Abstract
Background: The sale of over-the-counter (OTC) medicines from pharmacies can help individuals self-manage symptoms. However, some OTC medicines may be abused, with addiction and harms being increasingly recognised. This review describes the current knowledge and understanding of OTC medicine abuse.
Approach: Comprehensive search of international empirical and review literature between 1990 and 2011.
Findings: OTC medicine abuse was identified in many countries and although implicated products varied, five key groups emerged: codeine-based (especially compound analgesic) medicines, cough products (particularly dextromethorphan), sedative antihistamines, decongestants and laxatives. No clear patterns relating to those affected or their experiences were identified and they may represent a hard-to-reach group, which coupled with heterogeneous data, makes estimating the scale of abuse problematic. Associated harms included direct physiological or psychological harm (e.g. opiate addiction), harm from another ingredient (e.g. ibuprofen-related gastric bleeding) and associated social and economic problems. Strategies and interventions included limiting supplies, raising public and professional awareness and using existing services and Internet support groups, although associated evaluations were lacking. Terminological variations were identified.
Conclusions: OTC medicine abuse is a recognised problem internationally but is currently incompletely understood. Research is needed to quantify scale of abuse, evaluate interventions and capture individual experiences, to inform policy, regulation and interventions.
Background
The mechanisms by which individuals can obtain medicines include not only their traditional prescribing by doctors, but also the ability to purchase medicines directly. The most obvious example of this is the community or retail pharmacy, where the metonymic term over-the-counter (OTC) originates and is used to describe such medicines. Such availability has been argued to offer benefits in terms of convenient access to, and choice of, medicines as well as involving individuals as active participants in their own health and the treatment of illness (Bond & Bradley, Citation1996; Nettleton, Citation2006). The range of medicines available is often more restrictive compared to prescribed medicines, and there are often limitations to indications and doses, although there has been a trend towards increasing deregulation of medicines from prescription to OTC supply and most recently availability from Internet pharmacies (Bessell et al., Citation2003). There has been a tendency for the public to perceive OTC medicines to be safer than prescription medicines (Bissell et al., Citation2001; Hughes et al., Citation2002; Raynor et al., Citation2007), but it has been recognised that OTC medicines have the potential for harm as well as benefit (Lessenger & Feinberg, Citation2008). This may result in what has been variously referred to as the misuse or abuse of OTC medicines and their potential to cause addiction and dependency. A number of specific OTC medicines and therapeutic groups have been implicated and in a recent review for doctors, for example, Lessenger and Feinberg (Citation2008) suggested medicines such as stimulants, laxatives, sedatives and dissociative substances such as dextromethorphan as being liable to abuse. They noted that in relation to abused drugs, “the literature is sparse about OTC medicines” and their review tellingly omits opiate-based OTC analgesics. The latter are available for purchase in many countries and combine codeine or dihydrocodeine with either ibuprofen or paracetamol and have led to particular concerns about addiction and also gastric or hepatic damage, respectively (Reay, Citation2009; Frei et al., 2010).
As Lessenger and Feinberg (Citation2008) noted, there is a relative lack of literature relating to OTC medicines that may be abused, and only one previous review has been undertaken (Reed et al., 2011); this was limited only to codeine-based OTC medicines and certain prescribed medicines and focused mainly on the context for England but similarly concluded that there was little current OTC evidence relating to the prevalence of misuse and dependence and treatment. The aim of this article, therefore, is to undertake a comprehensive review of the international empirical and other relevant literatures, to describe current knowledge and understanding about the range of OTC abuse. Specific objectives were to identify the different types of OTC medicines implicated, the scale of OTC abuse, the characteristics of those affected, harms associated with OTC medicine abuse and also approaches to dealing with it in terms of policy and interventions.
Review strategy
A thematic literature review approach was adopted, since there were a range of questions identified which a systematic review would have been inappropriate for, and also because including both review and empirical literature was considered advantageous in mapping out the breadth of understanding in this area.
Initial searches were conducted using ISI Web of Science, CINAHL, EMBASE and Medline together with specific searches of journals such as the Pharmaceutical Journal using combinations of the following terms: “over the counter”, “OTC”, “medicine”, “drug”, “misuse”, “abuse”, “addiction”, “dependency” and “non-prescription”. Additional searches were then undertaken based on identified medicines, and these included “codeine”, “pseudoephedrine”, “dextromethorphan”, “antihistamine”, “laxative” and also specific products, such as “Nurofen Plus” or “Coricidin”, for example, as they were identified in the literature. Reference lists of included publications were also checked and further searching was undertaken as a result. Additional grey literature was explored by strategies such as extensively contacting researchers in the field, to identify current research and non-peer-reviewed research publications. Additional non-peer-reviewed journal literature such as official organisation documents were also identified by searching OpenSIGLE, key organisations such as the Royal Pharmaceutical Society of Great Britain, the Medicines and Health Care products Regulatory Authority and the Proprietary Association of Great Britain together with more general searches of common search engines such as Google. Searches were undertaken for publications from 1990 to 2011 and inclusion criteria included publications published in English, empirical, review or opinion pieces. Exclusion criteria included non-English language publications and reference exclusively to prescribed or illicitly obtained medicines. Prescribed medicines were specifically excluded since, whilst this represents an important category, it covers very different mechanisms of governance.
Literature review findings
A total of 53 publications were identified, including 25 empirical studies, 11 case reports, 11 reviews articles, 1 book chapter, 1 doctoral thesis, 1 parliamentary enquiry and 3 key publications from organisations. The empirical studies represented 10 countries, with the United Kingdom (England, Wales, Scotland and Northern Ireland) being the most studied, followed by the United States (). The earliest identified study was conducted in 1996. A range of methods had been used in empirical studies, with various scales of surveys being most commonly used, as well as primary data collection of treatment centres and secondary data collection of emergency department presentations. Qualitative methods were identified in only two empirical studies and several studies reported on findings from pilot stages only (Fleming et al., Citation2004; Sweileh et al., Citation2004; Orriols et al., Citation2009). The findings are now described in more detail, organised in relation to the objectives described earlier – types of medicine implicated, scale of OTC abuse, associated harms, characteristics of those affected and approaches to dealing with OTC abuse – with an additional theme relating to terminology also being included.
