Abstract
The inhibitory effects of some drugs on 6-phosphogluconate dehydrogenase from human erythrocytes have been investigated. For this purpose, initially, erythrocyte 6-phosphogluconate dehydrogenase was purified 3364 times in a yield of 58% by using ammonium sulfate precipitation and 2′,5′-ADP Sepharose 4B affinity gel. A temperature of +4°C was maintained during the purification process. Enzyme activity was determined with the Beutler method by using a spectrophotometer at 340 nm. This method was utilized for all kinetic studies. Many commonly used drugs were investigated in this study. Some drugs (ketotifen (Ki: 8.3 ± 1.7 μM), dacarbazine (Ki: 10.1 ± 0.7 μM), meloxicam (Ki: 50.9 ± 13.2 μM), furosemide (Ki: 127 ± 37.8 μM), methotrexate (Ki: 136.7 ± 25.3 μM), metochloropramide hydrochloride (Ki: 2.1113 ± 0.6979 mM), ritodrine hydrochloride (Ki: 6.0353 ± 1.2783 mM), and gadopentetic acid (Ki: 73.4 ± 21.9 mM)) inhibited enzyme activity in vitro. Ki constants for the enzyme were found by means of Lineweaver–Burk graphs. All drugs showed non-competitive inhibition. In addition, IC50 values of the drugs were determined by plotting activity percent vs [I].
Introduction
6-Phosphogluconate dehydrogenase (E.C.1.1.1.44; 6PGD), the third enzyme in the pentose phosphate metabolic pathway, catalyzes the conversion of 6-PGA (6-phosphogluconate) and NADP+ (nicotinamide adenine dinucleotide phosphate) to d-ribulose-5-phosphate and NADPH, which protects the cell against oxidizing agents by producing reduced glutathione (GSH)Citation1,Citation2. NADPH is also a coenzyme participating in the synthesis of a number of biomolecules such as fatty acids, steroids, and some amino acidsCitation3,Citation4. In the case of lack of NADPH, the concentration of GSH in living cells declines, resulting in cell death. GSH is indirectly produced by 6PGD; therefore, 6PGD can be defined as an indirect antioxidant enzymeCitation4,Citation5. Many agents are known to activate or inhibit enzymes in vitro and in vivoCitation6–9, so affecting metabolic pathways. Inhibition of 6PGD leads to decreased NADPH and GSH, which will cause cell damage especially in older erythrocytes, resulting in some problems in living cellsCitation6–8.
The effects of many commonly used drugs (netilmicin sulfate, cefepime, amikacin, isepamycin, chloramphenicol, ceftazidim, teicoplanin, ampicillin, ofloxacin, levofloxacin, cefotaxime, penicillin G, gentamicin sulfate, ciprofloxacin, cefozin, decefin, streptomycin, combisid, meronem, larnoxicam, metronidazole, imipenem, ornidazole, vancomycin, clindamycin, and amoxicillin) on 6PGD enzyme activities have been investigatedCitation10,Citation11. However, no reports could be found in the literature on the effects of ketotifen, dacarbazine, meloxicam, furosemide, methotrexate, metochloropramide hydrochloride, ritodrine hydrochloride, and gadopentetic acid on human erythrocyte 6PGD, although vitamin C has been reported to stimulate 6PGD in vitro and in vivoCitation10.
This study was aimed at the purification of human erythrocyte 6PGD, and determination of the effects of some commonly used drugs on human red blood cell 6PGD activity.
Materials and methods
Materials
6-PGA, NADP+, Tris-HCl, and the other chemicals were from Sigma-Aldrich Co. (Germany), and the drugs were purchased from Hoechst Marion Roussel (Turkey).
Activity determination
6PGD activity was measured spectrophotometrically at 25°C as described by BeutlerCitation12. Briefly, the enzyme sample was added to a 1 mL (final volume) incubation mixture containing 0.1 M Tris-HCl + 0.5 mM ethylenediaminetetraacetic acid (EDTA) (pH 8.0), 10 mM MgCl2, 0.2 mM NADP+, and 0.6 mM 6-PGA, and the increase in absorption at 340 nm due to the reduction of NADP+ was measured at 25°C. One enzyme unit represents the reduction of 1 µmol of NADP+ per min at 25°C, pH 8.0.
