Abstract
A series of 4-thiazolidinones bearing a sulfonamide group (4a–w) were prepared by cyclizing various 5-bromo-2-methoxy-N’-[(1E)-arylmethylene/arylethylidene]benzenesulfonohydrazides. All the compounds were characterized by IR, 1H NMR, and elemental analysis. The compounds were tested for their anticonvulsant activity utilizing MES and scPTZ animal models. The majority of the compounds exhibited significant activity against both animal models; however, compounds 4c, 4m, and 4o displayed promising activity and could be considered as leads for further investigations.
Introduction
Epilepsy is a common chronic neurological disorder that is characterized by recurrent unprovoked seizuresCitation1. Different types of epilepsy are not based on a single underlying mechanism but are multifactorial in origin. It has been postulated that these seizures are transient signs and/or symptoms due to abnormal, excessive, or synchronous neuronal activity in the brainCitation2. In a considerable number of epilepsies, genetic or familial disposition also plays an important role in seizure precipitation. Epilepsy is usually controlled but not cured with medication, and the literature reveals that over 30% of people with epilepsy do not have seizure control, even with the best available medicationsCitation3,Citation4. Thus, the search for new anticonvulsant drugs continues to be an active area of investigation in medicinal chemistry.
Antiepileptic drugs belong to many different chemical classes of compoundsCitation5,Citation6. The most common structural elements of the older generation of clinically active antiepileptic drugs derived from hydantoins, oxazolidinediones, succinimides, and glutarimides can be defined as a nitrogen-containing heteroatomic system bearing one or two phenyl rings and at least one carbonyl groupCitation7,Citation8. Moreover, several sulfonamide/sulfamate derivatives (acetazolamide, topiramate, zonisamide, etc.) have already been in clinical use, and the anticonvulsant effects of these or related sulfonamides are probably due to CO2 retention followed by inhibition of red-cell and brain enzymesCitation9–12.
Previously we have reported several heterocyclic compounds showing considerable anticonvulsant activityCitation13–15. In the course of our investigations on heterocyclic moieties aimed at developing newer anticonvulsants, a number of sulfonamides incorporating thiazolidin-4-ones have been synthesized and evaluated for anticonvulsant activity. The presence of sulfur in the sulfonamide as well as thiazolidin-4-one moieties of the titled compounds was expected to increase the lipophilicity, consequently elevating drug concentration in the brain.
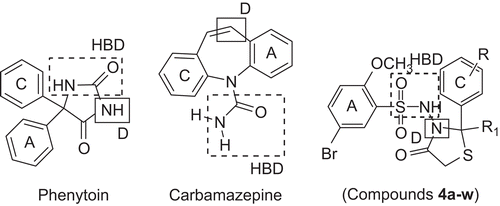
All the synthesized compounds showed the four pharmacophoric elements () that are necessary for good anticonvulsant activity as suggested by Pandeya et al.Citation16. These elements are present in many currently used antiepileptic drugs. They are the hydrophobic domain (A), hydrogen bonding domain (HBD), electron donor moiety (D), and distal hydrophobic domain (C). The attachment of a second aryl ring designated as the distal ring to the proximal aryl ring to increase the van der Waals bonding at the binding site and to increase potency has also been reportedCitation17. The present work further gives impetus to these observations. Distance mapping of the synthesized compounds was also performed with the help of the given model.
Materials and methods
Chemistry
Melting points were taken in open capillary tubes and are uncorrected. 1H nuclear magnetic resonance (NMR) spectra (400 MHz) were recorded on a Bruker model DRX 400 NMR spectrometer in dimethylsulfoxide (DMSO)-d6 using tetramethylsilane (TMS) as internal standard. Infrared (IR) spectra were recorded on a Bio-Rad FTS 135 spectrometer using a KBr pellet. Elemental analysis was performed using a Vario EL III CHNS analysis system (Elementar, Germany). Thin layer chromatography (TLC) was carried out using silica gel 60 F254 plates (Merck). All the chemicals and solvents used were obtained from Merck.
General procedure for the synthesis of titled compounds (4a–w)
5-Bromo-2-methoxybenzenesulfonyl chloride (1) p-Bromoanisole (0.10 mol, 18.7 g) was gradually added to chlorosulfuric acid (3 mol, 20 mL) at 0°C with constant stirring. The reaction mixture was left for 1 h at room temperature and then poured on crushed ice to give a white powder.