Table I. Summary of empirical studies
Table II. Examples of medicines/therapeutic groups implicated in OTC abuse
Medicines implicated in OTC abuse
OTC medicine abuse was identified in many countries and although implicated products varied, five key groups emerged: codeine-based (especially compound analgesic) medicines, cough products (particularly dextromethorphan), sedative antihistamines, decongestants and laxatives. This variation may be related to both geographical variation and methodological and study design factors.
Geographical variation was evident and different products were subject to abuse in different countries. This appeared to be associated with variation in the availability of products, such as codeine-based analgesic or cough medicines in several countries but not in the United States, for example; specific trends, such as adolescent dextromethorphan abuse in the United States; and variation in regulation, such as availability of prescription medicines for purchase in some countries. In Jordan, for example, antibiotics and benzodiazepines were commonly cited by pharmacists as being abused, as regulations restricting their supply were not always enforced (Albsoul-Younes et al., Citation2010). Despite such international variation, common themes emerged and this Jordanian study typified several others in identifying five key groups of non-prescription medicines that were implicated in OTC abuse namely: sympathomimetic decongestants, cough products, analgesics, antihistamines and laxatives (see ). These reflected a similar categorisation made by Matheson et al. (Citation2002) and MacFadyen et al. (Citation2001), who identified Nytol (a brand of diphenhydramine, an antihistamine) as the product of misuse most suspected by pharmacists in Scotland, and, like Hughes et al. (1999b), these were broadly similar to the methodological design of studies such as Orriols et al. (Citation2009), who grouped their survey of pharmacy customers into whether they purchased codeine (an analgesic), dextromethorphan (a cough suppressant), pseudoephedrine (a decongestant) or an antihistamine. Cough products (and especially dextromethorphan) appeared to be the focus of several studies and data from the United States (Steinman, Citation2006; Levine, Citation2007; Peters et al., Citation2007; Substance Abuse and Mental Health Services Administration, Citation2008; Ford, Citation2009).
Methodologically, studies varied as to whether they focused on a particular product or sought to capture the range of products involved. It was also apparent that sampling influenced the emergent data, and, for example, studies that used pharmacists appeared to generate more detailed and varied descriptions of medicines that may be abused or misused (Hughes et al., 1999b; Matheson et al., Citation2002) compared to patient/customer/public accounts (Wazaify et al., Citation2005; Ajuoga et al., Citation2008; Major & Vincze, Citation2010), reflecting pharmacists’ knowledge of products and brands.
Scale of OTC medicine abuse
Attempts to describe the extent of OTC medicine abuse have been made using a variety of methods and data sources, which were often geographically related, but reflected heterogeneous participant groups and data. These included pharmacists’ perceptions of abuse (often in UK studies), data from drug treatment centres and poisons centres (e.g. in the United States), sales of codeine-containing medicines, perceptions of members of the public and self-reported abuse from specific groups such as US adolescents and gym users. The heterogeneous nature of these data sources makes assessing the international scale of OTC medicine abuse difficult to determine and making comparisons between countries difficult.
Data relating to the United Kingdom have been obtained from various sources. One of the most frequently referred to in the literature (Phelan & Akram, Citation2002; Ford & Good, Citation2007; Reay, Citation2009) involved the data reported from the UK-based on-line support group, Overcount, indicating the number of individuals who have registered with the site. This figure had been quoted as ranging from “more than 4000” (Ford & Good, Citation2007) to 16,000 (Reay, Citation2009), but specific details of the data were not provided in either source and no further information about it were identified in this review. Several UK studies have explored the experiences and perceptions of pharmacists in relation to OTC medicine misuse and abuse and estimates of the extent of the problem were presented as a result. The earliest identified study involved a postal survey of pharmacists in a county in England (Paxton & Chapple, Citation1996), which reported that 69% of pharmacists considered there to be some form of OTC medicine misuse in their pharmacies. Matheson et al. (Citation2002) reported on two postal surveys of pharmacists in Scotland undertaken in 1995 and 2000, which reported pharmacists’ belief that OTC product misuse was occurring in their area as 67.8% and 68.5%, respectively. Also involving Scottish pharmacists and a postal survey, MacFadyen et al. (Citation2001) reported that 31% of pharmacists perceived there to be frequent misuse and 58% perceived occasional misuse. This study also estimated that a mean of 5.6 patients were suspected of misusing medicines for each pharmacy in an “average week”, with the maximum being 40 in one pharmacy. In Wales, Pates et al. (Citation2002) also used a postal survey design and reported that 66% of respondents believed the presence of a problem in their area. In Northern Ireland, Hughes et al. (1999b) reported that pharmacist estimates of abuse in the previous 3 months ranged from 0 to 700, with a median of 10 and a mode of 6. Wazaify et al. (Citation2006) reported that six pharmacists identified 196 clients suspected of OTC abuse/misuse over 6 months. Geographically, urban pharmacies were associated with more suspected abuse than rural ones in two Scottish studies (MacFadyen et al., Citation2001; Matheson et al., Citation2002) and Mattoo et al. (Citation1997) reported that of those attending a clinic in India for addiction to codeine cough syrups, 80% were urban residents. Others studies identified no difference (Hughes et al., 1999b).