Preparation of hemolysate
Fresh human blood samples were collected in tubes containing EDTA, then centrifuged (15 min, 2500 × g), and the plasma and buffy coat (leukocytes) removed. The packed red cells were washed three times with KCl (0.16 M), hemolyzed with five times the volume of ice-cold water, and then centrifuged (4°C, 10,000 × g, for 30 min), to remove the ghosts and intact cellsCitation11.
Ammonium sulfate precipitation
The hemolysate was subjected to precipitation with ammonium sulfate (gradient 35–65%). Enzyme activity was determined both in the supernatant and in the precipitate for each respective precipitation. The precipitated enzyme was dissolved in phosphate buffer (50 mM, pH 7.0), and gave a clear solution which contained partially purified enzymeCitation11.
Purification of 6PGD
The ammonium sulfate fraction (35–65%) of the hemolysate obtained as above was loaded onto a 2′;,5′-ADP Sepharose 4B affinity column and the flow rate was adjusted to 20 mL/h. The column was then sequentially washed with a 25 mL buffer of 0.1 M K-acetate and 0.1 M K-phosphate (pH 6.0) and 25 mL of a buffer of 0.1 M K-acetate and 0.1 M K-phosphate (pH 7.85). Washing continued until an absorbance of 0.05 at 280 nm was obtained. Elution of the enzyme was carried out with a mixture containing 80 mM K-phosphate, 80 mM KCl, 5 mM NADP+, and 10 mM EDTA (pH 7.85). Enzyme activity was measured in all fractions, and the activity-containing fractions were pooled, then dialyzed in 50 mM K-acetate + 50 mM K-phosphate buffer (pH 7.0) for 2 h with two changes of buffer. All procedures were performed at 4°CCitation11.
Protein determination
The protein content in all samples was quantified spectrophotometrically at 595 nm according to Bradford’s methodCitation13, using bovine serum albumin as standard.
SDS polyacrylamide gel electrophoresis
Enzyme purity was examined using Laemmli’s procedureCitation14 with 3% and 8% acrylamide concentrations for running and stacking gel, respectively. Bovine albumin (66,000), chicken ovalbumin (45,000), and bovine carbonic anhydrase (29,000) were used as standards (Sigma: MW-SDS-200) (see ).
In vitro effect of drugs
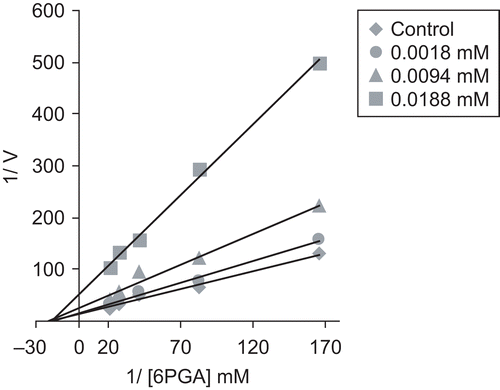
In order to determine the effects of some drugs on human 6PGD, concentrations of ketotifen (0.0018–0.0282 mM), dacarbazine (0.0049–0.054 mM), meloxicam (0.02–0.285 mM), furosemide (0.03–0.6 mM), methotrexate (0.036–0.55 mM), metochloropramide hydrochloride (0.83–8.335 mM), ritodrine hydrochloride (1.54–15.4 mM), and gadopentetic acid (24.99–249.935 mM) were added to the reaction mixture and the enzyme activity was measured. An experiment in the absence of drug was used as control (100% activity). The IC50 values were obtained from activity (%) vs. drug concentration plots (for example ). In order to determine the Ki values, the substrate (6-PGA) concentrations were 0.006, 0.012, 0.024, 0.036, 0.048 mM for ketotifen, dacarbazine, meloxicam, furosemide, methotrexate, and 0.003, 0.006, 0.012, 0.024, 0.036 mM for metochloropramide hydrochloride, ritodrine hydrochloride, gadopentetic acid. Inhibitor (drug) solutions were added to the reaction mixture at three different fixed concentrations. Lineweaver–Burk graphsCitation15 were drawn using 1/V vs. 1/[S] values, and the Ki values were calculated from these graphs (see ). Regression analysis graphs were drawn using percentage inhibition values vs. drug concentration using a statistical package (SPSS for Windows; version 10.0) on a computer (Student t-test; n = 3).
