Abstract
Two novel series of oxadiazole and oxadiazoline analogs possessing an indole nucleus were synthesized for their potential anti-inflammatory activity. The structures of the compounds were elucidated by elemental and spectral (IR, 1H-NMR, 13C-NMR, and MS) analysis. Most of the test compounds demonstrated appreciable anti-inflammatory activities. The anti-inflammatory activity of oxadiazoles at doses of 100 mg/kg was shown by their ability to provide 27–66%, 14–32%, and 20-51%. protection against carrageenan-induced rat paw edema, moist cotton pellet-induced, and dry cotton pellet-induced granuloma, respectively. On the other hand, the anti-inflammatory properties of oxadiazolines at doses of 100 mg/kg were reflected by their ability to provide 20-56%, 11–26%, and 25–47% protection against carrageenan-induced rat paw edema, moist cotton pellet-induced, and dry cotton pellet-induced granuloma, respectively. The ulcerogenic potential of the compounds was determined. Structure–activity relationships among synthesized compounds were also established.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used for the treatment of pain, fever, and inflammation, particularly arthritisCitation1,Citation2. The pharmacological activity of NSAIDs, including indomethacin, is related to the suppression of prostaglandin biosynthesis from arachidonic acid by inhibiting the cyclooxygenase (COX) enzymes. On chronic use of NSAIDs, one prominent side effect is the formation of gastric ulcers. This adverse effect may be attenuated in the presence of an inhibitor of 5-lipoxygenase (5-LO). 1,3,4-Oxadiazoles have been found to possess anti-inflammatory properties by virtue of a dual mechanism, i.e. inhibiting both COX and LO, reducing gastric ulcer formationCitation3,Citation4.
Numerous studies have been performed with the aim of exploring the anti-inflammatory properties of 1,3,4-oxadiazole analogsCitation5–12. These studies have shown that 1,3,4-oxadiazole analogs are equipotent with phenylbutazone, naproxen, and other NSAIDs. With the aim of finding a COX/LO dual inhibitor, which may have improved efficacy and fewer side effects compared with existing NSAIDs, we considered it of interest to synthesize novel 1,3,4-oxadiazole analogs to investigate their anti-inflammatory activities.
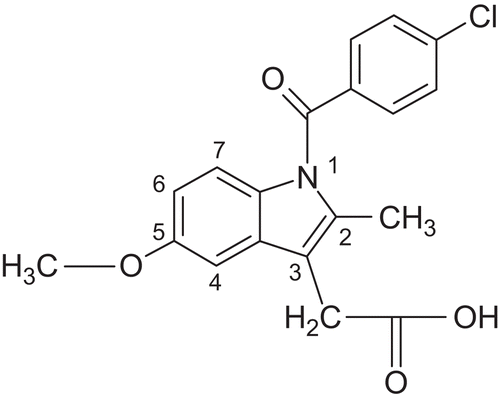
On the other hand, the various indole derivatives are well documented in the literature as potent anti-inflammatory agentsCitation13,Citation14. We designed title compounds with an indole nucleus in addition to a 1,3,4-oxadiazole nucleus, as indomethacin is one of the established anti-inflammatory drugs containing the indole nucleus. The long-term use of indomethacin causes gastric bleeding, which is also attributed to the presence of the carboxylic acid moiety in its structureCitation3, so we designed test compounds with an indole nucleus but not possessing a carboxylic group. We replaced the carboxylic acid moiety present at position 3 of the indole ring of indomethacin with other substitutents containing the 1,3,4-oxadiazole nucleus. We attempted to observe the effect of elimination from indomethacin of the 4-chlorobenzoyl group attached to the N-methyl group present at position 2 and the methoxy group present at position 5 on its biological activity (). Overall, it was interesting to observe the effect of combining indole and 1,3,4-oxadiazole nuclei in a single compound due to their different modes of action. The synthetic strategy employed in preparation of the title compounds is novel because no oxadiazole and oxadiazoline analogs with indol-1H-yloxymethyl substitution have been synthesized using oxidative cyclization with bromination in the presence of acetic acid.
Materials and methods
Chemistry
All the chemicals and reagents were obtained from Aldrich (USA), Himedia (India), and Spectrochem Pvt Ltd (India). Melting points were determined using the open capillary method and are uncorrected. Elemental analysis was done using an elemental analyzer Heraeus Carlo Erba-1108, infrared (IR) spectra were recorded on a PerkinElmer IR spectrophotometer (KBr disk) (PerkinElmer, Beaconsfield, UK), 13C-nuclear magnetic resonance (NMR) spectra on a Bruker DRX-300 NMR spectrometer (dimethylsulfoxide (DMSO)-d6, tetramethylsilane (TMS); Bruker Bioscience, Billerica, MA, USA), and electrospray mass spectra on a Micromass Quattro II triple-quadrupole mass spectrometer (methanol; Micromass, Manchester, UK). The title compounds were prepared using the synthetic strategy described in .
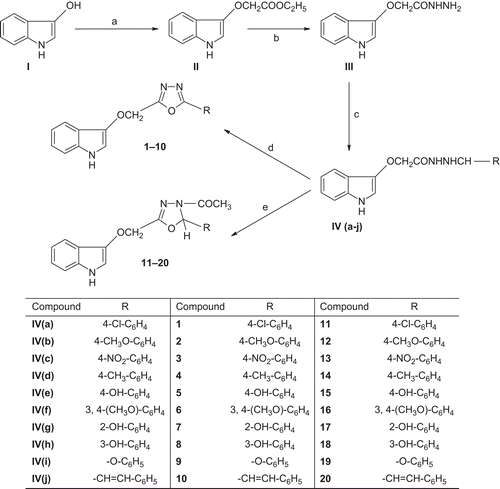
General procedure for synthesis of ethyl-(1H-indol-3-yloxy)-acetate (II)
1H-Indol-3-ol (0.01 mol) was refluxed with ethylchloroacetate (0.01 mol) in the presence of anhydrous acetone (30 mL) and anhydrous potassium carbonate (0.02 mol) for 12 h on a water bath. The reaction mixture was filtered and excess solvent was removed by distillation under reduced pressure. The crude product obtained was filtered and recrystallized with ethanol. Yield: 65%; m.p. 213°C; IR (KBr, cm−1): 3034.7 (aromatic C-H), 3234.1 (N-H of indole), 1602.4, 1503.9 (aromatic C-C), 2910.4 (aliphatic C-H str), 1743.3 (ester C=O str); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.4 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 4.9 (s, 2H, OCH2), 4.2 (q, J = 6.5 Hz, 2H, OCH2CH3), 1.4 (t, J = 7.2 Hz, 3H, OCH2CH3); ESMS (methanol) m/z 219 (M+).