Data relating to the United States have been reported from a range of sources, ranging from specifically collected national level data, to surveys of specific groups, such as gym users, nicotine gum users and high school students. The Annual National Survey on Drug Use and Health (NSDUH) has provided data relating to specific issues such as, for example, abuse of OTC cough medicines amongst adolescents (Substance Abuse and Mental Health Services Administration, Citation2008), which revealed that in 2006 around 3.1 million people aged 12–25 stated that they had used an OTC cough and cold medicine to “get high” for a non-medical reason. This appeared to involve dextromethorphan, a cough suppressant, in 140 different products. Emergency department admissions were used by the Drug Abuse Warning Network (DAWN) to provide national-level data relating to the involvement of dextromethorphan in admissions (Substance Abuse and Mental Health Services Administration, Citation2010). This revealed that for 2004 0.7% (n = 12,584) of all emergency department admissions involved dextromethorphan and that the rate of visits was significantly higher amongst adolescents (aged 12–20) than other age groups. The third national-level data collected in the United States involved that collected in the treatment episode data set (TEDS) for treatment admissions by the Drug and Alcohol Services Information System (DASIS) (Substance Abuse and Mental Health Services Administration, Citation2004). Data from 2002 revealed that, as primary sources of abuse, only 4% of the 1.9 million admissions related to prescription or OTC medicines, which were described as including cough products, aspirin, sleep aids, diphenhydramine and other antihistamines. Of these, OTC medicines accounted for only 1% (n = 600) of admissions, and the authors noted that:
OTC medications are relatively rare as primary substances of abuse. They are more commonly noted as secondary or tertiary substances of abuse upon admission. (Substance Abuse and Mental Health Services Administration, Citation2004)
A more recent study from the United States also used drug treatment admissions (Gonzales et al., Citation2010), but reported on the state of California only. Prescription and OTC medicines in this study accounted for 6841 (3.2%) of admissions, with adolescents (12–18-year-olds) accounting for 1.5% of overall admissions. As in the above national-level study, the Californian study found OTC medicines to be relatively low, representing only 1.9% (n = 139) of the total prescribed and OTC medicine admissions. These were found to be statistically more likely to be reported by adolescents, who were more likely to cite “self” for referral to treatment than older clients, who cited “others” more often. The authors identified methodological concerns about the recording of such data, noting that OTC and prescription medicine recording by treatment staff was inconsistent, and may be due to not only the relatively recent inclusion of such data but also two further factors:
First, new prescription and OTC medications come on the market frequently. Second, there is wide variability in prescription and OTC drugs in relation to brand names, generic names, chemical names, and street names, which can change over time. (Gonzales et al., Citation2010)
Steinman (Citation2006) focused on the adolescent US population and surveyed 39,345 high school students in one county and reported 4.7% as having occasionally misused OTC medicines, with 2.1% reporting use in the past month; the study did not explore the types of product involved. Hughes et al. (Citation2004) identified 20% of those using nicotine replacement therapy (NRT) gum for more than 90 days as being addicted, and Ajuoga et al. (Citation2008) identified 37.2% of HIV positive patients as misusing OTC products. Kanayama et al. (Citation2001) used data from a survey of gym users and national data on fitness club membership to estimate a national incidence of 1.5 million individuals using adrenal hormones and 2.8 million using ephedrine.
The situation in Jordan was studied by Albsoul-Younes et al. (Citation2010), who adopted similar methods to UK studies, and found that 94.1% of pharmacists suspected some abuse or misuse of OTC products, and a mean estimate of “abusers” in the last 3 months per pharmacy to be 18.6 for regular, and 15.4 for new customers. From a total of 710 patients attending treatment clinics in Cape Town, South Africa in a 6-month period, Myers, Siegfried and Parry (Citation2003) identified 17 cases involving OTC codeine abuse.
Wazaify et al. (Citation2005) surveyed members of the public in Northern Ireland and described almost one-third of participants as having personally encountered OTC abuse (based on either personal experience, knowledge or observation). The most recent study identified (Nielsen et al., Citation2010) involved an on-line survey of 909 Australian individuals who used codeine and identified 138 (17.3%) as being “likely to be codeine dependent” using a severity of dependence scale. Two studies sampled pharmacy customers: in France, Orriols et al. (Citation2009) questioned 53 pharmacy customers using surveys about their codeine use in the previous month and identified 15% as misusing, 7.5% as abusing and 7.5% as being dependent. Major and Vincze (Citation2010) randomly surveyed pharmacy customers in Hungary and reported that almost one-third had personally experienced OTC abuse. With a specific focus on analgesic use, Agaba et al. (Citation2004) randomly sampled an area in Nigeria and reported analgesic abuse in 22.6% of respondents. They collected data on patients’ self-reported weekly use and overall duration and defined abuse as being a cumulative lifetime use exceeding 5000 “pills”.
OTC medicine sales data were identified in two reviews. Almarsdóttir and Grimsson (Citation2000) used secondary data and reported on a significant rise in codeine sales between 1993 and 1998 in Iceland and attributed this not to the hypothesised influence of legislative changes but to an increased Western consumption of medicines generally or more specifically OTC codeine abuse reporting by treatment centres. Reed et al. (2011) reported on national UK sales data relating to codeine-containing OTC medicines from a trade association. Data indicated that 21.4 million packs of codeine-containing OTC medicines were sold during 2008. This represented an increase from 19.5 million packets in 2006 but trends were not identifiable due to the limited data available.
Who is addicted to OTC medicines? Data relating to those who may be addicted to OTC medicines were obtained from several different sources. Several studies relied on the perceptions of pharmacists, whilst others relied on sampling the public, pharmacy customers or those suspected of actual abuse. Several studies analysed the case reports obtained from addiction centres. Overall, there was no consensus as to who may be affected by OTC medicine abuse. Amongst the first type, Akram (Citation2000) summarised several early UK studies as involving “middle-aged females”, whereas Albsoul-Younes et al. (Citation2010) reported that Jordanian pharmacists perceived the majority of abusers to be 26–50-year-old males. Similarly, Sweileh et al. (Citation2004) reported pharmacists as perceiving males to be more likely products than females in all categories except laxatives, in the 20–40-year-old age range. Other studies provided more equivocal pharmacist perceptions, and Pates et al. (Citation2002) noted that 54% of pharmacists considered all types of people to be suspected of OTC misuse, although female customers were more likely to be suspected of abusing or misusing laxatives. Of the remainder, there was variation in the ages suspected and Ajuoga et al. (Citation2008) found no association between OTC product misuse amongst HIV positive US patients and age, gender, ethnicity or education status.
Some studies, however, did include designs that permitted the collection of demographic data. Myers et al. (Citation2003), for example, examined details of patients attending a drug treatment centre in Cape Town, South Africa. It should be noted that in this study, although some data pertained to an OTC-specific medicine (codeine), the main findings did not present OTC medicines and those on prescription separately. This was also the case for data collected in the United States by the DAWN (Substance Abuse and Mental Health Services Administration, Citation2010). Steinman (Citation2006) reported that female students misused OTC medicines more than males, and misuse was also higher amongst older white students and Native American youths. Agaba et al. (Citation2004) reported those abusing analgesics to be slightly older than those who did not abuse. Nielsen et al. (Citation2010) compared codeine-dependent users and codeine users and, although not reporting any statistical data, found the former to be younger, with lower educational level, less likely to be in full-time employment but more likely to have used illicit substances and had family history of alcohol or drug problems.
