Abstract
A series of 2,3-disubstituted 3H-quinazolin-4-ones was synthesized. Antimicrobial activities of the synthesized compounds were investigated against Gram (+ve) and Gram (–ve) bacteria, including B. subtilis, S. aureus, S. flexneri, P. aeruginosa, and S. typhi, and six fungi, namely Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani, and Candida glabrata using the broth microdilution method. Compounds 9, 11, and 12 showed significant activities against the selected bacterial cultures, while 7–10, 12, 15, and 16 showed good to moderate antifungal activities. Compound 11 exhibited strongest leishmanicidal activity against Leishmania major (MHOM/PK/88/DESTO) promastigotes, while other compounds showed weak to moderate leishmanicidal activities.
Introduction
The discovery of new antimicrobial and antileishmanial compounds from diversified sources such as microorganisms, animals, and plants and through synthetic means is an active area of scientific research around the world. The increasing prevalence of multi-drug resistance (MDR) pathogens with reduced susceptibility to the existing arsenal of antibiotics has increased the spectrum of non-treatable ailments, and has added urgency to the new drug discovery effortsCitation1. Leishmaniasis is a tropical disease caused by protozoa of the Leishmania genus. These protozoa cause a disease with different clinical forms, namely, cutaneous, hyperergic, mucocutaneous, and anergic diffuse leishmaniasisCitation2. Pentavalent antimonial (SbV) compounds are the common drugs in practice for the treatment of leishmaniasis, but they cause renal and cardiac toxicity. This disease is also treated with various diamidines and pentamidine, formulated as a salt of pentamidine isethionate, which again has its serious side effectsCitation3. Alternative pharmaceutical formulations have been used in order to reduce the toxicity of these drugsCitation4. The lack of an effective antileishmanial drug has necessitated the search for more effective, new chemotherapeutic agents with better activity and least toxicity. In this pursuit, we have synthesized some 2,3-disubstituted 3H-quinazolin-4-ones and have determined their leishmanicidal and antimicrobial activities. Quinazolin-4(3H)-ones are a large group of heterocyclic compounds possessing diverse and interesting biological activitiesCitation5,Citation6.
3-Arylquinazolin-4(3H)-one in particular has been extensively utilized as a core structure in the field of medicinal chemistryCitation7–9. The quinazolin-4(3H)-one nucleus is a basic unit found in various naturally occurring bioactive alkaloids such as febrifugine, isofebrifugine, vascinone, and othersCitation10. The chemistry of this group of alkaloids has found significant importance in medicinal chemistry and has been comprehensively reviewedCitation11–14. However, antileishmanial activities of this group of alkaloids have not been explored. We therefore, in continuation of our synthetic work on quinazolin-4(3H)-one based bioactive compoundsCitation15,Citation16, report herein the synthesis and antimicrobial and antileishmanial activities of some 2,3-disubstituted quinazolin-4(3H)-ones.
Experimental
Chemistry
All the reagents and solvents were obtained from commercial suppliers and were used after preliminary purification wherever that was needed according to their standard methods of purification. Melting points were determined in open capillaries using Gallenkamp melting point apparatus and are uncorrected. All the reactions were monitored using aluminum-supported precoated thin layer chromatography (TLC; Merck 60 F254) silica plates, and detection on TLC was accomplished with the help of an ultraviolet (UV) lamp (254 nm).1H-nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 400 MHz or 300 MHz instrument in CDCl3, CD3OD, or dimethylsulfoxide (DMSO)-d6 as solvent. Mass spectra were recorded on a Jeol MAT 312 instrument, while infrared (IR) spectra were recorded on Bio-Rad FTS-Win IR apparatus, using a KBr disk.
2-Phenyl-4H-3,1-benzoxazine-4-one (2)
To a well-stirred solution of anthranilic acid (2 g, 6 mmol), dissolved in dry pyridine (20 mL), was added, drop-wise, benzoyl chloride (3.5 mL) with continuous stirring for 30 min at room temperature. After completion of reaction, the mixture was poured into saturated NaHCO3 solution. The product precipitated out was filtered, washed with water, and recrystallized from dilute ethanol. Yield: 95%; mp = 108–110°C; IR (KBr) νmax cm−1: 2922 (C-H), 1759 (C=O), 1602 (C=N), 1557 (C=C), 1470 (C-H, bend), 1309 (C-H, bend), 1224 (C-O), 1172 (C-C); EI-MS m/z (%): 223 (M+, 100), 146 (15), 120 (13), 105 (100), 77 (83), 51 (18); 1H-NMR (400 MHz, CDCl3): δ 8.26 (d, 1H, J = 8), 7.82 (m, 4H), 7.56 (m, 4H).