General procedure for synthesis of 2-(1H-indol-3-yloxy)-acetatohydrazide (III)
The compound II (0.01 mol) was refluxed with hydrazine hydrate (0.01 mol) in absolute ethanol (30 mL) for 8 h. On cooling the reaction mixture in an ice bath, crude product was separated out. It was filtered and recrystallized with ethanol. Yield: 62%; m.p. 202°C; IR (KBr, cm−1): 3039.3 (aromatic C-H), 3231.5 (N-H of indole), 1601.8, 1503.6 (aromatic C-C), 1691.7 (amide C=O str), 3427.1 (amide NH str); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 4.9 (s, 2H, OCH2), 2.2 (t, J = 3.2 Hz, 1H, NHNH2), 7.9 (d, J = 4.4 Hz, 2H, NHNH2); ESMS (methanol) m/z 205 (M+).
General procedure for synthesis of 4-(4-substitutedbenzyl)-2-(1H-indol-3-yloxy)-acetohydrazide (IV)
A solution of III (0.01 mol) was prepared in 50 mL of absolute ethanol in a round-bottomed flask. The required aldehyde (0.01 mol) dissolved in 20 mL of absolute ethanol was added, dropwise, and the mixture was refluxed for 5–6 h. The solid mass, which separated out on cooling, was filtered and finally recrystallized from appropriate proportions of dimethylformamide (DMF) and water.
Compound IV(a) Yield: 60%; m.p. 232°C; IR (KBr, cm−1): 3033.5 (aromatic C-H), 3230.9 (N-H of indole), 1602.8, 1502.2 (aromatic C-C), 1694.4 (amide C=O str), 3435.6 (amide NH str), 836.9 (C-H def (para) disubstituted benzene), 723.6 (C-Cl str); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.4 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 4.8 (s, 2H, OCH2), 2.3 (d, J = 2.4 Hz, 1H, NHNHCH2), 8.0 (q, J = 3.6 Hz, 1H, NHNH), 3.7 (d, J = 4.1 Hz, 2H, NHNHCH2); ESMS (methanol) m/z 330 (M+).
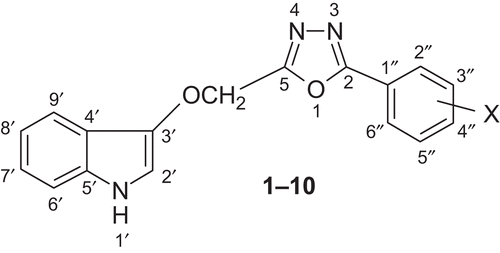
General procedure for synthesis of 3-{[5-(4-substitutedphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole (1–10)
A mixture of IV (0.01 mol), anhydrous sodium acetate (0.02 mol), and glacial acetic acid was placed in a round-bottomed flask equipped with a separating funnel for the addition of bromine. Bromine (0.6 mL) in glacial acetic acid (5 mL) was added slowly while stirring magnetically. After 2 h of stirring, the solution was poured on crushed ice, and the resulting solid was separated, dried, and recrystallized from ethanol ().
3-{[5-(4-Chlorophenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 1 Yield: 60%; m.p. 248°C; IR (KBr, cm−1): 3052.1 (aromatic C-H), 1090.1 (C-O of 1,3,4-oxadiazole nucleus), 1639.7 (C=N of 1,3,4-oxadiazole nucleus), 3230.7 (N-H of indole), 1601.8, 1502.5 (aromatic C-C), 834.2 (C-H def (para) disubstituted benzene), 719.8 (C-Cl str); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 123.8 (C-2′), 102.3 (C-3′), 128.6 (C-4′), 136.2 (C-5′) 111.5 (C-6′), 119.8 (C-7′), 121.7 (C-8′), 120.9 (C-9′), 171.3 (C-2), 168.2 (C-5), 128.6 (C-2′′ and C-6′′), 129.5 (C-3′′ & C-5′′), 133.9 (C-4′′), 134.8 (C-1′′), 73.2 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.2 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 7.2–7.5 (m, 4H, ArH), 5.4 (s, 2H, OCH2); ESMS (methanol) m/z 326 (M+). Anal. Calcd. for C19H16N3O3Cl: C, 61.71; H, 4.36; N, 11.36. Found: C, 61.63; H, 4.28; N, 11.42%.
3-{[5-(4-Methoxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 2 Yield: 65%; m.p. 255°C; IR (KBr, cm−1): 3043.7 (aromatic C-H str), 1087.4 (C-O of 1,3,4-oxadiazole nucleus), 1637.5 (C=N of 1,3,4-oxadiazole nucleus), 3226.4 (N-H of indole), 1602.6, 1504.3 (aromatic C-C str), 1256.3 (C-O of OCH3), 836.1 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.0 (C-2′), 102.2 (C-3′), 128.4 (C-4′), 136.1 (C-5′) 111.3 (C-6′), 119.9 (C-7′), 121.8 (C-8′), 121.2 (C-9′), 171.2 (C-2), 168.0 (C-5), 128.1 (C-2′′ and C-6′′), 114.5 (C-3′′ and C-5′′), 162.2 (C-4′′), 128.9 (C-1′′), 73.4 (OCH2 connecting indole and oxadiazole rings), 56.3 (OCH2); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.3 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 6.8–7.4 (m, 4H, ArH), 5.4 (s, 2H, OCH2), 3.8 (s, 3H, OCH3); ESMS (methanol) m/z 321 (M+). Anal. Calcd. for C18H15N3O3: C, 67.28; H, 4.71; N, 13.08. Found: C, 67.21; H, 4.78; N, 13.16%.
3-{[5-(4-Nitrophenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 3 Yield: 62%; m.p. 263°C; IR (KBr, cm−1): 3041.5 (aromatic C-H str), 1091.7 (C-O of 1,3,4-oxadiazole nucleus), 1642.6 (C=N of 1,3,4-oxadiazole nucleus), 3222.5 (N-H of indole), 1601.6, 1504.2 (aromatic C-C str), 1542.5, 1353.8 (N=O str in Ar NO2 group), 835.5 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.4 (C-3′), 128.3 (C-4′), 136.3 (C-5′) 111.5 (C-6′), 119.7 (C-7′), 121.9 (C-8′), 121.4 (C-9′), 171.1 (C-2), 168.3 (C-5), 128.0 (C-2′′ and C-6′′), 124.4 (C-3′′ and C-5′′), 149.2 (C-4′′), 142.7 (C-1′′), 73.6 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.3 Hz, 1H, NH of indole), 7.4 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 7.3–8.3 (m, 4H, ArH), 5.3 (s, 2H, OCH2); ESMS (methanol) m/z 336 (M+). Anal. Calcd. for C17H12N4O4: C, 60.71; H, 4.30; N, 16.66. Found: C, 60.63; H, 4.36; N, 16.73%.