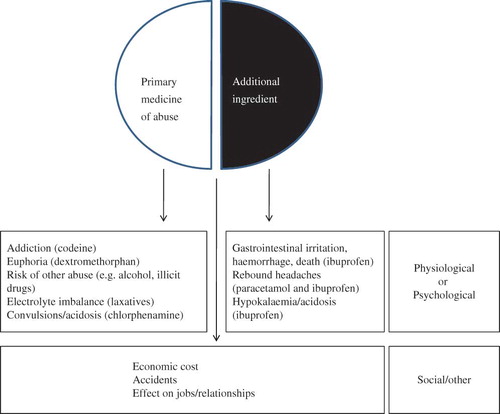
Harms related to OTC medicine abuse
A range of problems and harms associated with OTC medicine abuse were identified and these comprised three broad categories (). First, there were direct harms related to the pharmacological or psychological effects of the drug of abuse or misuse. Second, there were physiological harms related to the adverse effects of another active ingredient in a compound formulation. Both these types of harm led to concerns about overdoses and presentation at emergency services. Third, there were those harms related to other consequences, such as progression to abuse of other substances, economic costs and effects on personal and social life. Direct harms included addiction and dependence to an opiate such as codeine (Mattoo et al., Citation1997; Orriols et al., Citation2009; Nielsen et al., Citation2010). Other direct problems included convulsions and acidosis due to a codeine and antihistamine (diphenhydramine) containing antitussive medicine (Murao et al., Citation2008) and tachycardia, hypertension and lethargy due to abuse of Coricidin cough and cold tablets (dextromethorphan and chlorphenamine) (Banerji & Anderson, Citation2001). Lessenger and Feinberg (Citation2008) produced a comprehensive list of physical findings of nonmedical use of abused OTC products, noting agitation with nicotine gum, caffeine and ephedra, priapism with ephedrine and pseudoephedrine, psychiatric effects with dextromethorphan, euphoric psychosis with Coricidin and chlorphenamine and gastrointestinal disturbances with laxatives. Also within this category of direct harms were concerns raised about chronic rebound headache associated with repeated use of analgesics.
In relation to harms from other ingredients, two analgesic combination products – paracetamol and codeine (co-codamol) and ibuprofen and codeine – were considered problematic, with ibuprofen-containing medicine being particularly highlighted (Chetty et al., Citation2003; Dyer et al., Citation2004; Lambert & Close, Citation2005; Ford & Good, Citation2007; Dobbin & Tobin, Citation2008; Dutch, Citation2008; Ernest et al., Citation2010; Frei et al., Citation2010; Robinson et al., Citation2010). Dutch (Citation2008) and Ford and Good (Citation2007) reported on two hospital and three primary care presentations, respectively, of patients who had used a combination analgesic containing ibuprofen and codeine. Ford and Good (Citation2007) noted the side effects relating to ibuprofen and Dutch (Citation2008) reported both patients having perforated gastric ulcers. Hypokalaemia secondary to renal acidosis was identified as a result of abuse of this combination product (Chetty et al., Citation2003; Dyer et al., Citation2004; Lambert & Close, Citation2005; Ernest et al., Citation2010). Dobbin and Tobin (Citation2008) reported on 77 cases reported through personal networks of one of the authors where harm and dependence to ibuprofen and codeine OTC products had occurred. They identified similar clinical presentations as noted above and one death.
In relation to other consequences, several studies have referred to the association of OTC medicine abuse and the use of illicit substances (Levine, Citation2007; Reay, Citation2009) or obtaining codeine supplies from “street” supplies (Sproule et al., Citation1999). Tinsley and Watkins (Citation1998) reported on seven patients with dependence (according to DSM-IV criteria for amphetamine-like abuse) to ephedrine or pseudoephedrine and reported adverse social consequences in relation to losing jobs, family-marital stresses, relapse into alcohol misuse, motor vehicle violations and accidents.
Interventions and support
A range of strategies were identified that were aimed at minimising the harm associated with OTC medicine abuse, and supporting and treating affected individuals, although there was no evidence of any associated evaluation of these. Strategies ranged from pharmacy-based approaches reported by pharmacists in their actual work, to suggested interventions such as increasing awareness of the problem, providing additional training, to allowing pharmacists to provide treatment withdrawal programmes.
Many empirical studies that surveyed pharmacists sought their practical strategies and a number of common approaches emerged (Matheson et al., Citation2002; Pates et al., Citation2002; Albsoul-Younes et al., Citation2010). These included removing products from sight, claiming products were not in stock or not stocked anymore, alerting or counselling customers to the abuse potential of products, refusing sales, suggesting customers contact their doctor and supplying only limited amounts. A Delphi survey of experts in the field of addiction and OTC medicines also identified similar strategies (McBride et al., Citation2003), as well as broader strategies based on raising public awareness, establishing an official body to monitor Internet sales, limiting advertising and making warnings on packets more visible. Fleming et al. (Citation2004) developed a harm reduction model that comprised a manual and treatment algorithms for involving a customer’s doctor, the appropriate signposting for opioid, laxative and antihistamine abuse. Lack of pharmacist confidence and general practitioner (GP) engagement and competing work demands were identified as barriers. Wazaify et al. (Citation2006) reported that the same model led to some clients agreeing to stop using a medicine, using an alternative and being referred to their doctor for prescribing. No clients were recruited to enable collection of quality of life data. Raising awareness was recognised as being necessary amongst both the public (McBride et al., Citation2003; Reay, Citation2009) and health care professionals such as doctors (Williams & Kokotailo, Citation2006; Lessenger & Feinberg, Citation2008; Reay, Citation2009). A harm reduction strategy was proposed by Temple (Citation1996) whereby pharmacists would set a contract with individuals experiencing OTC medicine abuse to have regular supplies of medicines, reducing over time and involving detailed record keeping and adequate communication between pharmacies and involving drug team coordinators.
The All Party Parliamentary Drug Misuse Group (APPDMG) in the United Kingdom (Reay, Citation2009) concluded that increased recognition and support were needed for the voluntary groups that provided support for those with an OTC problem. Two specific websites – Overcount and CodeineFree – were identified and considered to provide a valuable service that was not formally recognised (Reay, Citation2009).