General method for the preparation of compounds 3–6
Substituted benzoic acids in a slight excess of freshly distilled thionyl chloride (10 mL) catalyzed with 2–3 drops of dimethyl formamide (DMF) were boiled under reflux for 2–3 h. The mixture was allowed to cool below room temperature, and was then added drop-wise to a well-stirred solution of anthranilic acid in dry pyridine (15 mL). The stirring was continued for 30 min or till precipitate formation was complete. The mixture was then added to saturated NaHCO3 solution and shaken well. The precipitated solid was filtered, washed with aqueous NaHCO3 solution followed by distilled water, dried, and recrystallized from a suitable solvent to yield the corresponding 2-aryl-4H-3,1-benzoxazin-4-one (3–6) in 80–96% yield.
2-(4-Nitrophenyl)-4H-3,1-benzoxazin-4-one (3)
4-Nitrobenzene (1.2 g, 0.007 mol), thionyl chloride (10 mL), anthranilic acid (0.5 g, 0.0036 mol), and pyridine (15 mL). Yield: 89%; mp = 202–204°C; IR (KBr) νmax cm−1: 1709 (C=O, str.), 1600 (C=N, str.), 1471 (C=C-C, str.), 1344 (C-O, str.), 1233 (C-C, str.), 747, 690, 669 (C-H, bend, arom.); EI-MS m/z (%): 268 (M+, 28), 253 (44), 236 (7), 235 (15), 207 (48), 179 (19), 178 (27), 167 (9), 105 (91), 104 (17), 102 (32), 92 (10), 77 (39), 76 (100), 63 (17), 51 (20); 1H-NMR (400 MHz, CDCl3), δ: 8.34 (d, 2H, J = 9, Ar-H’s), 8.02 (d, 1H, J = 9, Ar-H’s), 7.98 (m, 3H, Ar-H’s), 7.81(t, 1H, J = 8.7), 7.39 (t, 1H, J = 8.5).
2-(4-Methylphenyl)-4H-3,1-benzoxazin-4-one (4)
4-Methyl benzoic acid (3.9 g, 0.029 mol), thionyl chloride (10 mL), and anthranilic acid (2 g, 0.0155 mol). Yield: 92%; mp = 144°C; IR (KBr) νmax cm−1: 3262 (C-H), 1725 (C=O), 1516 (C=N), 1464 (C=C-C), 1264 (C-O), 1198 (C-C): EI-MS m/z (%): 237 (M+, 92), 146 (21), 120 (18), 119 (100), 91 (38), 76 (10), 65 (10); 1H-NMR (300 MHz, CDCl3), δ: 8.21 (d, 1H, J = 8.6), 8.11 (d, 1H, J = 8.7), 7.97 (d, 2H, J = 8), 7.82 (t, 1H, J = 8.4), 7.44 (t, 1H, J = 8.0), 7.18 (d, 2H, J = 8.4), 2.36 (s, 3H, CH3).
2-(4-Chlorophenyl)-4H-3,1-benzoxazin-4-one (5)
4-Chlorobenzoic acid (0.029 mol), thionyl chloride (10 mL), and anthranilic acid (2 g, 0.0155 mol). Yield: 89%; mp = 158–160°C; IR (KBr) νmax cm−1: 3039 (C-H), 1716 (C=O), 1557 (C=N), 1470 (C=C), 1224 (C-O), 1173 (C-C), 765 (C-Cl); EI-MS m/z (%): 259 (M+2, 3), 257 (M+, 8), 243 (10), 241 (61), 215 (2), 213 (5), 146 (24), 139 (5), 113 (4), 111 (13), 76 (91), 50 (5); 1H-NMR (300 MHz, CDCl3), δ: 8.23 (d, 1H, J = 9), 8.15 (d, 1H, J = 9), 7.94 (d, 2H, J = 8), 7.8 (t, 1H, J = 8.5), 7.51–7.42 (m, 3H).