Results
Purification of the enzyme led to a specific activity of 13.12 EU/mg protein, a yield of 58%, and 3364-fold purification (). Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) was performed after purification of the enzyme, and the electrophoretic pattern is shown in . IC50 values of ketotifen, dacarbazine, meloxicam, furosemide, methotrexate, metochloropramide hydrochloride, ritodrine hydrochloride, and gadopentetic acid were 0.0084, 0.0120, 0.0550, 0.1250, 0.1420, 3.2700, 3.6600, and 209.10 mM, and the Ki constants were 8.3 ± 1.7 μM (non-competitive), 10.1 ± 0.7 μM (non-competitive), 50.9 ± 13.2 μM (non-competitive), 127 ± 37.8 μM (non-competitive), 136.7 ± 25.3 μM (non-competitive), 2.1113 ± 0.6979 mM (non-competitive), 6.0353 ± 1.2783 mM (non-competitive), and 73.4 ± 21.9 mM (non-competitive), respectively (). Representative graphs are shown for ketotifen ( and ).
Table 1. Purification scheme of 6-phosphogluconate dehydrogenase from human erythrocytes.
Table 2. Ki values obtained from Lineweaver–Burk graphs for 6PGD in the presence of three fixed inhibitors and five substrate concentrations for different drugs.
Discussion
It is known that many drugs have adverse effects on the body when used for therapeutic or other purposesCitation16 which may be dramatic and systematicCitation17. A good example is that of pamaquine used for malaria treatment, which caused severe adverse effects in patients within a few days, resulting in black urination, hyperbilirubinemia, a dramatic decrease in blood Hb levels, and finally death, which occurred in cases of severe G6PD (glucose 6-phosphate dehydrogenase) deficiencyCitation18. Therefore, investigation of the effects of some drugs on the enzyme activity of human erythrocyte 6PGD is very important.
Here, human erythrocyte 6PGD enzyme was purified with a 58% yield and 3364-fold purification in 5–6 hours using 2′,5′-ADP Sepharose 4B affinity gel chromatography (). SDS-PAGE showed that high purity for the enzyme had been obtained (). The inhibitory effects of some drugs on 6PGD activity in humans have been reported in several investigationsCitation9–11. For example, it has been reported that netilmicin sulfate, cefepime, amikacin, isepamycin, chloramphenicol, ceftazidim, teicoplanin, ampicillin, ofloxacin, levofloxacin, cefotaxime, penicillin G, gentamicin sulfate, ciprofloxacin, cefozin, decefin, streptomycin, combisid, meronem, larnoxicam, metronidazole, imipenem, ornidazole, vancomycin, clindamycin, and amoxicillin inhibit human erythrocyte 6PGDCitation10,Citation11, and that ampicillin and amikacin inhibit rat red-cell 6-PGACitation9. However, to the best of our knowledge, the inhibitory effects of the drugs examined here on human erythrocyte 6PGD have not been studied. In order to show the inhibitory effects, while the most suitable parameter is the Ki constant, some researchers use the IC50 valueCitation9,Citation10. Therefore, in this study, both the Ki and IC50 parameters of these drugs for 6PGD were determined. IC50 values of ketotifen, dacarbazine, meloxicam, furosemide, methotrexate, metochloropramide hydrochloride, ritodrine hydrochloride, and gadopentetic acid were 0.0084, 0.0120, 0.0550, 0.1250, 0.1420, 3.2700, 3.6600, and 209.10 mM, respectively. Ki constants of ketotifen, dacarbazine, meloxicam, furosemide, methotrexate, metochloropramide hydrochloride, ritodrine hydrochloride, and gadopentetic acid were 8.3 ± 1.7 μM (non-competitive), 10.1 ± 0.7 μM (non-competitive), 50.9 ± 13.2 μM (non-competitive), 127 ± 37.8 μM (non-competitive), 136.7 ± 25.3 μM (non-competitive), 2.1113 ± 0.6979 mM (non-competitive), 6.0353 ± 1.2783 mM (non-competitive), and 73.4 ± 21.9 mM (non-competitive), respectively (). In this study, the drugs inhibited 6PGD activity, compared with the control group. The drugs can cause non-competitive inhibition by binding to other sites affecting the three-dimensional structure of the enzymeCitation20.