Definitions and terminology
Considerable terminological variation was apparent in the identified literature. Some literature referred only to the term “misuse” and appeared to use this generically, to describe all forms of problematic OTC medicine use in pharmacies (MacFadyen et al., Citation2001; Matheson et al., Citation2002; Pates et al., Citation2002; Myers et al., Citation2003; Ajuoga et al., Citation2008). As Akram (Citation2000) noted, however, this is unfortunate because it does not distinguish between misuse and abuse as separate problems, although some attempts to do this were identified in the literature:
Misuse is defined as using an OTC product for a legitimate medical reason but in higher doses or for a longer period than recommended, e.g. taking more of a painkiller than recommended to treat headache. Abuse is the non-medical use of OTC drugs, e.g. to experience a ‘high’ or lose weight. (Wazaify et al., Citation2005, p. 170)
According to Fleming et al. (Citation2004), misuse applied to potentially all medicines, whereas abuse related to specific medicines, such as laxatives, antihistamines and codeine-based products. There was no mention in the literature of the transition between misuse and abuse, as has been recognised in the medical prescribing situation of involuntary addiction (Reay, Citation2009). Further distinctions were identified within these broad categories and, for example, with misuse, it was argued to be possible to view this as resulting from using a medicine at a higher than recommended dose, or using it to treat symptoms for which the medicine is not indicated (Abbott & Fraser, Citation1998); with OTC medicine abuse, a distinction has also been made between sole OTC medicine abuse and substitution, where an individual is dependent on another medicine, often an illicit drug, and uses an OTC product when the other is unavailable (Abbott & Fraser, Citation1998; Temple, Citation2003).
Several studies did draw upon the wider literature relating to clinical classification such as DSM-IV (American Psychiatric Association, Citation2000) or ICD-10 (World Health Organisation, Citation1992) in specifically contrasting the terms abuse and dependence or “pharmacodependence” (Orriols et al., Citation2009) and misuse and dependence (Hughes et al., Citation2004). Several studies used the word “dependant” in relation to some use of the word codeine (Tinsley & Watkins, Citation1998; Orriols et al., Citation2009). The term “addiction” was identified in some literature (Hughes et al., Citation2004; Reay, Citation2009), but was infrequently used overall and, as Reay (Citation2009) noted, this may have occurred due to the perceived stigmatising effect that the term and that of “addict” might have on those affected. One mixed methods study (Nielsen et al., Citation2010) used the DSM-IV definition of dependence (but not abuse) as inclusion criteria for their qualitative interviews with codeine-dependent individuals and described some users having “therapeutic dependence” to doses at or less than the maximum, often over a prolonged period.
An additional and significant definitional point concerned the terms used to describe not only the condition but the actual individual themselves, who were affected by OTC medicine problems. Within the empirical literature, this related partly to the study design and sample and included the use of the word “patient” in studies where the participants were those attending hospitals to seek treatment (Mattoo et al., Citation1997; Myers et al., Citation2003) and the term “client” in a study which studied a pharmacy-based intervention (Fleming et al., Citation2004). Two studies referred to those affected by OTC medicine abuse and/or misuse as “customers” (McBride et al., Citation2003; Albsoul-Younes et al., Citation2010) reflecting the commercial nature of OTC medicine sales, although Albsoul-Younes et al. (Citation2010) also used the term “abusers” uniquely. One further definition offered was that relating to individuals who “manage their drug use as part of their normal daily routine” and were termed “recreational users”, to describe a heterogeneous group of individuals who may be abusing anabolic steroids, and “soft drugs” such as cannabis or LSD, or OTC medicines (Scottish Specialist in Pharmaceutical Public Health, Citation2004).
Discussion
This review of the literature has revealed a number of themes and data to inform understanding of OTC medicine abuse, However, what is perhaps most apparent is the extent of the omissions in the extant literature, particularly as they relate to the lack of:
qualitative methods that may be appropriate for exploring individual perspectives;
reliable quantitative data in some countries;
fully evaluated or implemented interventions;
data relating to Internet supplies; and
consensus over definitional terms.
These concerns are now considered in turn, before a number of specific suggestions for further research and policy involvement are proposed.
The various definitions described previously have a number of implications for research and understanding in this area. First, whilst they can positively reflect a range of different types of societal medicine use, they may also lead to confusion, particularly if, like some studies did, there are not accurate and consistent attempts to distinguish between them. This may be further complicated by the origins of these terms, with some such as “dependency” and “abuse” being associated with a clinical or diagnostic perspective (American Psychiatric Association, Citation2000), “addiction” carrying a societal broader interpretation and “misuse” being associated with pharmacy studies particularly. This reflects enduring debates about and changes to terminology in the wider addiction literature, including the WHO’s adoption of “dependence” over “addiction” (World Health Organisation, Citation1964) nearly half a century ago, to recent debates about these terms in the DSM-IV and proposed DSM-V (Dean & Rud, Citation1984; O’Brien et al., Citation2006). Underscoring this definitional variation are also fundamental issues about stigma, identify and also agency. The use of the term “dependency” and not “addiction” has been argued to have occurred due to issues of stigma of the latter (Dean & Rud, Citation1984; Erickson & Wilcox, Citation2006; Reay, Citation2009) as well as the issue of an “addict” or “spoilt” identity (Goffman, Citation1990; McIntosh & Mckeganey, Citation2000). In terms of agency, it is interesting to reflect on the distinction between misuse and abuse in some of the extant literature, since this appears to recognise a difference between intentionally experimenting with a medicine (to elicit a different effect) and abusing it, and unintentionally deviating from standard use (taking at different dose or indication) and therefore misusing it. Whether these can be adequately mapped onto additional concerns about the loss of control in addiction, as argued by Reith (Citation2004), for example, are additional issues. One further omission is the absence of any reference to pseudo-addiction in the OTC literature identified in this review. Pseudo-addiction has been defined as the under-treatment of pain (Bell & Salmon, Citation2009), which may lead to symptoms that are similar to dependency and which reveal a potentially even more complex area.