2-(4-Bromophenyl)-4H-3, 1-benzoxazin-4-one (6)
4-Bromobenzoic acid (6 g, 0.029 mol), thionyl chloride (10 mL), and anthranilic acid (2 g, 0.0155 mol). Yield: 87%; mp = 184°C; IR (KBr) νmax cm−1: 3021 (C-H), 1726 (C=O), 1602 (C=N), 1546 (C=C), 1415 (C-H), 1309 (C-O), 1172 (C-C); EI-MS m/z (%): 303 (M+2, 48), 301 (M+, 56), 287 (4), 285 (40), 259 (12), 257 (16), 182 (4), 181 (11), 180 (6), 168 (9), 166 (13), 146 (81), 120 (20), 118 (22), 105 (92), 92 (11), 76 (100), 63 (25), 57 (29), 51 (42); 1H-NMR (300 MHz, CDCl3), δ: 8.23 (d, 1H, J = 8.9), 8.12 (d, 1H, J = 8.7), 7.93 (dd, 2H, J1,2 = 8.7, J3,4 = 9.5), 7.81 (t, 1H, J = 9.2), 7.66 (m, 2H), 7.45 (d, 1H, J = 8.3).
General procedure for the synthesis of 2,3-disubstituted quinazolin-4(3H)-ones (7–17)
An appropriate 2-aryl-4H-3,1-benzoxazin-4-one (2–6) and primary aromatic/aliphatic amine were refluxed in a suitable high-boiling solvent for 3–7 h. After completion of the reaction, as monitored by TLC, excess of the solvent was evaporated under reduced pressure and the reaction mixture was then poured into ice-cold water. The precipitated product was filtered out and recrystallized from the appropriate solvent.
3-(2-Hydroxyethyl)-2-(4-nitrophenyl) quinazolin-4(3H)-one (7)
2-(4-Nitrophenyl)-4H-3,1-benzoxazin-4-one (2 g, 0.0074 mol), monoethanolamine (2 mL, 0.0476 mol), and dry benzene (10 mL). Reflux time (3 h). Yield: 87%; mp = 228°C; IR (KBr) νmax cm−1: 3450 (O-H), 2924 (C-H), 1676 (C=O), 1640 (C=N), 1582 (C=C), 1347 (C-N, C-O), 1256 (C-C), 853, 768, 698 (arom. =C-H, bend); EI-MS m/z (%): 311 (M+, 23), 295 (53), 280 (35), 266 (100), 254 (7), 226 (29), 146 (12), 122 (6), 120 (44), 119 (13), 105 (5), 104 (41), 15 (15), 76 (19), 75 (7), 64 (5), 50 (4); 1H-NMR (400 MHz, CDCl3): δ: 8.68 (d, 1H, J = 8.28), 8.305 (t, 2H, J = 8.8), 8.1325 (t, 2H, 8.6), 7.632–7.495 (m, 3H), 3.724 (t, 2H, J = 5), 3.53 (t, 2H, J = 5.2), 3.31(b, s, 1H, OH).
3-Amino-2-(4-chlorophenyl) quinazolin-4(3H)-one (8)
2-(4-Chlorophenyl)-4H-3,1-benzoxazin-4-one (5) (2 g, 0.0077 mol), hydrazine hydrate 80% (5 mL), and dry benzene (15 mL). Reflux time (2h). Yield: 90%; mp = 189–190°C; IR (KBr) νmax: 3212 (N-H), 1769 (C=O), 1660 (C=N, C=C), 1245 (C-O, C-N), 1172 (C-C), 768 (C-Cl); EI-MS m/z (%): 273 (M+2, 50), 272 (53), 271 (M+, 100), 270 (86), 257 (4), 255 (13), 243 (100), 242 (61), 214 (4), 213 (5), 202 (24), 178 (73), 90 (3), 77 (5), 76 (8), 50 (4); 1H-NMR (300 MHz, CDCl3), δ: 8.30 (d, 1H, J = 6), 7.79–7.77 (m, 4H), 7.57–7.44 (m, 3H), 5.05 (b, s, 2H).
3-Amino-2-phenylquinazolin-4(3H)-one (9)
2-Phenyl-4H-3,1-benzoxazine-4-one (2) (2 g, 0.0089 mol), hydrazine hydrate 80% (5 mL), and dry benzene (15 mL). Reflux time (6 h). Yield: 86%, mp = 236–238°C; IR (KBr) νmax cm−1: 3207 (N-H), 1768 (C=O), 1602 (C=N), 1484 (C=C), 1245 (C-O, C-C); EI-MS m/z (%): 237 (100), 236 (34), 222 (26), 221 (12), 208 (59), 180 (18), 179 (7), 152 (7), 119 (37), 105 (10), 103(8), 77 (28), 63 (5), 50 (14); 1H-NMR (300 MHz, CDCl3): δ: 8.35 (d, 1H, J = 8), 7.82 (m, 4H), 7.56 (m, 4H), 4.43 (s, 2H).