5-Bromo-2-methoxybenzenesulfonohydrazide (2) The sulfonyl chloride (1) (0.03 mol, 10 g) treated with hydrazine hydrate (98%, 3 mol, 6 mL) in aqueous ethanol (10 mL) at 0°C was set aside overnight at 0°C. The hydrazide was obtained as a white powder.
5-Bromo-2-methoxy-N’-[(1E)-arylmethylene/arylethylidene]benzenesulfonohydrazides (3a–w) To the hydrazide (2) (0.11 mol) in glacial acetic acid (5 mL) ethanol (10 mL) was added and refluxed with aromatic aldehydes and ketones (0.11 mol) for 5–8 h. The reaction mixture was cooled to room temperature and kept overnight. The solid collected was washed with methanol, dried in open air, and recrystallized from methanol to get the hydrazones (3a–w).
5-Bromo-2-methoxy-N-(2-alkyl-4-oxo-2-aryl-1,3-thiazolidin-3-yl)benzenesulfonamides (4a–w) A mixture of hydrazones (3a–w) (0.01 mol) and thioglycolic acid (0.01 mol) in 25 mL dioxane was taken in a round-bottomed flask. To this solution a pinch of zinc chloride was added and the reaction mixture was refluxed for 8–10 h. The mixture was then poured on crushed ice and the solid so obtained was filtered, washed with water, dried in air, and recrystallized from dioxane.
5-Bromo-2-methoxy-N-(2-methyl-4-oxo-2-phenyl-1,3-thiazolidin-3-yl)benenesulfonamide (4a) IR (KBr) cm−1: 3458 (N-H), 3024 (C-H), 1723 (C = O), 1312 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.01 (s, 3H, CH3), 3.74 (s, CH2-thia.), 3.83 (s, 3H, OCH3), 7.04–7.06 (d, 1H, Ar-H), 7.23–7.67 (m, 5H, Ar-H), 7.89–7.91 (d, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 10.57 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(2-hydroxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4b) IR (KBr) cm−1: 3237 (O-H), 3236 (N-H), 3019 (C-H), 1683 (C = O), 1319 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.00 (s, 3H, CH3), 3.65 (s, CH2-thia.), 3.81 (s, 3H, OCH3), 7.00–7.08 (m, 3H, Ar-H), 7.14 (s, 1H, OH), 7.49–7.63 (m, 2H, Ar-H), 7.89–7.91 (d, 1H, Ar-H), 8.07 (s, 1H, Ar-H), 9.87 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(3-hydroxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4c) IR (KBr) cm−1: 3450 (O-H), 3447 (N-H), 2961 (C-H), 1716 (C = O), 1302 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 1.98 (s, 3H, CH3), 3.69 (s, CH2-thia.), 3.78 (s, 3H, OCH3), 6.67–6.73 (m, 1H, Ar-H), 7.04–7.06 (d, 1H, Ar-H), 7.23 (s, 1H, Ar-H), 7.35–7.41 (m, 1H, Ar-H) 7.89–7.91 (d, 1H, Ar-H), 8.03 (s, 1H, Ar-H), 8.23 (s, 1H, OH) 9.79 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(4-hydroxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4d) IR (KBr) cm−1: 3546 (OH), 3543 (N-H), 2919 (C-H), 1769 (C = O), 1308 (NH-SO2); 1H NMR (DMSO-d6) δppm: 2.03 (s, 3H, CH3), 3.71 (s, CH2-thia.), 3.80 (s, 3H, OCH3), 7.07-7.09 (d, 1H, Ar-H), 7.10–7.12 (d, 2H, Ar-H), 7.54–7.56 (d, 2H, Ar-H), 7.82–7.84 (d, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 9.84 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(2,4-dihydroxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4e) IR (KBr) cm−1: 3439 (O-H), 3435 (N-H), 2998 (C-H), 1669 (C = O), 1311 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.00 (s, 3H, CH3), 3.72 (s, CH2-thia.), 3.81 (s, 3H, OCH3), 6.45 (s, 1H, Ar-H), 7.04–7.15 (m, 5H, Ar-H and 2 × OH), 7.88–7.90 (d, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 9.91 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(2-methoxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl] benzenesulfonamide (4f) IR (KBr) cm−1: 3316 (N-H), 3053 (C-H), 1719 (C = O), 1318 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.01 (s, 3H, CH3), 3.69 (s, CH2-thia.), 3.84 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.69–7.21 (m, 5H, Ar-H), 7.83–7.85 (d, 1H, Ar-H), 8.12 (s, 1H, Ar-H), 10.21 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(3-methoxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl] benzenesulfonamide (4g) IR (KBr) cm−1: 3428 (N-H), 3037 (C-H), 1673 (C = O), 1301 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.01 (s, 3H, CH3), 3.70 (s, CH2-thia.), 3.81 (s, 3H, OCH3), 3.94 (s, 3H, OCH3) 7.04–7.06 (d, 1H, Ar-H), 7.23–7.67 (m, 5H, Ar-H), 7.81–7.83 (d, 1H, Ar-H), 8.06 (s, 1H, Ar-H), 10.18 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(4-methoxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl] benzenesulfonamide (4h) IR (KBr) cm−1: 3433 (N-H), 2942 (C-H), 1659 (C = O), 1307 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 1.94 (s, 3H, CH3), 3.76 (s, CH2-thia.), 3.72 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 7.06–7.08 (d, 1H, Ar-H), 7.23–7.25 (d, 1H, Ar-H) 7.56–7.59 (d, 1H, Ar-H), 7.87–7.89 (d, 1H, Ar-H), 8.01 (s, 1H, Ar-H), 9.51 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(2,4-dimethoxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl] benzenesulfonamide (4i) IR (KBr) cm−1: 3435 (N-H), 2998 (C-H), 1715 (C = O), 1319 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 1.94 (s, 3H, CH3), 3.53 (s, 3H, OCH3), 3.69 (s, 2H, CH2-thia.), 3.78 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 6.62 (s, 1H, Ar-H), 6.78–6.80 (d, 1H, Ar-H), 7.01–7.03 (d, 1H, Ar-H), 7.47–7.49 (d, 1H, Ar-H), 7.83–7.95 (d, 1H, Ar-H), 8.05 (s, 1H, Ar-H), 9.38 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(3,4-dimethoxyphenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl] benzenesulfonamide (4j) IR (KBr) cm−1: 3428 (N-H), 3052 (C-H), 1722 (C = O), 1315 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 1.98 (s, 3H, CH3), 3.70 (s, 6H, 2 × OCH3), 3.73 (s, 2H, CH2-thia.), 3.81 (s, 3H, OCH3), 7.00–7.11 (m, 4H, Ar-H), 7.82–7.84 (d, 1H, Ar-H), 7.96 (s, 1H, Ar-H), 9.41 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(2-chlorophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4k) IR (KBr) cm−1: 3437 (N-H), 2983 (C-H), 1719 (C = O), 1310 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.05 (s, 3H, CH3), 3.76 (s, 2H, CH2-thia.), 3.82 (s, 3H, OCH3), 7.08–7.10 (d, 1H, Ar-H), 7.29–7.53 (m, 4H, Ar-H), 7.89–7.91 (d, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 10.46 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(3-chlorophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4l) IR (KBr) cm−1: 3376 (N-H), 3055 (C-H), 1689 (C = O), 1306 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.03 (s, 3H, CH3), 3.73 (s, 2H, CH2-thia.), 3.84 (s, 3H, OCH3), 7.07–7.09 (d, 1H, Ar-H), 7.16–7.54 (m, 4H, Ar-H), 7.93–7.95 (d, 1H, Ar-H), 8.13 (s, 1H, Ar-H), 10.39 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(4-chlorophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4m) IR (KBr) cm−1: 3453 (N-H), 2978 (C-H), 1712 (C = O), 1305 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.07 (s, 3H, CH3), 3.78 (s, 2H, CH2-thia.), 3.87 (s, 3H, OCH3), 7.01–7.03 (d, 1H, Ar-H), 7.58–7.60 (d, 2H, Ar-H), 7.69–7.71 (d, 2H, Ar-H), 7.92–7.94 (d, 1H, Ar-H), 