3-{[5-(4-Methylphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 4 Yield: 61%; m.p. 242°C; IR (KBr, cm−1): 3048.2 (aromatic C-H str), 1093.5 (C-O of 1,3,4-oxadiazole nucleus), 1645.1 (C=N of 1,3,4-oxadiazole nucleus), 3223.7 (N-H of indole), 1603.8, 1502.3 (aromatic C-C str), 2927.6 (aliphatic C-H str), 1429.4 (aliphatic C-H def), 830.3 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.1 (C-2′), 102.6 (C-3′), 128.2 (C-4′), 136.4 (C-5′) 111.2 (C-6′), 119.9 (C-7′), 121.8 (C-8′), 121.3 (C-9′), 171.0 (C-2), 168.1 (C-5), 126.9 (C-2′′ and C-6′′), 129.6 (C-3′′ and C-5′′), 137.8 (C-4′′), 133.7 (C-1′′), 73.6 (OCH2 connecting indole and oxadiazole rings), 21.2 (CH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 7.1–7.4 (m, 4H, ArH), 5.4 (s, 2H, OCH2), 2.4 (s, 3H, ArCH3); ESMS (methanol) m/z 305 (M+). Anal. Calcd. for C18H15N3O2: C, 70.81; H, 4.95; N, 13.76. Found: C, 70.94; H, 4.90; N, 13.83%.
3-{[5-(4-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 5 Yield: 64%; m.p. 273°C; IR (KBr, cm−1): 3040.2 (aromatic C-H str), 1092.6 (C-O of 1,3,4-oxadiazole nucleus), 1640.1 (C=N of 1,3,4-oxadiazole nucleus), 3226.7 (N-H of indole), 1602.6, 1503.5 (aromatic C-C str), 3453.8 (O-H str of alcoholic group), 1159.2 (C-O str of alcoholic group), 835.2 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.3 (C-2′), 102.2 (C-3′), 128.1 (C-4′), 136.2 (C-5′) 111.2 (C-6′), 120.1 (C-7′), 121.9 (C-8′), 121.2 (C-9′), 171.2 (C-2), 168.2 (C-5), 128.5 (C-2′′ and C-6′′), 116.3 (C-3′′ and C-5′′), 157.5 (C-4′′), 129.2 (C-1′′), 73.7 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.2 (d, J = 3.1 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.8–7.3 (m, 4H, ArH), 5.2 (s, 2H, OCH2), 5.2 (s, 1H, OH); ESMS (methanol) m/z 307 (M+). Anal. Calcd. for C17H13N3O3: C, 61.44; H, 4.26; N, 13.67. Found: C, 61.51; H, 4.29; N, 13.73%.
3-{[5-(3,4-Dimethoxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 6 Yield: 59%; m.p. 249°C; IR (KBr, cm−1): 3043.5 (aromatic C-H str), 1088.1 (C-O of 1,3,4-oxadiazole nucleus), 1640.3 (C=N of 1,3,4-oxadiazole nucleus), 3224.9 (N-H of indole), 1601.7, 1504.9 (aromatic C-C str), 1256.6 (C-O of OCH3); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.5 (C-3′), 128.2 (C-4′), 136.5 (C-5′) 111.3 (C-6′), 120.1 (C-7′), 121.9 (C-8′), 121.1 (C-9′), 171.1 (C-2), 168.3 (C-5), 129.9 (C-1′′), 120.5 (C-2′′) 115.8 (C-3′′), 147.7 (C-4′′), 148.2 (C-5′′), 113.7 (C-6′′), 73.6 (OCH2 connecting indole and oxadiazole rings), 56.6 (OCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.7–6.9 (m, 3H, ArH), 5.3 (s, 2H, OCH2), 3.8 (s, 3H, OCH3); ESMS (methanol) m/z 351 (M+). Anal. Calcd. for C19H17N3O4: C, 64.95; H, 4.88; N, 11.96. Found: C, 64.85; H, 4.82; N, 11.85%.
3-{[5-(2-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 7 Yield: 54%; m.p. 234°C; IR (KBr, cm−1): 3039.1 (aromatic C-H str), 1093.6 (C-O of 1,3,4-oxadiazole nucleus), 1640.4 (C=N of 1,3,4-oxadiazole nucleus), 3230.7 (N-H of indole), 1603.6, 1502.8 (aromatic C-C str), 3448.4 (O-H str of alcoholic group), 1151.0 (C-O str of alcoholic group), 741.5 (C-H def (ortho) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.4 (C-3′), 128.3 (C-4′), 136.1 (C-5′) 111.2 (C-6′), 120.2 (C-7′), 121.8 (C-8′), 121.2 (C-9′), 171.3 (C-2), 168.2 (C-5), 123.6 (C-1′′), 155.8 (C-2′′) 116.3 (C-3′′), 129.8 (C-4′′), 121.5 (C-5′′), 128.6 (C-6′′), 73.4 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.3 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.8–7.4 (m, 4H, ArH), 5.4 (s, 2H, OCH2), 5.1 (s, 1H, OH); ESMS (methanol) m/z 307 (M+). Anal. Calcd. for C17H13N3O3: C, 66.44; H, 4.26; N, 13.67. Found: C, 66.52; H, 4.29; N, 13.75%.
3-{[5-(3-Hydroxyphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 8 Yield: 56%; m.p. 252°C; IR (KBr, cm−1): 3044.7 (aromatic C-H str), 1093.5 (C-O of 1,3,4-oxadiazole nucleus), 1647.0 (C=N of 1,3,4-oxadiazole nucleus), 3225.2 (N-H of indole), 1604.1, 1503.6 (aromatic C-C str), 3446.6 (O-H str of alcoholic group), 1158.2 (C-O str of alcoholic group), 816.3 (C-H def (meta) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.3 (C-2′), 102.2 (C-3′), 128.0 (C-4′), 136.3 (C-5′) 111.1 (C-6′), 120.2 (C-7′), 121.9 (C-8′), 121.3 (C-9′), 171.4 (C-2), 168.1 (C-5), 137.8 (C-1′′), 114.3 (C-2′′) 157.9 (C-3′′), 115.6 (C-4′′), 130.5 (C-5′′), 119.8 (C-6′′), 73.4 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.2 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.7–7.2 (m, 4H, ArH), 5.3 (s, 2H, OCH2), 5.1 (s, 1H, OH); ESMS (methanol) m/z 307 (M+). Anal. Calcd. for C17H13N3O3: C, 66.44; H, 4.26; N, 13.67. Found: C, 66.53; H, 4.33; N, 13.78%.
3-[(5-Phenoxy-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 9 Yield: 58%; m.p. 263°C; IR (KBr, cm−1): 3047.1 (aromatic C-H str), 1088.3 (C-O of 1,3,4-oxadiazole nucleus), 1637.2 (C=N of 1,3,4-oxadiazole nucleus), 3224.8 (N-H of indole), 1602.5, 1504.6 (aromatic C-C str), 762.6 (C-H def monosubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.1 (C-3′), 128.4 (C-4′), 136.1 (C-5′) 111.3 (C-6′), 120.2 (C-7′), 122.1 (C-8′), 121.3 (C-9′), 171.1 (C-2), 168.3 (C-5), 121.2 (C-2′′ and C-6′′), 129.4 (C-3′′ and C-5′′), 124.6 (C-4′′), 154.2 (C-1′′), 73.5 (OCH2 connecting indole and oxadiazole rings); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.8–7.1 (m, 5H, ArH), 5.3 (s, 2H, OCH2); ESMS (methanol) m/z 307 (M+). Anal. Calcd. for C17H13N3O3: C, 66.44; H, 4.26; N, 13.67. Found: C, 66.37; H, 4.28; N, 13.61%.