3-(4-Iodophenyl)-2-phenylquinazolin-4(3H)-one (10)
2-Phenyl-4(H)-3,1-benzoxazine-4-one (2) (1 g, 0.00448 mol), 4-iodoaniline (1 g, 0.00448 mol), and dry benzene (10 mL). Reflux time (2 h) Yield: 88%; mp = 233–235°C; IR (KBr) νmax cm−1: 1664 (C=O), 1603 (C=N), 1534 (C=C, conjugated), 1257 (C-C), 565 (C-I); EI-MS m/z (%): 424 (12), 348 (3), 297 (3), 222 (96), 209 (10), 181 (5), 146 (11), 120 (11), 105 (100), 92 (14), 77 (24), 65 (5), 51 (7); 1H-NMR (300 MHz, CDCl3): δ: 8.4 (d, 1H, J = 7.8), 7.98 (d, 2H, J = 15), 7.85–7.81 (t, 1H, J = 8), 7.6–7.54 (m, 4H), 7.5–7.45 (m, 3H), 7.3–7.28 (d, 2H, J = 11.5).
3-Benzyl-2-phenylquinazolin-4(3H)-one (11)
2-Phenyl-4H-3,1-benzoxazine-4-one (2) (1 g, 0.00448 mol), freshly distilled benzyl amine (0.015 mL, 0.00896 mol), and dry benzene (10 mL). Yield: 89%; mp = 155–157°C; IR (KBr) νmax cm−1: 2929 (C-H), 1668 (C=O), 1519 (C=C, conjugated), 1305 (C=C-C); FAB-MS (–ve): 311; EI-MS m/z (%): 312 (M+), 252 (4), 223 (49), 195 (10), 139 (10), 120 (60), 105 (100), 92 (32); 1H-NMR (300 MHz, CDCl3): δ: 8.82 (d, 1H, J = 8), 8.042 (d, 2H, J = 8), 7.53–7.47 (m, 5H), 7.35–7.31 (m, 5H), 7.09 (t, 1H, J = 8), 4.63 (s, 2H, CH2).
3-Mesityl-2-phenylquinazolin-4(3H)-one (12)
2-Phenyl-4H-3,1-benzoxazine-4-one 2 (1 g, 0.00448 mol), 2,4,6-trimethylaniline, and dry benzene (10 mL). Reflux time (8 h). Recrystallized from ethanol. Yield: 73%; mp = 210–213°C; IR (KBr) νmax cm−1: 2934 (C-H), 1641 (C=O), 1496 (C=C), 1255 (C-C); EI-MS m/z (%): 340 (M+, 12), 254 (10), 222 (60), 146 (5), 135 (100), 120 (18), 105 (58), 92 (5), 76 (20), 65 (4); 1H-NMR (300 MHz, CDCl3): δ: 8.7 (d, 1H, J = 8), 8.02 (d, 2H, J = 8), 7.95 (t, 1H, J = 8), 7.63–7.42 (m, 5H), 6.96 (s, 2H), 2.3 (s, 3H), 2.2 (s, 6H).
3-Amino-2-(4-methylphenyl) quinazolin-4(3H)-one (13)
2-(4-Methylphenyl)-4H-3,1-benzoxazin-4-one 4 (2 g), hydrazine hydrate 80% (5 mL), and dry benzene (15 mL). Reflux time (8 h). Yield: 85%; mp = 165°C; IR (KBr) νmax cm−1: 3308 (N-H), 1665 (C=O), 1530 (C=N), 1251 (C-O, C-N), 769, 695, 660 (arom, C-H, bend); EI-MS m/z (%): 251 (M+, 4), 138 (100), 222 (2), 200 (5), 146 (19), 120 (96), 91 (38), 82 (10), 65 (18), 1H-NMR (300 MHz, CDCl3), δ; 8.35 (d, 1H, J = 8), 7.93 (d, 2H, J = 8), 7.64 (m, 3H), 7.45 (d, 1H, J = 8), 7.15 (q, 1H, J = 8), 4.08 (s, 2H), 2.348 (s, 3H, CH3).