8.16 (s, 1H, Ar-H), 10.47 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(2,4-dichlorophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4n) IR (KBr) cm−1: 3438 (N-H), 2982 (C-H), 1719 (C = O), 1314 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.05 (s, 3H, CH3), 3.76 (s, 2H, CH2-thia.), 3.85 (s, 3H, OCH3), 7.05–7.07 (d, 1H, Ar-H), 7.40–7.42 (d, 1H, Ar-H), 7.90–7.92 (d, 1H, Ar-H), 7.95 (s, 1H, Ar-H), 8.10 (s, 1H, Ar-H), 8.21–8.23 (d, 1H, Ar-H), 10.53 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(4-bromophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4o) IR (KBr) cm−1: 3445 (N-H), 3051 (C-H), 1689 (C = O), 1318 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.03 (s, 3H, CH3), 3.77 (s, 2H, CH2-thia.), 3.86 (s, 3H, OCH3), 7.09–7.11 (d, 1H, Ar-H), 7.64–7.66 (d, 2H, Ar-H), 7.96–7.98 (d, 1H, Ar-H), 8.00–8.02 (d, 1H, Ar-H), 8.15 (s, 1H, Ar-H), 10.37 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(2-nitrophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4p) IR (KBr) cm−1: 3419 (N-H), 2998 (C-H), 1710 (C = O), 1321 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.17 (s, 3H, CH3), 3.72 (s, 2H, CH2-thia.), 3.85 (s, 3H, OCH3), 7.13–7.15 (d, 1H, Ar-H), 7.56–7.87 (m, 4H, Ar-H), 7.93–7.95 (d, 1H, Ar-H), 8.17 (s, 1H, Ar-H), 10.71 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(3-nitrophenyl)-2-methyl-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzene sulfonamide (4q) IR (KBr) cm−1: 3431 (N-H), 2984 (C-H), 1715 (C = O), 1319 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.09 (s, 3H, CH3), 3.75 (s, 2H, CH2-thia.), 3.87 (s, 3H, OCH3), 7.09–7.11 (d, 1H, Ar-H), 7.76–7.80 (t, 1H, Ar-H), 7.90–7.92 (d, 1H, Ar-H), 8.04–8.19 (m, 3H, Ar-H), 8.31 (s, 1H, Ar-H), 10.69 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-[2-(4-nitrophenyl)-4-oxo-1,3-thiazolidin-3-yl]-2-methoxy benzenesulfonamide (4r) IR (KBr) cm−1: 3438 (N-H), 2997 (C-H), 1718 (C = O), 1315 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.06 (s, 3H, CH3), 3.79 (s, 2H, CH2-thia.), 3.84 (s, 3H, OCH3), 7.07–7.09 (d, 1H, Ar-H), 7.91–7.93 (d, 1H, Ar-H), 7.95–7.97 (d, 2H, Ar-H), 8.19 (s, 1H, Ar-H), 8.32–8.34 (d, 1H, Ar-H), 10.73 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-(4-oxo-2-phenyl-1,3-thiazolidin-3-yl)benzenesulfonamide (4s) IR (KBr) cm−1: 3443 (N-H), 2988 (C-H), 1712 (C = O), 1306 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 3.67 (s, 2H, CH2-thia.), 3.80 (s, 3H, OCH3), 6.25 (s, 1H, CH-thia.), 7.05–7.07 (d, 1H, Ar-H), 7.30–7.52 (m, 5H, Ar-H), 7.85–7.97 (d, 1H, Ar-H), 7.97 (s, 1H, Ar-H), 8.19 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(2-methylphenyl)-4-oxo-1,3-thiazolidin-3-yl]benzene sulfonamide (4t) IR (KBr) cm−1: 3419 (N-H), 2971 (C-H), 1718 (C = O), 1311 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.58 (s, 3H, CH3), 3.63 (s, 2H, CH2-thia.), 3.79 (s, 3H, OCH3), 6.16 (s, 1H, CH-thia.), 7.02–7.60 (m, 5H, Ar-H), 7.85–7.87 (d, 1H, Ar-H), 7.94 (s, 1H, Ar-H), 8.15 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(3-methylphenyl)-4-oxo-1,3-thiazolidin-3-yl]benzene sulfonamide (4u) IR (KBr) cm−1: 3426 (N-H), 2985 (C-H), 1689 (C = O), 1317 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.33 (s, 3H, CH3), 3.65 (s, 2H, CH2-thia.), 3.82 (s, 3H, OCH3), 6.29 (s, 1H, CH-thia.), 6.99–7.32 (m, 5H, Ar-H), 7.82–7.84 (d, 1H, Ar-H), 7.91 (s, 1H, Ar-H), 8.21 (s, 1H, NH, D2O exchangeable).