Methodologically, quantitative approaches have dominated, illustrated by the use of cross-sectional descriptive survey designs, often using self-completion postal surveys of pharmacist participants in UK studies. Response rates appear to have varied significantly using this approach, and whilst Matheson et al. (Citation2002) reported very good response rates across two surveys using a prepaid envelope and two reminder letters, and Hughes et al. (1999b) received responses from just under half of pharmacists sampled using two mailings. These studies reflect a trend to using pharmacists proxies and hence obtaining data that reported on pharmacists’ perceptions of the problem and the profile of those they considered to be affected, which as Orriols et al. (Citation2009) noted is “much too subjective to obtain reliable qualitative and quantitative data”. Although not explicitly noted by the researchers, this may reflect a belief that those who are abusing or misusing OTC medicines may be a hard-to-reach or covert (Reay, Citation2009) group and hence using pharmacist proxies is perhaps perceived as being more appropriate. However, several study designs have involved sampling those suspected of abusing/misusing OTC medicines, either via pharmacies (Phelan & Akram, Citation2002; Orriols et al., Citation2009), at targeted venues such as gyms (Kanayama, et al., Citation2001) or by post (Sproule et al., Citation1999). Although these represent less subjective accounts of the problem, they have resulted in poor response rates except in the study by Orriols et al. (Citation2009), who argued that allowing purchasers to complete a questionnaire away from the pharmacy and return it via post, as compared to completing it in the pharmacy, meant those who were abusing or misusing could complete the forms anonymously. However, Orriols et al. (Citation2009) were disappointed by the poor level of pharmacy participation, which may be related to the need for the pharmacies involved to undertake the administration of the questionnaires, as was identified in other studies (Wazaify et al., Citation2006).
Of particular note is that qualitative methods have been neglected and only one identified study used focus groups (Björnsdóttir et al., Citation2009) and one which reported the use of semi-structured interviews (Mattoo et al., Citation1997) presented detailed statistical data and the absence of qualitative data suggested this was a structured survey design. Nielsen et al. (Citation2010) used qualitative interviews and reported a range of different types of abuse of codeine, as well as barriers to treatment, illustrating the unique data that this method can generate. Adopting such methods may reveal further insights that could help understanding of the contested definitional issues raised above, as well as providing more than the proxy summaries of those perceived to be affected, as offered by some pharmacist-participant studies.
The use of secondary data sources, such as those in various US reports (Substance Abuse and Mental Health Services Administration, Citation2004, Citation2008, Citation2010) and using details of patients attending drug treatment centres in South Africa (Myers et al., Citation2003), for example, offers potentially more robust statistical information on the extent of the problem. However, such data are not unproblematic and in the case of some US data, for example, prescription and OTC medicines were often reported together.
Linked to the source of this last type of secondary data is any evaluation or indeed thorough detail of treatment options for those affected by OTC medicine abuse. Empirical studies have identified a range of often pragmatic solutions, but evidence-based interventions and attendant evaluations are a clear omission in this field.
Finally, the emergence of new forms of medicine supply, such as via the Internet, in what Fox et al. (Citation2005) termed the “second moment” of “e-pharmacy” has not been studied, despite being recognised as a potential threat (McBride et al., Citation2003). Such developments may not only stretch the metonymic accuracy of the term OTC, but also require a redefinition of what such supplies involve, as such supplies transcend national boundaries and attendant regulation in many cases (Bessell et al., Citation2003) and may challenge the international patterns identified.
In relation to policy, this review confirms that there is a problem in a number of countries but concerns about what is being investigated – whether this is misuse, abuse, dependency, addiction or pseudo-addiction – coupled with a lack of systematic data on the scale of the problem make appropriate and proportionate policy-based interventions difficult to consider. There exists a tension between making OTC medicines available to individuals to increase their access to medicines and enabling them to self-manage conditions and accepting that there is some degree of risk of such products being misused or abused, with potentially serious consequences for some. Raising awareness of potential problems of OTC medicines, as the recent response in the United Kingdom has illustrated in terms of making purchasers aware of the possibility of addiction, would appear a prudent response. But whilst this may arguably warn those using products for the first time, for those with an existing problem, more support may be needed in the clinical pathway.
Conclusion
This review of the literature relating to OTC medicine abuse has revealed that there is a recognised problem internationally involving a range of medicine and potential harms. Methodological concerns have emerged in relation to the use of proxy, self-report and non-OTC specific data and the relative lack of qualitative research involving individual experiences of OTC medicine abuse. These represent urgent areas where research is needed; to explore the extent of the problem and to provide insights into those affected, coupled wih providing clarification of the type of problem being investigated. Such research is needed to inform policy, regulation and the preparedness of a range of health care professionals to avoid harm to those who purchase OTC medicines that may be liable to abuse.
Declaration of interest
This review was part of a larger study that was funded by the Pharmacy Practice Research Trust. The author reports no conflict of interest. The author alone is responsible for the content and writing of this paper.
References
- Abbott, F. V., & Fraser, M. I. (1998). Use and abuse of over-the-counter analgesic agents. Journal of Psychiatry & Neuroscience, 23(1), 13–34.
- Agaba, E. I., Agaba, P. A., & Wigwe, C. M. (2004). Use and abuse of analgesics in Nigeria: A community survey. Nigerian Journal of Medicine, 13(4), 379–382.
- Ajuoga, E., Sansgiry, S. S., Ngo, C., & Yeh, R. F. (2008). Use/misuse of over-the-counter medications and associated adverse drug events among HIV-infected patients. Research in Social & Administrative Pharmacy, 4(3), 292–301.
- Akram, G. (2000). Over-the-counter medication: An emerging and neglected drug abuse? Journal of Substance Use, 5(2), 136–142.
- Akram, G., & Roberts, K. (2003). Pharmacists’ management of over-the-counter medication requests from methadone patients. Journal of Substance Use, 8(4), 215–222.
- Albsoul-Younes, A., Wazaify, M., Yousef, A.-M., & Tahaineh, L. (2010). Abuse and misuse of prescription and nonprescription drugs sold in community pharmacies in Jordan. Substance Use & Misuse, 45(9), 1319–1329.
- Almarsdóttir, A. B., & Grimsson, A. (2000). Over-the-counter codeine use in Iceland: The impact of increased access. Scandinavian Journal of Public Health, 28(4), 270–274.
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association.
- Banerji, S., & Anderson, I. (2001). Abuse of Coricidin HBP cough & cold tablets: Episodes recorded by a poison center. American Journal of Health-System Pharmacy, 58(19), 1811–1814.
- Bell, K., & Salmon, A. (2009). Pain, physical dependence and pseudoaddiction: Redefining addiction for “nice” people? International Journal on Drug Policy, 20(2), 170–178.
- Bessell, T. L., Anderson, J. N., Silagy, C. A., Sansom, L. N., & Hiller, J. E. (2003). Surfing, self-medicating and safety: Buying non-prescription and complementary medicines via the Internet. Quality & Safety in Health Care, 12(2), 88–92.
- Bissell, P., Ward, P. R., & Noyce, P. R. (2001). The dependent consumer: Reflections on accounts of the risks of non-prescription medicines. Health, 5(1), 5–30.