3-({5-[2-Phenylethenyl]-1,3,4-oxadiazol-2-yl}methoxy)-1H-indole 10 Yield: 61%; m.p. 284°C; IR (KBr, cm−1): 3041.6 (aromatic C-H str), 1092.7 (C-O of 1,3,4-oxadiazole nucleus), 1643.5 (C=N of 1,3,4-oxadiazole nucleus), 3223.8 (N-H of indole), 1600.3, 1502.6 (aromatic C-C str), 768.2 (C-H def monosubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.0 (C-2′), 102.4 (C-3′), 128.3 (C-4′), 136.5 (C-5′) 111.4 (C-6′), 120.3 (C-7′), 122.2 (C-8′), 121.2 (C-9′), 171.3 (C-2), 168.1 (C-5), 126.4 (C-2′′ and C-6′′), 128.5 (C-3′′ and C-5′′), 127.8 (C-4′′), 134.9 (C-1′′), 73.6 (OCH2 connecting indole and oxadiazole rings), 124.9 (CH=CH-C6H5), 130.7 (CH=CH-C6H5); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.5 Hz, 1H, NH of indole), 7.4 (d, 1H, J = 2.9 Hz, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 7.1–7.3 (m, 5H, ArH), 5.2 (s, 2H, OCH2); ESMS (methanol) m/z 317 (M+). Anal. Calcd. for C19H15N3O2: C, 71.91; H, 4.76; N, 13.24. Found: C, 71.79; H, 4.68; N, 13.26%.
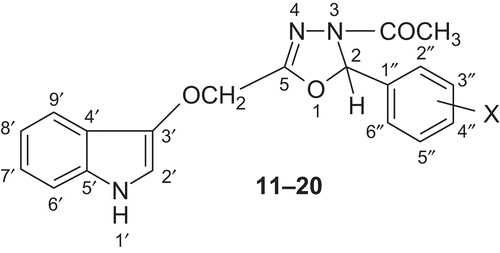
General procedure for synthesis of 3-{[4-acetyl-5-(4-substitutedphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole (11–20)
A mixture of IV (0.01 mol) and acetic anhydride (30 mL) was refluxed for 1 h. Excess acetic acid (byproduct) and acetic anhydride were removed by distillation under reduced pressure, and the residue obtained was recrystallized from ethanol ().
3-[(4-Acetyl-5-(4-chlorophenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 11 Yield: 58%; m.p. 242°C; IR (KBr, cm−1): 3044.2 (aromatic C-H), 1088.6 (C-O of 1,3,4-oxadiazoline nucleus), 1642.9 (C=N of 1,3,4-oxadiazoline nucleus), 3227.1 (N-H of indole), 1603.1, 1503.5 (aromatic C-C), 831.7 (C-H def (para) disubstituted benzene), 1710.5 (C=O str), 723.8 (C-Cl str); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 123.9 (C-2′), 102.1 (C-3′), 128.4 (C-4′), 136.2 (C-5′) 111.3 (C-6′), 119.9 (C-7′), 121.8 (C-8′), 121.1 (C-9′), 76.3 (C-2), 155.7 (C-5), 128.7 (C-2′′ and C-6′′), 128.9 (C-3′′ and C-5′′), 132.1 (C-4′′), 140.7 (C-1′′), 74.5 (OCH2 connecting indole and oxadiazoline rings), 170.4 (COCH3), 23.6 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 7.1–7.2 (m, 4H, ArH), 4.3 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.4 (s, 3H, COCH3); ESMS (methanol) m/z 369 (M+). Anal. Calcd. for C19H16N3O3Cl: C, 61.71; H, 4.36; N, 11.36. Found: C, 61.79; H, 4.33; N, 11.30%.
3-[(4-Acetyl-5-(4-methoxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 12 Yield: 60%; m.p. 257°C; IR (KBr, cm−1): 3040.2 (aromatic C-H str), 1088.0 (C-O of 1,3,4-oxadiazoline nucleus), 1641.7 (C=N of 1,3,4-oxadiazoline nucleus), 3228.8 (N-H of indole), 1600.4, 1503.1 (aromatic C-C str), 1258.5 (C-O of OCH3), 1712.8 (C=O str), 834.9 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 123.7 (C-2′), 102.2 (C-3′), 128.1 (C-4′), 136.3 (C-5′) 111.2 (C-6′), 120.1 (C-7′), 121.9 (C-8′), 121.2 (C-9′), 76.1 (C-2), 155.5 (C-5), 128.3 (C-2′′ and C-6′′), 113.8 (C-3′′ and C-5′′), 160.5 (C-4′′), 133.9 (C-1′′), 74.6 (OCH2 connecting indole and oxadiazoline rings), 170.6 (COCH3), 23.8 (COCH3), 56.2 (OCH2); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.1–7.6 (m, 4H, indolic protons), 6.7–7.1 (m, 4H, ArH), 4.4 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.5 (s, 3H, COCH3), 3.8 (s, 3H, OCH3); ESMS (methanol) m/z 365 (M+). Anal. Calcd. for C20H19N3O4: C, 65.74; H, 5.24; N, 11.50. Found: C, 65.84; H, 5.28; N, 11.57%.
3-[(4-Acetyl-5-(4-nitrophenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 13 Yield: 63%; m.p. 266°C; IR (KBr, cm−1): 3035.8 (aromatic C-H str), 1093.2 (C-O of 1,3,4-oxadiazoline nucleus), 1643.1 (C=N of 1,3,4-oxadiazoline nucleus), 3220.2 (N-H of indole), 1602.7, 1503.6 (aromatic C-C str), 1541.7, 1352.3 (N=O str in Ar NO2 group), 1709.1 (C=O str), 838.2 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.2 (C-3′), 128.1 (C-4′), 136.3 (C-5′) 111.2 (C-6′), 120.1 (C-7′), 121.9 (C-8′), 121.0 (C-9′), 75.8 (C-2), 155.3 (C-5), 128.2 (C-2′′ and C-6′′), 123.5 (C-3′′ and C-5′′), 147.2 (C-4′′), 149.5 (C-1′′), 74.4 (OCH2 connecting indole and oxadiazoline rings), 171.1 (COCH3), 23.4 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.3 (d, J = 3.6 Hz, 1H, NH of indole), 7.3 (d, J = 3.1 Hz, 1H, H-2′ of indole), 7.1-7.6 (m, 4H, indolic protons), 7.4–8.2 (m, 4H, ArH), 4.2 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.6 (s, 3H, COCH3); ESMS (methanol) m/z 380 (M+). Anal. Calcd. for C19H16N4O5: C, 60.00; H, 4.24; N, 14.73. Found: C, 60.13; H, 4.27; N, 14.81%.