5-Bromo-2-methoxy-N-[2-(4-methylphenyl)-4-oxo-1,3-thiazolidin-3-yl]benzene sulfonamide (4v) IR (KBr) cm−1: 3429 (N-H), 2991 (C-H), 1701 (C = O), 1320 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 2.29 (s, 3H, CH3), 3.68 (s, 2H, CH2-thia.), 3.76 (s, 3H, OCH3), 6.21 (s, 1H, CH-thia.), 7.05–7.07 (d, 1H, Ar-H), 7.21–7.23 (d, 2H, Ar-H), 7.59–7.61 (d, 2H, Ar-H) 7.85–7.87 (d, 1H, Ar-H), 7.96 (s, 1H, Ar-H), 8.13 (s, 1H, NH, D2O exchangeable).
5-Bromo-N-{2-[4-(dimethylamino)phenyl]-4-oxo-1,3-thiazolidin-3-yl}-2-methoxy benzene sulfonamide (4w) IR (KBr) cm−1: 3464 (N-H), 2983 (C-H), 1712 (C = O), 1323 (NH-SO2); 1H NMR (DMSO-d6) δ ppm: 3.06 (s, 6H, 2 × CH3), 3.58 (s, 2H, CH2-thia.), 3.74 (s, 3H, OCH3), 6.19 (s, 1H, CH-thia.), 6.73–6.75 (d, 2H, Ar-H) 7.07–7.09 (d, 1H, Ar-H), 7.21–7.23 (d, 2H, Ar-H), 7.85–7.87 (d, 1H, Ar-H), 7.89 (s, 1H, Ar-H), 8.07 (s, 1H, NH, D2O exchangeable).
Pharmacology
Anticonvulsant activity
The investigations were conducted on Swiss albino mice of either sex (25–30 g). Food and water were withdrawn prior to the experiments. All the compounds (4a–w) were dissolved in polyethylene glycol. Initially all compounds were administered intraperitoneally (ip) at doses of 30, 100, and 300 mg/kg to mice. Activity was established using the maximal electroshock seizure (MES) and subcutaneous pentylenetetrazole (scPTZ) tests according to the protocol of the Antiepileptic Drug Development Program (ADD), Epilepsy Branch, National Institute of Health, Bethesda, MD, USACitation18,Citation19.
MES test Mice were prescreened 24 h beforehand by delivering a maximal electroshock of 50 mA, 60 Hz, and 0.2 s duration by means of corneal electrodes. A drop of 0.9% sodium chloride was instilled in each eye prior to application of the electrodes in order to prevent death of the animal. The abolition of the hind-limb tonic extensor component of the seizure in half or more of the animals was defined as protection.
scPTZ test The scPTZ test utilized a dose of pentylenetetrazole 70 mg/kg. This produced clonic seizures lasting for a period of at least 5 s. The test compounds were administered at the three graded doses, i.e. 30, 100, and 300 mg/kg ip. At the anticipated time, the convulsant was administered subcutaneously. Animals were observed over a 30 min period. The absence of clonic spasm in half or more of the animals in the observed time period indicated a compound’s ability to abolish the effect of pentylenetetrazole on the seizure threshold.
Toxicity studies
Neurotoxicity The minimal motor impairment was measured in mice by the rotarod testCitation20. The mice were trained to stay on an accelerating rotarod of diameter 3.2 cm that rotated at 10 rpm. Neurotoxicity was indicated by the inability of the animal to maintain equilibration on the rod for at least 1 min in each of three trials. The dose at which 50% of the animals were unable to balance themselves and fell off the rotating rod was determined.
Ethanol potentiation test Mice were treated with the test compound and 1 h later with ethanol 2.5 g/kg ip. This dose of ethanol did not induce a lateral position in the control animals. The number of animals that were in the lateral position after receiving ethanol in each group was determinedCitation21.
In vitro GABA-transminase inhibition assay
Since several sulfonamide derivatives have already been found to show anticonvulsant activity due to CO2 retention followed by inhibition of red-cell and brain enzymes, the most active compounds were tested in vitro against the γ-aminobutyric acid-transaminase (GABA-T) enzyme and the inhibition was assessed. The GABA-T enzyme was isolated from Pseudomonas fluorescens using a method described earlierCitation22. The assay was performed for a 4 h time period according to the standard protocolCitation23.