2-(4-Nitrophenyl)-3-(1,3-thiazol-2-yl)quinazolin-4(3H)-one(14)
2-(4-Nitrophenyl)-4H-3,1-benzoxazine-4-one (0.2 g, 0.000813 mol), 2-aminothiazole (0.08 g), and dry pyridine (10 mL). Reflux time (5 h). Yield: 97%; mp = 231°C; IR (KBr) νmax cm−1: 1645 (C=O), 1575 (C=N), 1520 (C=C), 1301 (C-H, bend), 1245 (C-O), 1234 (C-C), 834, 756, 681 (C-H, arom., bend): EI-MS m/z (%): 350 (M+, 67), 319 (23), 294 (15), 281 (12), 267 (93), 253 (18), 229 (11), 145 (83), 132 (11), 120 (71), 105 (100); 1H-NMR (300 MHz, CDCl3): δ: 8.28 (d, 1H, J = 8.5), 8.127 (d, 2H, J = 8.5), 8.063 (d, 1H, J = 7.9), 7.632 (d, 2H, J = 7.8), 7.492 (t, 1H, J = 7.3), 7.13 (t, 1H, J = 7.7), 7.01 (d, 1H, J = 4), 6.70 (d, 1H, J = 3.5).
3-(2-Hydroxyethyl)-2-phenylquinazolin-4(3H)-one (15)
2-Phenyl-4H-3,1-benzoxazine-4-one (2), (2 g, 0.008968 mol), monoethanolamine (2 mL, 0.032 mol), and dry benzene (10 mL). Reflux time (6 h). Recrystallized from ethanol. Yield: 85%; mp = 164–166°C; IR (KBr) νmax: 3344 (O-H), 2280 (C-H), 1664 (C=O), 1496 (C=C), 1427 (C=C, conjugated), 1255 (C-C); EI-MS m/z (%): 266 (M+, 25), 254 (11), 249 (7), 235 (10), 224 (40), 223 (43), 222 (14), 190 (6), 189 (39), 180 (4), 179 (22), 167 (3), 149 (6), 146 (28), 120 (16), 111 (7), 106 (12), 105 (100), 97 (11), 85 (11), 83 (10), 77 (81), 71 (16), 57 (25), 55 (13), 51 (14), 44 (100), 43 (15), 40 (5); 1H-NMR (300 MHz, CD3OD): δ: 8.622 (d, 1H, J = 8.3), 7.997 (d, 2H, J = 8.2), 7.795 (d, 1H, J = 7), 7.6–7.51 (m, 4H), 7.22–7.17 (t, 1H, J = 8.1), 3.74–3.70 (t, 2H, J = 5.7), 3.55–3.51 (t, 2H, J = 5.7), 3.29 (s, 1H).
3-(4-Bromophenyl)-2-phenylquinazolin-4(3H)-one (16)
2-Phenyl-4(H)-3,1-benzoxazine-4-one (1 g, 0.00448 mol),4-bromoaniline (0.78 g, 0.00448 mol), and dry pyridine (10 mL). Reflux time (6 h). Recrystallized from ethanol. Yield: 41%; mp = 228–230°C; IR (KBr) νmax cm−1: 3066 (=C-H), 1603 (C=O), 1492 (C=C, conjugated), 1258 (C-C), 757 (C-Br); EI-MS m/z (%): 379 (M+2, 14), 377 (M+, 10), 301 (81), 221 (100), 208 (47), 180 (3), 157 (27), 105 (100), 92 (6), 77 (34), 65 (37), 57 (3); 1H-NMR (300 MHz, CDCl3): δ: 8.40 (d, 2H, J = 8.2), 8.01 (d, 1H, J = 13), 7.93–7.79 (m, 2H), 7.65 (t, 1H, J = 8), 7.58 (t, 1H, J = 8.7), 7.49–7.40 (m, 6H).
3-(4-Methylphenyl)-2-phenylquinazolin-4(3H)-one (17)
2-Phenyl-4(H)-3,1-benzoxazine-4-one 2 (1 g, 4.48 mmol), 4-methylaniline (0.015 mL, 8.96 mmol), and dry benzene (10 mL). Reflux time (30 min). Yield: 73%; mp = 186–188°C; IR (KBr) νmax cm−1: 2982 (C-H), 1664 (C=O), 1512 (C=C), 1448 (C=C-C), 1232 (C-C); EI-MS m/z (%): 312 (M+, 3), 222 (18), 210 (4), 179 (8), 145 (18), 120 (36), 105 (100), 92 (19), 77 (73), 51 (13); 1H-NMR (300 MHz, CDCl3): δ: 8.70 (d, 1H, J = 8), 8.02 (d, 2H, J = 8), 7.57–7.45 (m, 6H), 7.21–7.02 (m, 4H), 2.34 (s, 3H, CH3).