3-[(4-Acetyl-5-(4-methylphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 14 Yield: 62%; m.p. 223°C; IR (KBr, cm−1): 3045.1 (aromatic C-H str), 1090.5 (C-O of 1,3,4-oxadiazoline nucleus), 1641.7 (C=N of 1,3,4-oxadiazoline nucleus), 3227.7 (N-H of indole), 1601.6, 1501.8 (aromatic C-C str), 2929.0 (aliphatic C-H str), 1422.7 (aliphatic C-H def), 1713.8 (C=O str), 834.4 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 123.7 (C-2′), 102.2 (C-3′), 128.1 (C-4′), 136.3 (C-5′) 111.1 (C-6′), 119.8 (C-7′), 121.9 (C-8′), 121.0 (C-9′), 75.8 (C-2), 155.3 (C-5), 127.3 (C-2′′ and C-6′′), 129.2 (C-3′′ and C-5′′), 135.9 (C-4′′), 139.5 (C-1′′), 74.5 (OCH2 connecting indole and oxadiazoline rings), 170.4 (COCH3), 23.8 (COCH3), 21.3 (CH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.3 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.9–7.1 (m, 4H, ArH), 4.5 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.8 (s, 3H, COCH3), 2.3 (s, 3H, ArCH3); ESMS (methanol) m/z 349 (M+). Anal. Calcd. for C20H19N3O3: C, 68.75; H, 5.48; N, 12.03. Found: C, 68.81; H, 5.55; N, 12.08%.
3-[(4-Acetyl-5-(4-hydroxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 15 Yield: 56%; m.p. 235°C; IR (KBr, cm−1): 3042.6 (aromatic C-H str), 1093.9 (C-O of 1,3,4-oxadiazoline nucleus), 1642.6 (C=N of 1,3,4-oxadiazoline nucleus), 3229.3 (N-H of indole), 1601.7, 1502.9 (aromatic C-C str), 3451.8 (O-H str of alcoholic group), 1160.5 (C-O str of alcoholic group), 1707.4 (C=O str), 836.9 (C-H def (para) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.1 (C-2′), 102.3 (C-3′), 128.2 (C-4′), 136.1 (C-5′) 111.3 (C-6′), 119.8 (C-7′), 121.8 (C-8′), 121.0 (C-9′), 76.1 (C-2), 155.8 (C-5), 128.8 (C-2′′ and C-6′′), 115.7 (C-3′′ and C-5′′), 155.2 (C-4′′), 135.3 (C-1′′), 74.3 (OCH2 connecting indole and oxadiazoline rings), 170.6 (COCH3), 23.7 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.6 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.6–7.0 (m, 4H, ArH), 4.4 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.7 (s, 3H, COCH3), 5.3 (s, 1H, OH); ESMS (methanol) m/z 351 (M+). Anal. Calcd. for C19H17N3O4: C, 64.95; H, 4.88; N, 11.96. Found: C, 64.88; H, 4.85; N, 11.92%.
3-[(4-Acetyl-5-(3, 4-dimethoxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 16 Yield: 54%; m.p. 265°C; IR (KBr, cm−1): 3039.1 (aromatic C-H str), 1090.4 (C-O of 1,3,4-oxadiazoline nucleus), 1642.9 (C=N of 1,3,4-oxadiazoline nucleus), 3220.7 (N-H of indole), 1602.8, 1503.5 (aromatic C-C str), 1713.1 (C=O str), 1259.7 (C-O of OCH3); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 123.9 (C-2′), 102.3 (C-3′), 128.0 (C-4′), 136.2 (C-5′) 111.2 (C-6′), 120.2 (C-7′), 121.9 (C-8′), 121.0 (C-9′), 76.5 (C-2), 155.3 (C-5), 135.8 (C-1′′), 120.6 (C-2′′) 114.9 (C-3′′), 145.5 (C-4′′), 147.6 (C-5′′), 113.9 (C-6′′), 74.5 (OCH2 connecting indole and oxadiazoline rings), 171.2 (COCH3), 23.9 (COCH3), 56.4 (OCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.6 Hz, 1H, NH of indole), 7.4 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.6–6.7 (m, 4H, ArH), 4.4 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.8 (s, 3H, COCH3), 3.8 (s, 3H, OCH3); ESMS (methanol) m/z 395 (M+). Anal. Calcd. for C21H21N3O5: C, 63.79; H, 5.35; N, 10.63. Found: C, 63.71; H, 5.38; N, 10.69%.
3-[(4-Acetyl-5-(2-hydroxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 17 Yield: 60%; m.p. 231°C; IR (KBr, cm−1): 3039.1 (aromatic C-H str), 1090.2 (C-O of 1,3,4-oxadiazoline nucleus), 1644.0 (C=N of 1,3,4-oxadiazole nucleus), 3232.2 (N-H of indole), 1602.8, 1500.9 (aromatic C-C str), 3445.2 (O-H str of alcoholic group), 1156.3 (C-O str of alcoholic group), 1711.6 (C=O str), 741.5 (C-H def (ortho) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.1 (C-3′), 128.2 (C-4′), 136.0 (C-5′) 111.3 (C-6′), 120.1 (C-7′), 122.2 (C-8′), 120.8 (C-9′), 68.3 (C-2), 155.6 (C-5), 129.7 (C-1′′), 156.5 (C-2′′) 115.2 (C-3′′), 127.7 (C-4′′), 120.9 (C-5′′), 128.7 (C-6′′), 74.4 (OCH2 connecting indole and oxadiazoline rings), 171.4 (COCH3), 23.8 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.6–7.0 (m, 4H, ArH), 4.6 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.8 (s, 3H, COCH3), 5.2 (s, 1H, OH); ESMS (methanol) m/z 351 (M+). Anal. Calcd. for C19H17N3O4: C, 64.95; H, 4.88; N, 11.96. Found: C, 65.07; H, 4.83; N, 11.91%.
3-[(4-Acetyl-5-(3-hydroxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 18 Yield: 55%; m.p. 242°C; IR (KBr, cm−1): 3041.1 (aromatic C-H str), 1092.7 (C-O of 1,3,4-oxadiazoline nucleus), 1643.4 (C=N of 1,3,4-oxadiazoline nucleus), 3229.5 (N-H of indole), 1602.6, 1502.8 (aromatic C-C str), 3448.9 (O-H str of alcoholic group), 1160.5 (C-O str of alcoholic group), 1715.3 (C=O str), 818.0 (C-H def (meta) disubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.1 (C-2′), 102.0 (C-3′), 128.3 (C-4′), 136.2 (C-5′) 111.1 (C-6′), 120.3 (C-7′), 121.8 (C-8′), 120.9 (C-9′), 74.1 (C-2), 155.5 (C-5), 143.6 (C-1′′), 114.5 (C-2′′) 157.3 (C-3′′), 113.9 (C-4′′), 129.8 (C-5′′), 119.6 (C-6′′), 75.8 (OCH2 connecting indole and oxadiazoline rings), 171.2 (COCH3), 23.6 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.1 (d, J = 3.4 Hz, 1H, NH of indole), 7.3 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.6–7.0 (m, 4H, ArH), 4.5 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.7 (s, 3H, COCH3), 5.1 (s, 1H, OH); ESMS (methanol) m/z 351 (M+). Anal. Calcd. for C19H17N3O4: C, 64.95; H, 4.88; N, 11.96. Found: C, 64.86; H, 4.82; N, 11.90%.