- Björnsdóttir, I., Almarsdóttir, A. B., & Traulsen, J. M. (2009). The lay public’s explicit and implicit definitions of drugs. Research in Social & Administrative Pharmacy, 5(1), 40–50.
- Bond, C. M., & Bradley, C. (1996). Over the counter drugs: The interface between the community pharmacist and patients. British Medical Journal, 312(7033), 758–760.
- Bryant-Waugh, R., Turner, H., & East, P. (2005). Over-the-counter laxatives and eating disorders: A survey of pharmacists’ and other retailers’ views and practice. Pharmaceutical Journal, 275, 87–91.
- Chetty, R., Baoku, Y., Mildner, R., Banerjee, A., Vallance, D., Haddon, A.& Labib, M. (2003). Severe hypokalaemia and weakness due to Nurofen misuse. Annals of Clinical Biochemistry, 40(Pt. 4), 422–423.
- Dean, J., & Rud, F. (1984). The drug addict and the stigma of addiction. Substance Use & Misuse, 19(8), 859–869.
- Dobbin, M., & Tobin, C. L. (2008). Over-the-counter ibuprofen/codeine analgesics: Misuse and harm. Melbourne, VIC: Drugs Policy and Services Branch Department of Human Services.
- Dutch, M. J. (2008). Nurofen plus misuse: An emerging cause of perforated gastric ulcer. Medical Journal of Australia, 188(1), 56–57.
- Dyer, B. T., Martin, J. L., Mitchell, J. L., Sauven, N. C., & Gazzard, B. (2004). Hypokalaemia in ibuprofen and codeine phosphate abuse. International Journal of Clinical Practice, 58(11), 1061–1062.
- Erickson, C. K., & Wilcox, R. E. (2006). Please, not “Addiction” in DSM-V. The American Journal of Psychiatry, 163(11), 2015–2016.
- Ernest, D., Chia, M., & Corallo, C. E. (2010). Profound hypokalaemia due to Nurofen Plus and Red Bull misuse. Critical Care and Resuscitation, 12(2), 109–110.
- Fleming, G. F., McElnay, J. C., & Hughes, C. M. (2004). Development of a community pharmacy-based model to identify and treat OTC drug abuse/misuse: A pilot study. Pharmacy World & Science, 26(5), 282–288.
- Ford, C., & Good, B. (2007). Over the counter drugs can be highly addictive. British Medical Journal, 334(7600), 917–918.
- Ford, J. A. (2009). Misuse of over-the-counter cough or cold medications among adolescents: Prevalence and correlates in a national sample. Journal of Adolescent Health: Official Publication of the Society for Adolescent Medicine, 44(5), 505–507.
- Fox, N., Ward, K., & O’Rourke, A. (2005). The birth of the e-clinic. Continuity or transformation in the UK governance of pharmaceutical consumption? Social Science & Medicine, 61(7), 1474–1484.
- Frei, M. Y., Nielsen, S., Dobbin, M., & Tobin, C. L. (2010). Serious morbidity associated with misuse of over-the-counter codeine-ibuprofen analgesics: A series of 27 cases. Medical Journal of Australia, 193(5), 294–296.
- Goffman, E. (1990). Stigma: Notes on the management of spoiled identity. London: Penguin Group.
- Gonzales, R., Brecht, M.-L., Mooney, L., & Rawson, R. A. (2010). Prescription and over-the-counter drug treatment admissions to the California public treatment system. Journal of Substance Abuse Treatment, 40(3), 224–229.
- Hughes, G. F., Bell, H. M., & McElnay, J. C. (1999a). General practitioners’ awareness of the appropriate and inappropriate use of over-the-counter products. Pharmaceutical Journal, 263(7063), R29.
- Hughes, G. F., McElnay, J. C., Hughes, C. M., & McKenna, P. (1999b). Abuse/misuse of non-prescription drugs. Pharmacy World & Science, 21(6), 251–255.
- Hughes, J. R., Pillitteri, J. L., Callas, P. W., Callahan, R., & Kenny, M. (2004). Misuse of and dependence on over-the-counter nicotine gum in a volunteer sample. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 6(1), 79–84.
- Hughes, L., Whittlesea, C., & Luscombe, D. (2002). Patients’ knowledge and perceptions of the side-effects of OTC medication. Journal of Clinical Pharmacy Therapeutics, 27, 243–248.
- Kanayama, G., Gruber, A. J., Pope, H. G., Borowiecki, J. J., & Hudson, J. I. (2001). Over-the-counter drug use in gymnasiums: An underrecognized substance abuse problem? Psychotherapy and Psychosomatics, 70(3), 137–140.
- Lambert, A. P., & Close, C. (2005). Life-threatening hypokalaemia from abuse of Nurofen Plus. Journal of the Royal Society of Medicine, 98(1), 21.
- Lessenger, J. E., & Feinberg, S. D. (2008). Abuse of prescription and over-the-counter medications. Journal of the American Board of Family Medicine, 21(1), 45–54.
- Levine, D. A. (2007). “Pharming”: The abuse of prescription and over-the-counter drugs in teens. Current Opinion in Pediatrics, 19(3), 270–274.
- MacFadyen, L., Eadie, D., & McGowan, T. (2001). Community pharmacists’ experience of over-the-counter medicine misuse in Scotland. Journal of the Royal Society for the Promotion of Health, 121(3), 185–192.
- Major, C., & Vincze, Z. (2010). Consumer habits and interests regarding non-prescription medications in Hungary. Family Practice, 27(3), 333–338.
- Matheson, C., Bond, C. M., & Pitcairn, J. (2002). Misuse of over-the-counter medicines from community pharmacies: A population survey of Scottish pharmacies. Pharmaceutical Journal, 269(7206), 66–68.
- Mattoo, S. K., Basu, D., Sharma, A., Balaji, M., & Malhotra, A. (1997). Abuse of codeine-containing cough syrups: A report from India. Addiction, 92(12), 1783–1787.
- McBride, A. J., Pates, R., Ramadan, R., & McGowan, C. (2003). Delphi survey of experts’ opinions on strategies used by community pharmacists to reduce over-the-counter drug misuse. Addiction, 98(4), 487–497.
- McIntosh, J., & Mckeganey, N. (2000). Addicts’ narratives of recovery from drug use: Constructing a non-addict identity. Social Science & Medicine, 50(10), 1501–1510.