2-Methyl-3-(4-methylphenyl) quinazolin-4(3H)-one (18)
A mixture of methyl N-acetyl anthranilate (2 g, 0.0103 mol) and 4-methylaniline was boiled under reflux for 10 h in absolute ethanol (10 mL). After completion of reaction, monitored by TLC, ethanol was evaporated under reduced pressure and the mixture was allowed to cool by adding ice-cooled water. The precipitate formed upon cooling was filtered, dried, and recrystallized from ethanol. Yield: 67%; mp = 197–199°C; IR (KBr) νmax cm−1: 2935 (C-H), 1664 (C=O), 1534 (C=N), 1449 (C=C, conjugated), 1204 (C-C); EI-MS m/z (%): 250 (M+, 5), 235 (34), 222 (19), 210 (2), 179 (10), 145 (18), 105 (64), 105 (100), 92 (19), 77 (67), 65 (13), 51 (12); 1H-NMR (300 MHz, CDCl3): δ: 8.27 (d, 1H, J = 9), 8.02 (t, 1H, J = 8), 7.69–7.59 (m, 2H), 7.21 (d, 2H, J = 9), 7.024 (d, 2H, J = 8.7), 2.53 (s, 3H, CH3), 2.34 (s, 3H, CH3).
3-(2-Hydroxyethyl)-2-methylquinazolin-4(3H)-one (19)
Anthranilic acid (0.14 g, 1 mmol), triethylorthoacetate (0.23 mL, 1.2 mmol), monoethanolamine (0.3 mL, 1.2 mmol), and para-toluenesulfonic acid (1 g, 5.0 mmol) were stirred at room temperature under an argon atmosphere. After completion of the reaction as indicated by TLC, the reaction contents were poured into ice-cold water. The solid precipitated was filtered out, washed with water, and recrystallized from ethanol. A second crop of the product was obtained by extracting the filtrate twice with dichloromethane, and subsequently the solvent was removed under reduced pressure. Yield: 89%; m.p 134°C IR (KBr) νmax cm−1: 3250 (O-H), 1664 (C=O), 1534 (C=N, C=C), 1449 (C=C, conjugated), 1304 (C-O), 1255 (C-C), 757, 700, 664 (arom. =C-H, bend); EI-MS m/z (%): 204 (M+, 28), 185 (9), 173 (13), 161 (59), 169 (100), 159 (7), 146 (5), 145 (10), 143 (13), 119 (7), 118 (7), 116 (9), 92 (2), 90 (5), 77 (13), 76 (8), 50 (3); 1H-NMR (300 MHz, CDCl3): δ: 8.190 (d, 1H, J = 8), 7.38–7.682 (t, 1H, J = 7.9), 7.631 (d, 1H, J = 7.3), 7.45–7.39 (t, 1H, J = 7.7), 4.316–4.282 (t, 2H, J = 5.07), 4.02–3.99 (t, 2H, J = 5.3), 3.22 (b, s, 1H), 2.72 (s, 3H).
Antimicrobial activity
The synthesized compounds were assayed for their antimicrobial (antibacterial and antifungal) potential by the hole-diffusion methodCitation17,Citation18 followed by determination of their corresponding minimum inhibitory concentration (MIC) values against tested fungal and bacterial strains. The MICs of standard drugs and active test compounds were determined against test isolates by the broth microdilution technique.
Bactericidal bioassay (in vitro)
The synthesized compounds were screened for antibacterial activity against Escherichia coli (ATCC 25922), Bacillus subtilis (ATCC 6633), Shigella flexneri (clinical isolate), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and Salmonella typhi (ATCC 19430) by using literature protocolsCitation19,Citation20. In the agar well diffusion method, wells were dug in the media with the help of a sterile metallic borer with centers at least 24 mm part. Then, 2–8 h old bacterial inocula containing approximately 104–106 colony forming units (CFU)/mL were spread on the surface of nutrient agar with the help of a sterile cotton swab. The cotton swab was rotated firmly against the upper inside well of the tube to express excess fluid. The entire agar surface of the plate was streaked with the swab three times, turning the plate 60° between each streaking. The recommended concentration of test sample (2 mg/mL of DMSO) was then added in the respective well. Other wells supplemented with DMSO and reference antibacterial drugs served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 14–19 h. Potency was determined by measuring the diameter of the zone showing complete inhibition (mm). Growth inhibition and MIC values were calculated with reference to the positive control.