3-[(4-Acetyl-5-phenoxy-4,5-dihydro-1,3,4-oxadiazol-2-yl)methoxy]-1H-indole 19 Yield: 54%; m.p. 259°C; IR (KBr, cm−1): 3049.7 (aromatic C-H str), 1089.9 (C-O of 1,3,4-oxadiazoline nucleus), 1639.6 (C=N of 1,3,4-oxadiazoline nucleus), 3227.3 (N-H of indole), 1603.1, 1504.3 (aromatic C-C str), 1708.8 (C=O str), 760.7 (C-H def monosubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.1 (C-2′), 102.3 (C-3′), 128.1 (C-4′), 135.8 (C-5′) 111.2 (C-6′), 120.3 (C-7′), 121.9 (C-8′), 121.2 (C-9′), 101.6 (C-2), 155.7 (C-5), 114.3 (C-2′′ and C-6′′), 129.6 (C-3′′ and C-5′′), 120.6 (C-4′′), 163.4 (C-1′′), 73.7 (OCH2 connecting indole and oxadiazole rings), 171.3 (COCH3), 23.8 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.8 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 6.7–7.2 (m, 5H, ArH), 4.5 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.8 (s, 3H, COCH3); ESMS (Methanol) m/z 351 (M+). Anal. Calcd. for C19H17N3O4: C, 64.95; H, 4.88; N, 11.96. Found: C, 64.86; H, 4.84; N, 11.90%.
3-({4-Acetyl-5-[(E)-2-phenylethenyl]-4,5-dihydro-1,3,4-oxadiazol-2-yl}methoxy)-1H-indole 20 Yield: 57%; m.p. 274°C; IR (KBr, cm−1): 3044.7 (aromatic C-H str), 1093.3 (C-O of 1,3,4-oxadiazole nucleus), 1638.4 (C=N of 1,3,4-oxadiazole nucleus), 3221.5 (N-H of indole), 1601.8, 1503.7 (aromatic C-C str), 1712.7 (C=O str), 769.5 (C-H def monosubstituted benzene); 13C-NMR (75 MHz, DMSO-d6, TMS, δ ppm): 124.2 (C-2′), 102.1 (C-3′), 127.9 (C-4′), 136.2 (C-5′) 111.1 (C-6′), 120.4 (C-7′), 122.1 (C-8′), 121.3 (C-9′), 74.6 (C-2), 155.3 (C-5), 126.2 (C-2′′ and C-6′′), 128.6 (C-3′′ and C-5′′), 127.7 (C-4′′), 134.7 (C-1′′), 74.8 (OCH2 connecting indole and oxadiazole rings), 126.8 (CH=CH-C6H5), 128.2 (CH=CH-C6H5), 171.3 (COCH3), 23.9 (COCH3); 1H-NMR (300 MHz, DMSO-d6, TMS, δ ppm): 10.2 (d, J = 3.5 Hz, 1H, NH of indole), 7.3 (d, J = 2.9 Hz, 1H, H-2′ of indole), 7.0–7.6 (m, 4H, indolic protons), 7.1–7.3 (m, 5H, ArH), 4.6 (s, 2H, OCH2 connecting indole and oxadiazoline rings), 6.6 (s, 1H, H of oxadiazoline nucleus), 2.7 (s, 3H, COCH3); ESMS (methanol) m/z 361 (M+). Anal. Calcd. for C21H19N3O3: C, 69.79; H, 5.30; N, 11.63. Found: C, 69.68; H, 5.34; N, 11.57%.
Pharmacology
The anti-inflammatory activity of test compounds in acute and chronic inflammatory conditions was evaluated using the carrageenan-induced rat paw edema method and cotton pellet-induced granuloma method, respectively. The carrageenan-induced inflammation model is a COX-dependent reaction and is used to determine COX inhibition. The cotton pellet-induced granuloma method is widely used to evaluate the transudative and proliferative components of chronic inflammation.
For anti-inflammatory evaluation, adult albino rats of either sex weighing 150–175 g were divided in groups of six. The animals were maintained in colony cages at 25 ± 2°C, relative humidity of 45–55%, and under a 12 h light and 12 h dark cycle. All the animals were acclimatized for a week before use. All test compounds and indomethacin (reference drug) were administered orally, suspended in 1% carboxymethylcellulose (CMC). The acute oral toxicity test15 was performed for all the synthesized compounds according to the Organization of Economic Cooperation and Development (OECD) guidelines. For ulcerogenicity studies, aspirin was administered intraperitoneally as a suspension in 10% (v/v) Tween 80. Statistical analyses were carried out with the single tailed t-test. A level of p < 0.001 was adopted as the test of significance. The procedure employed for anti-inflammatory evaluation was reviewed and approved by the University Animal Ethical Committee.
Acute oral toxicity was performed following the OECD-423 guidelines (acute toxic class method). Swiss albino mice (n = 3) of either sex selected by random sampling were used for the study. The animals were fasted for 3–4 h with water ad libitum, after which the test compounds (suspended in CMC) were administered orally at the dose of 5 mg/kg and the mice observed for 3 days. If mortality was observed in 2–3 animals, the dose administered was assigned as the toxic dose. If mortality was observed in one animal, then the same dose was repeated to confirm the toxic dose. In the present study, mortality was not observed with the 5 mg/kg dose and the procedure was repeated for higher doses of 50, 300, and 2000 mg/kg. All three mice survived at the 2000 mg/kg dose, indicating that the compounds are nontoxic to animals.