The Ki constants show that ketotifen had the highest inhibitory effect, followed by dacarbazine, meloxicam, furosemide, methotrexate, metochloropramide hydrochloride, ritodrine hydrochloride, and gadopentetic acid. The IC50 values showed the same trend. From this investigation, by using the obtained Ki and IC50 values, undesirable side effects of these drugs on 6PGD activity and body metabolism and fatty acid synthesis can be reduced. According to these data, if it is required to administer ketotifen, dacarbazine, and meloxicam to patients, their dosage should be very well controlled to decrease hemolytic and other side effects due to possible inhibition of 6PGD.
Declaration of interest
This research was financed by grants from of the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project No: 108T213).
References
- Bianchi D, Bertrant O, Haupt K, Coello N. Effect of gluconic acid as a secondary carbon source on non-growing L-lysine producers cells of Corynebacterium glutamicum. Purification and properties of 6-phosphogluconate dehydrogenase. Enzyme Microb Technol 2001;28:754–9.
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry, 3rd ed. New York: Worth, 2000:558–60.
- Kahler SG, Kirkman HN. Intracellular glucose-6-phosphate dehydrogenase does not monomerize in human erythrocytes. J Biol Chem 1983;258:717–18.
- Srivastava SK, Beutler E. Glutathione metabolism of the erythrocyte. The enzymic cleavage of glutathione-haemoglobin preparations by glutathione reductase. Biochem J 1989;119:353.
- Kozar RA,Weibel CJ, Cipolla J, Klein AJ, Haber MM, Abedin MZ, et al. Antioxidant enzymes are induced during recovery from acute lung injury. Crit Care Med 2000;28:2486–91.
- Beutler E. Blood 1994;84:3613–36.
- Edward E, Morse MD. Toxic effects of drugs on erythrocytes. Ann Clin Lab Sci 1988;18:13–18.
- Jacobasch G, Rapoport SM. Hemolytic anemias due to erythrocyte enzyme deficiencies. Mol Aspects Med 1996;17:143–70.
- Ciftci M, Beydemir S, Yılmaz H, Bakan E. Effects of some drugs on rat erythrocyte 6-phosphogluconate dehydrogenase: An in vitro and in vivo study. J Pharmacol 2002;54:275–80.
- Akyuz M, Erat M, Ciftci M, Gumustekin K, Bakan N. Effects of some antibiotics on human erythrocyte 6-phosphogluconate dehydrogenase: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2004;19:361–5.
- Ciftci M, Adem A. Effects of some drugs on human erythrocyte 6-phosphogluconate dehydrogenase: an in vitro study. J Enzyme Inhib Med Chem 2007;22:751–4.
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. London: Academic Press, 1971:12.
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–51.
- Laemmli DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–3.
- Segel IE. Enzyme Kinetics. Toronto: John Wiley and Sons, 1975.
- Hochster R, Kates MM, Quastel JH. Metabolic Inhibitors. New York: Academic Press, 1972:71–89.
- Ciftci M, Kufrevioglu OI, Gundogdu M, Ozmen I. Effects of some antibiotics on enzyme activity of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 2000;41:109–13.
- Keha EE, Kufrevioglu OI. Biyokimya, 2nd ed. Istanbul: Aktif Yayinevi, 2009:356–66. [in Turkish]
- Gumustekin K, Ciftci M, Coban A, Altikat S, Aktas O, Gul M, et al. Effects of nicotine and vitamin E on glucose 6-phosphate dehydrogenase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem 2005;20:497–502.

![Figure 2. Activity % vs. [Ketotifen] regression analysis graph for human erythrocyte 6PGD in the presence of five different ketotifen concentrations.](/cms/asset/83ad7da7-5a43-49b0-8445-60aba1381bf6/ienz_a_425964_f0002_b.gif)