primary Sjögren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160:233–42.
- Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E, et al. Inefficacy of infliximab in primary Sjögren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjögren's Syndrome (TRIPSS). Arthritis Rheum. 2004;50:1270–6.
- Sankar V, Brennan MT, Kok MR, Leakan RA, Smith JA, Manny J, et al. Etanercept in Sjögren's syndrome: a twelve-week randomized, double-blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 2004;50:2240–5.
- Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M, et al. Efficacy and safety of belimumab in primary Sjögren's syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis. 2015;74:526–31.
- Singh N, Cohen PL. The T cell in Sjogren's syndrome: force majeure, not spectateur. J Autoimmun. 2012;39:229–33.
- Sumida T, Tsuboi H, Iizuka M, Hirota T, Asashima H, Matsumoto I, et al. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjögren's syndrome: a critical review. J Autoimmun. 2014;51:44–50.
- Adler S, Körner M, Förger F, Huscher D, Caversaccio MD, Villiger PM, et al. Evaluation of histological, serological and clinical changes in response to abatacept treatment of primary Sjögren's syndrome: a pilot study. Arthritis Care Res (Hoboken). 2013;65:1862–8.
- Meiners PM, Vissink A, Kroese FG, Spijkervet FK, Smitt-Kamminga NS, Abdulahad WH, et al. Abatacept treatment reduces disease activity in early primary Sjogren's syndrome (open-label proof of concept ASAP study). Ann Rheum Dis. 2014;73:1393–6.
- Tsuboi H, Matsumoto I, Hagiwara S, et al. Efficacy and safety of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis: rheumatoid arthritis with orencia trial toward Sjögren's syndrome Endocrinopathy (ROSE) trial - an open-label, one-year, prospective study - Interim analysis of 32 patients for 24 weeks. Mod Rheumatol. 2015;25:187–93.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
- Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K, et al. Revised Japanese criteria for Sjögren’s syndrome (1999): availability and validity. Mod Rheumatol. 2004;14:425–34.
- Yokosawa M, Tsuboi H, Nasu K, Hagiya C, Hagiwara S, Hirota T, et al. Usefulness of MR imaging of the parotid glands in patients with secondary Sjögren's syndrome associated with rheumatoid arthritis. Mod Rheumatol. 2015;25:415–20.
- Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, et al. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta-analysis. Arthritis Res Ther. 2011;13:R204.
- He J, Ding Y, Feng M, Guon J, Sun X, Zhao J, et al. Characteristics of Sjögren's syndrome in rheumatoid arthritis. Rheumatology (Oxford). 2013;52:1084–9.
- Matsudaira R, Tamura N, Sekiya F, Ogasawara M, Yamanaka K, Takasaki Y, et al. Anti-Ro/SSA antibodies are an independent factor associated with an insufficient response to tumor necrosis factor inhibitors in patients with rheumatoid arthritis. J Rheumatol. 2011;38:2346–54.
- Scarsi M, Paolini L, Ricotta D, Pedrini A, Piantoni S, Caimi L, et al. Abatacept reduces levels of switched memory B cells, autoantibodies, and immunoglobulins in patients with rheumatoid arthritis. J Rheumatol. 2014;41:666–72.