Carrageenan-induced rat paw edema test
The anti-inflammatory activity of the synthesized compounds was assessed using the rat paw edema assayCitation16 utilizing 0.1 mL of 1% carrageenan as a phlogistic agent. A mark was made on both hind paws just below the tibiotarsal junction so that the paw could be dipped in the mercury column of the plethysmograph up to the mark, to ensure constant paw volume. To each group of six animals, with the exception of the control group, the test (100 mg/kg of body weight) compounds were administered orally. The control group received an equivalent amount of CMC used as the solvent to dissolve the compounds. The standard drug indomethacin (10 mg/kg) was administered to one group. After 1 h, carrageenan (0.1 mL, 1% w/v solution in sterile saline) was injected into the subplantar tissue of the left paw of the control as well as the indomethacin group. The right paw served as the reference non-inflamed paw for comparison. The initial paw volume was measured using the plethysmograph within 30 s after the injection. The relative increase in paw volume was measured in control, standard, and treated groups 1, 2, and 3 h after carrageenan injection. After 3 h, the final paw volume of each animal was measured. The percent reduction in paw volume was calculated by subtracting the difference between the right and left hind paw volumes in the treated group from the difference in the control group and dividing by the difference in the control group. The anti-inflammatory activity of test compounds and the standard reference drug was determined using the formula: % anti-inflammatory activity = (1 – Vt/Vc) × 100, where Vt represents the mean increase in paw volume in rats treated with test compounds and Vc represents the mean increase in paw volume in the control group of rats ().
Table 1. Anti-inflammatory activitya of the synthesized oxadiazoles and oxadiazolines (100 mg/kg p.o.) using carrageenan-induced rat paw edema method and cotton pellet-induced granuloma method.
Cotton pellet-induced granuloma method
After shaving the fur, rats were anesthetized and 10 ± 1 mg of sterile cotton pellets were inserted, one in each axilla. The test compounds, indomethacin, and control vehicle were administered orally for 7 consecutive days from the day of cotton pellet implantation. On the 8th day, animals were anesthetized and the cotton pellets were removed surgically and freed from extraneous tissues. The moist cotton pellets were weighed and then dried at 60°C for 24 h, and then the dried cotton pellets were weighed again. The actual weight of the cotton pellets was subtracted from the weight of dried granuloma pellets. The increase in the weight of dried cotton pellets was taken as the measure of granuloma formation. The antiproliferative effects of test compounds were determined by comparing the results obtained in test groups with those in the control groupCitation17 ().
Evaluation of ulcerogenicity index
Albino rats of Wistar strain (150–175 g) of either sex were divided into different groups each of six animals. The ulceration was induced in albino rats as per the reported methodCitation18. One group was administered with aspirin intraperitonially (i.p.) in a dose of 200 mg/kg once daily for 3 days. The control groups of animals were administered only 10% v/v Tween 80 suspension (i.p.). The remaining group of animals was administered with test compounds i.p. in a dose of 20 mg/kg. On the 4th day, the pylorus was ligated as per the reported methodCitation19. Animals were starved for 36 h before the pylorus ligation procedure. Four hours after the ligation, animals were sacrificed. The stomach was removed and opened along the greater curvature. Ulcer index was determined by the reported methodCitation20 and is presented in .
Table 2. Ulcerogenicity index of the synthesized oxadiazole and oxadiazoline analogs (20 mg/kg i.p.).
Results and discussion
The structures of the compounds were elucidated on the basis of elemental analysis, infrared (IR), 1H-NMR, 13C-NMR, and electrospray mass spectroscopy (ESMS). The IR data of synthesized oxadiazole analogs clearly shows a C=N stretching band around 1640 cm−1 and C-O absorption band around 1090 cm−1, which indicates ring closure of the 1,3,4-oxadiazole ring. All the final compounds have strong absorption around 3045 cm−1, which is evidence of the presence of aromatic C-H bonds. The presence of aromatic C-C bonds was confirmed by observing absorption bands around 1602 and 1501 cm−1. The IR data also confirm the presence of specific functional groups in the final synthesized compounds. In the 13C-NMR spectra, C-2 and C-5 of the oxadiazole nucleus were seen around 168 and 171 ppm, respectively. All other carbons of the final compounds were also seen based on the expected chemical shift. The presence of a proton attached to the N of the indole ring (NH of indole) can be ascertained by the chemical shift around 10.1 with coupling constant around J = 3.4 Hz. The presence of a chemical shift with value 7.1–7.6 clearly indicates the presence of other protons of the indole ring. The chemical shifts for other protons were also seen as expected. The mass spectra of test compounds are in conformity with the assigned structures. The mass spectra of these compounds showed molecular ion peaks corresponding to their molecular formula.
The anti-inflammatory activity of oxadiazoles, i.e. 1–10, at doses of 100 mg/kg is indicated by their ability to provide 27–66%, 14–32%, and 20-51%. protection against carrageenan-induced rat paw edema, moist cotton pellet-induced granuloma, and dry cotton pellet-induced granuloma, respectively. On the other hand, the anti-inflammatory properties of oxadiazolines, i.e. 11–20, at doses of 100 mg/kg is reflected by their ability to provide 20-56%, 11–26%, and 25–47% protection against carrageenan-induced rat paw edema, moist cotton pellet-induced granuloma, and dry cotton pellet-induced granuloma, respectively (). Among the synthesized oxadiazoles, the maximum anti-inflammatory activity was exhibited by compound 5, with 66%, 32%, and 51% protection against carrageenan-induced rat paw edema, moist cotton pellet-induced granuloma, and dry cotton pellet-induced granuloma, respectively. In the case of oxadiazolines, the maximum activity was shown by compound 15, with 56%, 26%, and 47% protection against carrageenan-induced rat paw edema, moist cotton pellet-induced granuloma, and dry cotton pellet-induced granuloma, respectively. Indomethacin was used as the standard during anti-inflammatory evaluation of the test compounds. The significance level for synthesized compounds in anti-inflammatory evaluation was found to be p < 0.05 or p < 0.01. The results for indomethacin were also found to be statistically significant with the value of p < 0.0001. During evaluation of the ulcerogenicity index of the synthesized test compounds, the significance level was found to be p < 0.05.
The test compounds were not found to possess anti-inflammatory activity comparable to indomethacin, as they differ from indomethacin in three major structural modifications. The title compounds do not possess a 4-chlorobenzoyl group at the N of the indole ring, a methyl group at position 2 of the indole ring, and a methoxy group at position 5 of the indole ring, which seem to endow the biological activity of indomethacin. Moreover, we replaced the acetic acid group present at position 3 in indomethacin with other substitutents containing the 1,3,4-oxadiazole nucleus. In correlating the biological activity of the compounds with their structure, it was observed that the most potent anti-inflammatory compounds 5 and 15 had a hydroxy group on the benzene ring attached to C-2 of the oxadiazole and oxadiazoline moiety, respectively. The significant anti-inflammatory activity of compounds 5 and 15 may be attributed to the electronegativity of the hydroxy group, which can withdraw an electron more strongly than chloro, nitro, or other groups of the compounds. On the other hand, among all compounds, 4 and 14 had the weakest anti-inflammatory activity. Another notable point is that compounds with the methoxy group, i.e. 2 and 12, exhibited greater activity in comparison with compounds with the methyl group, i.e. 4 and 14. This may be because the methoxy group supplies its electron more strongly than the methyl group, as the former group contains an electronegative oxygen atom. Replacement of the methyl group on the aryl moiety with other groups, i.e. hydroxy, nitro, and chloro groups, resulted in a considerable increase in anti-inflammatory activity. Structure–activity relationships among the synthesized oxadiazole and oxadiazoline analogs indicate that the hydroxy, nitro, or chloro group in the aromatic ring and simultaneous presence of an indole ring are responsible for imparting significant anti-inflammatory activity to the test compounds.