Leishmanicidal bioassay (in vitro)
Leishmania major (MHOM/PK/88/DESTO) promastigotes, cultivated in bulk, were aseptically sedimented down at 3000 rpm, counted with the help of an improved incubator chamber under the microscope, and diluted with fresh medium to a final concentration of 2.0 × 10Citation6 parasites mL−1.
The compounds were dissolved in appropriate solvents and then diluted to a final concentration of 1.0 mg/mL of PBS (phosphate buffered saline; pH 7.4, containing 0.5% MeOH, 0.5% DMSO). The plates were then incubated at 21–22°C in the dark for 3–5 days, during which the control organism multiplied 3–6 times. Cultures were examined microscopically on an improved Neubauer chamber, and IC50 values of the compounds exerting antileishmanial activity were calculatedCitation21,Citation22.
Results and discussion
Chemistry
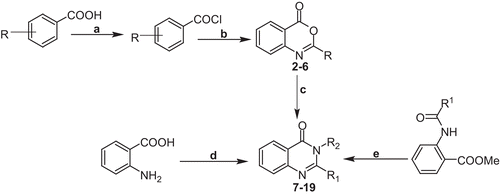
2-Aryl-4H-3,1-benzoxazine-4-ones have been reported for their significant biological activitiesCitation23,Citation24 as well as for being very good synthetic precursors in the synthesis of 2,3-disbstituted quianzolin-4(3H)-ones. The synthesis of 2-aryl-4H-3,1-benzoxazine-4-ones (2–6) was therefore accomplished using our recently developed three-component one-pot synthesisCitation15 starting from the easily available substituted benzoic acids (). The synthesized 2-aryl-4H-3,1-benzoxazine-4-ones were then refluxed with selected primary amines in appropriate solvents to accomplish the synthesis of 2,3-alkyl/aryl-quianzolin-4(3H)-ones (7–19). The synthesis of compounds 18 and 19 was accomplished by refluxing methyl N-acetyl anthranilate with toluene and using a solvent-free method recently developed by Narasimhulu et al.Citation25 ().
Scheme 1. General schematic synthesis of 2,3-disubstituted 3H-quinazolin-4-ones. Reagents and conditions: (a) thionyl chloride, DMF, reflux; (b) anthranilic acid, pyridine (dry), stir; (c) primary amine, pyridine/benzene, reflux 2–3 h; (d) triethylorthoacetate, para-toluenesulfonic acid (PTSA), primary amine, stir, r.t.; (e) primary amine, ethanol, reflux 2 h.
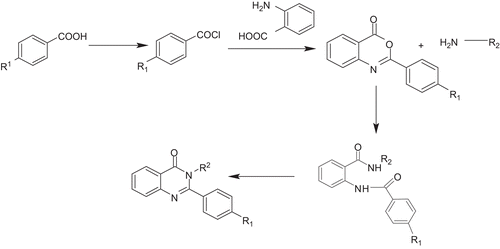
The mechanism of formation of 2,3-alkyl/aryl-quianzolin-4(3H)-ones (7–19) from 2-aryl-4H-3,1-benzoxazine-4-ones (2–6) is believed to involve the initial formation of diamide intermediates, which upon subsequent cyclization and dehydration give the target 2,3-alkyl/aryl-quianzolin-4(3H)-ones (, ).
Table 1. Synthesized 2,3-disubstituted quianzolin-4(3H)-ones 7–19 (see ).