Distance mapping
In conformational analysis of the older-generation clinically active anticonvulsant drugs such as phenytoin, carbamazepine, lamotrigene, rufinamide, remacemide, and phenobarbitone, a molecular model was suggested on the basis of molecular dynamics distance estimationsCitation24. According to these, an electron donor (D) should be at a distance of 3.2–5.1 Å from an aryl ring or any other hydrophobic unit (C) and 3.9–5.5 Å from the hydrogen bonding domain (HBD). For the molecular mechanics calculations, the ACD/Chemsketch/3-D viewer version 2.0 program was used to employ the CHARMM force fieldCitation8.
Results and discussion
Chemistry
The synthetic pathway giving access to the titled compounds (4a–w) is illustrated in . The synthesis of 5-bromo-2-methoxybenzenesulfonyl chloride (1) involved a reaction between p-bromoanisole and chlorosulfuric acid. In the subsequent step, 5-bromo-2-methoxybenzenesulfonohydrazide (2) was synthesized by the treatment of compound 1 with hydrazine hydrate. The aromatic aldehydes and ketones were refluxed with compound 2 in glacial acetic acid, yielding 5-bromo-2-methoxy-N’-[(1E)-arylmethylene/arylethylidene]benzenesulfonohydrazide (3a–w). In the last step, compounds 3a–w were cyclized in the presence of zinc chloride to afford the final compounds 4a–w. The physicochemical parameters of all the synthesized compounds are given in .
Scheme 1. Synthetic route to the titled compounds 4a–w. Reagents and conditions: (a) HClSO4, 0°C; (b) NH2NH2.H2O, 0°C, ethanol; (c) CH3COOH, RCOR1, reflux; (d) HSCH2COOH, ZnCl2, reflux.
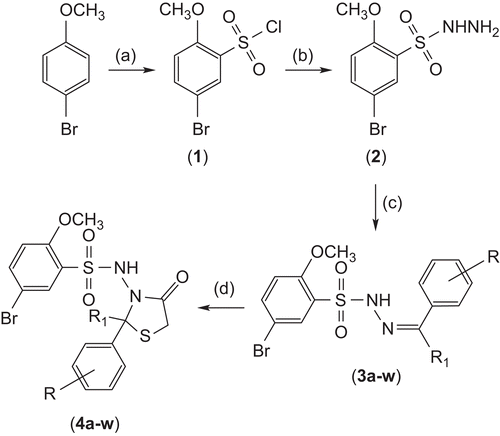
Table 1. Physical characterization data of compounds 4a–w.
All the synthesized compounds were well characterized by elemental analysis and spectroscopic data. In IR spectra, absorption bands for N-H, O-H, C-H, C = O, and NH-SO2 were found in the regions 3544–3236, 3055–2919, 1769–1659, and 1323–1301 cm−1, respectively. The 1H NMR spectra showed a distinct singlet at δ values 3.79–3.58 ppm assigned for CH2-thiazolidinones. Various singlets, doublets, triplets, and multiplets were found in aromatic zones. A singlet at δ values ranging from 10.73 to 8.07 ppm was assigned to N-H attached to the phenyl ring.
Pharmacology
Anticonvulsant evaluation of compounds 4a–w in mice utilizing MES and scPTZ models is summarized in together with the neurotoxicity and ethanol potentiation data. To obtain information about undesired side effects, the highly and moderately active compounds were subjected to neurotoxicity (rotorod) and ethanol potentiation tests.
Table 2. Anticonvulsant and neurotoxicity data of compounds 4a–w.
Preliminary evaluation of all the synthesized compounds was performed against two well-established seizure models, namely MES and scPTZ. All the compounds showed encouraging anticonvulsant activity. Compounds 4c, 4m, and 4o were found to be highly active against the MES test at a dose level of 30 mg/kg at 0.5 h time interval, indicative of their ability to prevent seizure spread at a relatively low dose. Compounds that exhibited moderate protection against the MES model at 100 mg/kg included 4b, 4d, 4e, 4j, 4k, 4l, 4q, 4s, and 4w, at 0.5 h. Thus, the majority of the compounds showed encouraging anticonvulsant activity at the 0.5 h interval, indicating rapid onset and shorter duration of action.