The ulcer index exhibited by all the synthesized compounds reveals that they possess lower ulcerogenic potential as compared to aspirin, the standard taken in the studies. All the test compounds showed only 30–45% of the ulcer index of aspirin (1.86 ± 0.64). On critical correlation of the ulcer index and structures of the compounds it was found that aryl substituents containing electron withdrawing groups showed a higher ulcer index with respect to other test compounds. The compounds 3-{[5-(4-methylphenyl)-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 4, 3-{[4-acetyl-5-(4-methylphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 14, and 3-({4-acetyl-5-[2-phenylethenyl]-4,5-dihydro-1,3,4-oxadiazol-2-yl}methoxy)-1H-indole 20 exhibited the least ulcer index among all the test compounds, i.e. 33%, 30%, and 34% of the ulcer index shown by aspirin, respectively. The ulcerogenic potential of the test compounds as shown in indicates that between the oxadiazole and oxadiazoline series, the latter series of compounds caused more ulcers in the test animals. This may be attributed to the presence of the acetyl group attached to one N of the oxadiazoline nucleus. The compound 3-{[4-acetyl-5-(4-hydroxyphenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl]methoxy}-1H-indole 15 showed the highest ulcer index (0.85 ± 1.89) among all the test compounds, which is about 46% of the ulcer index of aspirin.
Conclusion
Two novel series of oxadiazole and oxadiazoline analogs possessing an indole nucleus were synthesized for their potential anti-inflammatory activity, using the carrageenan-induced rat paw edema method and cotton pellet-induced granuloma method. In general, all the oxadiazoles have greater anti-inflammatory activity than their corresponding oxadiazoline analogs. On the other hand, it can also be concluded that all the oxadiazole and oxadiazoline analogs exhibit greater anti-inflammatory activity in the carrageenan-induced rat paw edema assay than in the cotton pellet-induced granuloma method, indicating that the studied compounds are more effective in acute inflammatory conditions than in chronic ones. The results of the present studies have clearly indicated that the presence of the indole nucleus in the oxadiazole and oxadiazoline analogs has a positive influence on their anti-inflammatory activity. In our laboratory, further research work is in progress on the design and optimization of molecules possessing 1,3,4-oxadiazole and indole nuclei together with different possible substitutions to explore their potential anti-inflammatory activity.
Acknowledgment
The help rendered by RSIC, CDRI Lucknow for spectral and elemental analysis is gratefully acknowledged.
Declaration of interest: The authors have declared no conflict of interest
References
- Palomer A, Cabre F, Pascual J, Campos J, Trugillo MA, Entrena A, et al. Identification of novel cyclooxygenase-2 selective inhibitors using pharmacophore models. J Med Chem 2002;45:1402–11.
- Talley JJ, Brown DL, Carter JS, Graneto MJ, Koboldt CM, Masferrer JL, et al. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, valdecoxib: a potent and selective inhibitor of COX-2. J Med Chem 2000;43:775–7.
- Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA 1998;95:13313–18.
- Warner TD, Giuliano F, Vaynovie I, Bukasa A, Mitchell JA, Vave JR. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in-vitro analysis. Proc Natl Acad Sci USA 1999;96:7563–8.
- Omar FA, Manfouz NM, Rahman MA. Design, synthesis and anti-inflammatory activity of some 1,3,4-oxadiazole derivatives. Eur J Med Chem 1996;31:819–25.
- Boschelli DH, Connor DT, Bornemeier DA, Dyer RD, Kennedy JA, Kuipers PJ, et al. 1,3,4-Oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazole analogs of the fenamates: in vitro inhibition of cyclooxygenase and 5-lipoxygenase activities. J Med Chem 1993;36:1802–10.
- Palaska E, Sahin G, Kelicen P, Durlu NT, Altinok G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazole-2-thiones. Farmaco 2002;57:101–7.
- Ramalingum T, Sattur PB. Synthesis and anti-inflammatory activity of benzal-3-pentadecylaryloxyalkylcarboxylic acid hydrazides and 2-benzalamino-5-(3-pentadecyl-aryloxyalkyl)–1,3,4 oxadiazoles. Eur J Med Chem 1990;25:541–4.
- Raman K, Singh HK, Salzman SK, Parmar SS. Substituted thiosemicarbazides and corresponding cyclized 1,3,4-oxadiazoles and their anti-inflammatory activity. J Pharm Sci 1983;82:167–9.
- Raman K, Parmar SS, Sulzman SK. Anti-inflammatory activity of substituted 1,3,4-oxadiazoles. J Pharm Sci 1989;78:999–1002.
- Sahin G, Palaska E, Kelicen P, Demirdamar R, Altinok G. Synthesis of some new 1-acylthio-semicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones and their anti-inflammatory activities. Arzneimittelforschung 2001;51:478–84.
- Mullican MD, Wilson MW, Connor DT, Kostlan CR, Schrier DJ, Dyer RD. Design of 5-(3, 5-di-tert-butyl-4-hydroxyphenyl) 1,3,4-thiadiazoles, -1,3,4 triazoles as orally-active, nonulcerogenic anti-inflammatory agents. J Med Chem 1993;36:1090–9.
- Misra U, Hitkari A, Saxena AK, Gurtu S, Shanker K. Biologically active indolylmethyl-1,3,4-oxadiazoles, 1,3,4-thiadiazoles, 4H-1,3,4-triazoles and 1,2,4-triazines. Eur J Med Chem 1996;31:629–34.
- Bhall M, Hitkari A, Gujrati VR, Bhall TN, Shanker K. Benzopyran-2-one derivatives: antiinflammatory, analgesic and antiproteolytic agents. Eur J Med Chem 1994;29:713–17.
- Ecobichon DJ. The Basis of Toxicology Testing. New York: CRC Press, 1997:43–86.
- Winter CA, Risely EA, Nuss GW. Carregeenin induced oedema in hind paw of the rat as assay for anti-inflammatory drugs. Exp Biol Med 1962;111:544–7.
- Olajide OA, Awe SO, Markinde JM. Effects of the aqueous extract of Bridelia ferruginea stem bark on carrageenan-induced oedema and granuloma tissue formation in rats and mice. J Ethnopharmacol 1999;66:113–17.
- Goyal RK, Chakrabarti A, Sanyal AK. The effect of biological variables on the anti-ulcerogenic effect of vegetable plantain banana. Planta Med 1985;29:85–8.
- Shay M, Komarov SA, Fels D, Meranze D, Grunstein H, Siplet H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 1945;5:43–61.
- Ganguly AK, Bhatnagar OP. Effect of bilateral adrenalectomy on production of restraint ulcers in the stomach of albino rats. Can J Phys Pharmacol 1973;51:748–50.