- Murao, S., Manabe, H., Yamashita, T., & Sekikawa, T. (2008). Intoxication with over-the-counter antitussive medication containing dihydrocodeine and chlorpheniramine causes generalized convulsion and mixed acidosis. Internal medicine (Tokyo, Japan), 47(11), 1013–1015.
- Myers, B., Siegfried, N., & Parry, C. D. H. (2003). Over-the-counter and prescription medicine misuse in Cape Town – Findings from specialist treatment centres. South African Medical Journal, 93(5), 367–370.
- Nettleton, S. (2006). The Sociology of health and illness (p. 352). Oxford: Polity.
- Nielsen, S., Cameron, J., & Pahoki, S. (2010). Over the counter codeine dependence final report 2010. Fitzroy, VIC: Turning Point Alcohol and Drug Centre.
- O’Brien, C. P., Volkow, N., & Li, T. K. (2006). What’s in a word? Addiction versus dependence in DSM-V. American Journal of Psychiatry, 163(5), 764–765.
- Orriols, L., Gaillard, J., Lapeyre-Mestre, M., & Roussin, A. (2009). Evaluation of abuse and dependence on drugs used for self-medication: A pharmacoepidemiological pilot study based on community pharmacies in France. Drug Safety, 32(10), 859–873.
- Pates, R., McBride, A. J., Li, S., & Ramadan, R. (2002). Misuse of over-the-counter medicines: A survey of community pharmacies in the South Wales health authority. Pharmaceutical Journal, 268(7184), 179–182.
- Paxton, R., & Chapple, P. (1996). Misuse of over-the-counter medicines: A survey in one English county. Pharmaceutical Journal, 256(6881), 313–315.
- Peters, Jr., R., Yacoubian, Jr., G. S., Rhodes, W., Forsythe, K. J., Bowers, K. S., Eulian, V. M., Mangum, C. A., O’Neal, J. D., Martin, Q., & Essien, E. J. (2007). Beliefs and social norms about codeine and promethazine hydrochloride cough syrup (CPHCS) use and addiction among multi-ethnic college students. Journal of Psychoactive Drugs, 39(3), 277–282.
- Phelan, M., & Akram, G. (2002). A community pharmacy-based survey of users of over-the-counter sleep aids. Pharmaceutical Journal, 269(7213), 287–290.
- Raynor, D., Blenkinsopp, A., Knapp, P., Grime, J., Nicolson, D., Pollock, K., Dorer, G., Gilbody, S., Dickinson, D., Maule, A. J., & Spoor, P. (2007). A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines. Health Technology Assessment, 11(5), 1–160.
- Reay, G. (2009). An inquiry into physical dependence and addiction to prescription and over-the-counter medication. London: All-Party Parliamentary Drugs Misuse Group.
- Reed, K., Bond, A., Witton, J., Cornish, R., Hickman, M., & Strang, J. (2011). The changing use of prescribed benzodiazepines and z-drugs, & of over-the-counter codeine-containing products in England: A structured review of published English & international evidence & available data to inform consideration of the extent of dependence. London: The National Addiction Centre, Kings College London.
- Reith, G. (2004). Consumption and its discontents: Addiction, identity and the problems of freedom. British Journal of Sociology, 55(2), 283–300.
- Robinson, G. M., Robinson, S., McCarthy, P., & Cameron, C. (2010). Misuse of over-the-counter codeine-containing analgesics: Dependence and other adverse effects. New Zealand Medical Journal, 123(1317), 59–64.
- Scottish Specialist in Pharmaceutical Public Health. (2004). Drugs misuse and community pharmacy: Issues for pharmaceutical care. Scotland: Scottish Specialist in Pharmaceutical Public Health.
- Sproule, B. A., Busto, U. E., Somer, G., Romach, M. K., & Sellers, E. M. (1999). Characteristics of dependent and nondependent regular users of codeine. Journal of Clinical Psychopharmacology, 19(4), 367–372.
- Steinman, K. (2006). High school students’ misuse of over-the-counter drugs: A population-based study in an urban county. Journal of Adolescent Health, 38(4), 445–447.
- Substance Abuse and Mental Health Services Administration, O. of A. S. (2004). The DASIS report. Characteristics of primary prescription and OTC treatment admissions: 2002. Rockville, MD: Substance Abuse and Mental Health Services Administration.
- Substance Abuse and Mental Health Services Administration, O. of A. S. (2008). The NSDUH report: Misuse of over-the-counter cough and cold medications among persons aged 12 to 25. Rockville, MD: Substance Abuse and Mental Health Services Administration.
- Substance Abuse and Mental Health Services Administration, O. of A. S. (2010). Drug abuse warning network, 2007: National estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration.
- Sweileh, W. M., Arafat, R. T., Al-Khyat, L. S., Al-Masri, D. M., & Jaradat, N. A. (2004). A pilot study to investigate over-the-counter drug abuse and misuse in Palestine. Saudi Medical Journal, 25(12), 2029–2032.
- Temple, D. (1996). A “harm reduction” model for community pharmacy. Chemist-and-Druggist, 245, 730–773.
- Temple, D. (2003). Misuse of over the counter medicines in the UK. In J. Sheridan & J. Strang ( Eds.), Drug misuse and community pharmacy (pp. 149–160). London: Taylor and Francis.
- Tinsley, J. A., & Watkins, D. D. (1998). Over-the-counter stimulants: Abuse and addiction. Mayo Clinic Proceedings, 73(10), 977–982.
- Wazaify, M., Hughes, C. M., & McElnay, J. C. (2006). The implementation of a harm minimisation model for the identification and treatment of over-the-counter drug misuse and abuse in community pharmacies in Northern Ireland. Patient Education and Counseling, 64(1–3), 136–141.
- Wazaify, M., Shields, E., Hughes, C. M., & McElnay, J. C. (2005). Societal perspectives on over-the-counter (OTC) medicines. Family Practice, 22(2), 170–171.
- Williams, J. F., & Kokotailo, P. K. (2006). Abuse of proprietary (over-the-counter) drugs. Adolescent Medicine Clinics, 17(3), 733–750, Abstract xiii.
- World Health Organisation. (1964). 13th report on the WHO expert committee on addiction-producing drugs. Geneva: World Health Organisation.
- World Health Organisation. (1992). ICD-10: International statistical classification of diseases and related health problems (10th rev. ed.). Geneva: World Health Organisation.