In the chemoshock investigation, those compounds that exhibited considerable activity in the MES test were chosen for scPTZ study. Compounds 4d, 4e, 4m, and 4w were found to be active after 0.5 h of drug administration at a dose of 100 mg/kg.
In neurotoxicity studies, ethanol potentiation and rotorod tests were employed to estimate the undesired effects such as sedation and ataxia produced by the compounds. The ethanol potentiation test was performed in parallel with the rotorod test to investigate the neurotoxic effects of the compounds, by inducing a lateral position in the animals. Compounds 4b, 4e, 4j, 4k, 4o, 4q, and 4w showed an interaction with ethanol, thereby potentiating the effect of ethanol, whereas compounds 4c, 4d, 4l, 4m, and 4s did not interact with ethanol. In the rotorod test, compounds 4c, 4e, 4j, 4o, 4q, and 4w were less neurotoxic, and the rest of the compounds did not exhibit neurotoxicity.
The correlation of lipophilicity (log P) and in vivo anticonvulsant activity as well as neurotoxicity was established and found to be non-linear. The lipophilicity of highly active compound 4c was found to be minimum (log P = 2.14), whereas the other highly active compounds 4m (log P = 3.47) and 4o (log P = 3.65) were moderately lipophilic. Paradoxically, the least active compound 4n showed high lipophilicity (log P = 4.08). Variable results were found between neurotoxicity and lipophilicity data.
In vitro GABA-T inhibition assay
In our quest to understand the mechanism involved in the anticonvulsant activity of the titled compounds, the three most active compounds (4c, 4m, and 4o) were subjected to the in vitro GABA-T inhibition assay. The GABA-transaminase enzyme has been found to be responsible for the metabolism of GABA, and therefore inhibition of the enzyme will result in an increased concentration of GABA in different brain regions. The results of the assay are presented in . All three compounds were found to inhibit the GABA-T enzyme at the 4 h time period. Compound 4c was found to inhibit GABA-T throughout the time points, but the maximum inhibition shown by 4c was 12% at 4 h. Compound 4m inhibited the enzyme at 2 h and maximum inhibition (8%) was observed at 4 h. Compound 4o exhibited maximum inhibition among these compounds. It showed inhibition at 1 h and continued to inhibit the enzyme significantly throughout the time points. The maximum inhibition of 30% at 4 h indicates the promising nature of this compound.
Table 3. In vitro GABA-transaminase inhibition assay of selected compounds.
Distance mapping
The present work further involved comparison of the structures of well known and structurally different compounds and the synthesized compounds. Comparison of the structures of the synthesized compounds and other molecules with anticonvulsant activity was performed to determine the structural elements essential for action. The compounds selected for this comparison have at least one aryl (C) hydrophobic domain, one electron donor (D), and a hydrogen bond acceptor/donor unit (HBD). In an initial study, molecular mechanics calculations using a force field based on CHARMM parameterization were performed to obtain an overview of their minimum conformation for bioactivity. shows the distances between the various groups postulated as essential for anticonvulsant action. The synthesized compounds were examined to check whether they reflected the conditions of the derived pharmacophore model. Analysis of the distance relationship showed that synthesized compounds 4a–w fulfilled the essential demands of the pharmacophore when compared with other known anticonvulsant drugs. In the case of the titled compounds, the distances C–D, C–HBD, and D–HBD were in conformity with the distances for active anticonvulsant drugs.
Table 4. Distance ranges between essential structural elements C, D, and HBD.
Conclusions
The sulfonamide derivatives of thiazolidin-4-one exhibited remarkable anticonvulsant activity with lesser neurotoxicity against the two animal models. Compounds 4c, 4m, and 4o were highly active in the MES test. In the scPTZ test, compounds 4m and 4o continued to be active, indicating the ability of these compounds to prevent more than one type of seizure. The anticonvulsant effects of these sulfonamide derivatives of 4-thiazolidinone are probably due to CO2 retention followed by inhibition of red-cell and brain enzymes.
Acknowledgments
The authors express their gratitude to Jamia Hamdard, New Delhi (India) for providing the necessary facility to carry out this research work.
Declaration of interest
One of the authors (M.F.A.) is thankful to the University Grants Commission (UGC), New Delhi for awarding a Junior Research Fellowship (JRF).
References
- Blume WT, Luders HO, Mizrahi E, Tassinari C, Boas WE, Engel J. Glossary of descriptive terminology for ictal semiology: report of the ILAE taskforce on application and terminology. Epilepsia 2001;42:1212–18.
- Fisher R, Boas WE, Blume W, Elger C, Genton P, Lee P. Epileptic seizures and epilepsy: definition proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470–2.
- Cascino GD. Epilepsy: contemporary perspective on evaluation and treatment. Mayo Clinic Proc 1994;69:1199–211.
- Engel J Jr. Surgery for seizures. N Engl J Med 1996;10:647–52.
- Saxena AK, Saxena M. In: Jucker E, ed. Progress in Drug Research. Basel: Birkhauser Verlag, 1995:185–291.
- Edafiogho IO, Scott KR. In: Wolff ME, ed. Burger’s Medicinal Chemistry and Drug Discovery. Chichester: John Wiley & Sons, 1996:175–sss260.
- Jones GP, Andrews PR. Structure–activity relations and receptor modelling of convulsant and anticonvulsant barbiturates from crystallographic data. J Chem Soc Perkin Trans II 1987:415–421.
- Wong MG, Defina JA, Andrews PR. Conformational analysis of clinically active anticonvulsant drugs. J Med Chem 1986;29:562–72.
- Masereel B, Rolin S, Abbate F, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valporyl and other lipophilic moieties. J Med Chem 2002;45:312–20.
- Thiry A, Dogne JM, Supuran CT, Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 2007;7:855–64.
- Thiry A, Dogne JM, Supuran CT, Masereel B. Anticonvulsant sulfonamides/sulfamates/sulfamides with carbonic anhydrase, inhibitory activity: drug design and mechanism of action. Curr Pharm Des 2008;14:661–71.
- Winum JY, Thiry A, Cheikh KE, Dogne JM, Montero JL, Vullo D, et al.Carbonic anhydrase inhibitors. Inhibition of isoforms I, II, IV, VA, VII, IX, and XIV with sulfonamides incorporating fructopyranose–thioureido tails. Bioorg Med Chem Lett 2007;17:2685–2691.
- Siddiqui N, Pandeya SN, Khan SA, Stables JP, Rana A, Alam M, et al. Synthesis and anticonvulsant activity of sulfonamide derivatives-hydrophobic domain. Bioorg Med Chem Lett 2007;17:255–9.
- Siddiqui N, Rana A, Khan SA, Bhat MA, Haque SE. Synthesis of benzothiazole semicarbazones as novel anticonvulsants – the role of hydrophobic domain. Bioorg Med Chem Lett 2007;17:4178–82.
- Rana A, Siddiqui N, Khan SA, Haque SE, Bhat MA. N-{[(6-Substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: synthesis and pharmacological evaluation. Eur J Med Chem 2008;43:1114–22.
- Pandeya SN, Yogeeswari P, Stables JP. Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur J Med Chem 2000;35:879–86.
- Yogeeswari P, Sriram D, Suniljit LRJ, Kumar SS, Stables JP. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur J Med Chem 2002;37:231–6.
- Swinyard EA, Woodhead JH, White SH, Franklin MR In: Levy RH, Mattson RH, Meldrum B, Penry JK, Dreifuss FE, eds.Antiepileptic Drugs, 3rd ed. New York: Raven Press, 1989:85–102.
- Kupferberg HJ. Antiepileptic drug development program: a cooperative effect of government and industry. Epilepsia 1989;30S:51–6.
- Clark CR, Wells MJM, Sansom RT, Norris GN, Dockens RC, Ravis WR. Anticonvulsant activity of some 4-aminobenzamides. J Med Chem 1984;27:779–82.
- Clerici F, Pocar D, Guido M, Loche A, Brufani VPM. Synthesis of 2-amino-5-sulfamyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J Med Chem 2001;44:931–6.
- Scott EM, Jakoby WB. Pyrrolidine metabolism: soluble gamma-aminobutyric transaminase and semialdehyde dehydrogenase. Science 1958;128:361–2.
- Lippert B, Metcalf BW, Jung MJ, Casara P. 4-Amino-hex-5-enoic acid, a selective catalytic inhibitor of 4-aminobutyyric aminotransferase in mammalian brain. Eur J Biochem. 1977;74:441–5.
- Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 1983;4:187–217.