Structures of the synthesized compounds were established on the basis of spectroscopic data. IR spectra of compounds 7, 15, and 19 showed absorption bands ranging 3250–3450 cm−1, indicating the presence of the OH group in the compounds. Absorption bands showing the presence of the N-H group, ranging 3207–3308 cm1, were found in the IR spectra of compounds 8, 9, and 13. Molecular masses of all the compounds were confirmed from electron ionization-mass spectrometry (EI-MS); also, the presence of isotopic distributions of chloro and bromo substitutents were confirmed from the M+2 peaks in the EI-MS spectra of the compounds. In the 1H-NMR spectra, characteristic absorption bands were observed ranging from 6.7 to 9 ppm, with coupling constants (J = 7–9 Hz) for the aromatic protons. The appearance of singlets at 3.31, 3.22, and 3.299 in the spectra of compounds 7, 15, and 19, respectively, depicted O-H, while singlets at 5.05, 4.43, and 4.08 confirmed the presence of NH2 in the spectra of compounds 8, 9, and 13.
Antibacterial activity
The synthesized compounds were tested for their in vitro antibacterial activity against both Gram (+) and Gram (–) bacteria cultures including B. subtilis, S. aureus, S. flexneri, P. aeruginosa, and S. typhi using the agar well diffusion protocolCitation19,Citation20. Imipenem was used as the standard antibiotic; in a typical experimental procedure, 24-h old culture was spread over Müller–Hinton agar (MHA) plates. Plates were incubated for 24 h at 37°C. Zones of inhibition were measured and compared with the standard drug. Most of the compounds were found to be inactive against the tested bacteria; among the series tested, compounds 9, 11, 12, and 19 showed significant activities against the tested bacterial culture (). Compound 9 was found to be more active against Salmonella typhi (ATCC 19430), MIC 0.29 μg/mL, than the standard imipenem having MIC = 0.7 μg/mL (). The MIC values of active compound 12 against B. subtilis (ATCC 6633), S. aureus (ATCC 25923), E. coli (ATCC 25922), and S. typhi (ATCC 19430) were found to be 0.15 μg/mL, 0.15 μg/mL, 0.13 μg/mL, and 0.11 μg/mL, respectively. Therefore, compound 12 was found to be more active against the selected strains than the standard drug. Compound 19 was found to be more potent against S. aureus (ATCC 25923), MIC = 0.9 μg/mL. In conclusion, compounds 9, 11, 12, and 19 were found to be more potent, and can therefore be used as a lead source for the development of better antibacterial agents.
Table 2. Antibacterial activities (mm) at 1 mg/mL.
Table 3. Antibacterial activities (MIC) of active compounds 9, 11, 12, and 13.
Antifungal activity
Antifungal activities were determined using the agar tube dilution protocolCitation26 against some human pathogens: Trichophyton longifusus (clinical isolate), Candida albicans (ATCC 2091), Aspergillus flavus (ATCC 32611), Mycosporum canis (ATCC 11622), Fusarium solani (ATCC 11712), and Candida glabrata (ATCC 90030). Compounds 9 and 15 were found to be inactive against all fungi, while the rest of the tested compounds showed weak to moderate activities against some fungi (). The MIC values of the active compounds 7, 8, 10, 12, and 16 are given in . The determined MIC value of compound 8 was 5.8 μg/mL against C. albicans (ATCC 2091), while the MIC value of the standard miconazole against Candida albicans (ATCC 2091) was 4.8 μg/mL. Compound 8 can therefore be used as a good starting point for the development of better antifungal agents against Candida albicans (ATCC 2091).
Table 4. Antifungal activities (% inhibition) at 200 μg/mL.
Table 5. Antifungal activities (MIC) of compounds 7, 8, 10, 12, and 18.
Leishmanicidal activity
Leishmanicidal activity was determined at 100 µg/mL levels. Amphotericin B was used as the standard drug, with IC50 = 0.50 µg/mL. Among the tested compounds, 7, 8, and 15 showed moderate activity while most others either showed weak activity or were inactive (). The compound 3-benzyl-2-phenylquinazolin-4(3H)-one (11) was found to be more potent (IC50 48 µg/mL), and is therefore anticipated to be a better new leishmanicidal candidate. The structure–activity relationship of the substituents is important. The chloro susbtituent on the phenyl group at position 2 enhances the activity; similarly, the alkyl group at the C-3 position increases the activity, as observed in compounds 8 and 7. A profound effect in compound 11 was observed due to the presence of the benzylic moiety at the C-3 position. In conclusion, we report here some new potent antileishmanial compounds and further work in this area is in progress.
Table 6. Leishmanicidal activities (IC50 values) of compounds 7–19.
Declaration of interest
The first two authors (M.A and R.K) acknowledge the financial support from the University of Peshawar in the form of institutional research grant and are thankful to the HEJ Research Institute of Chemistry (ICCBS) for the provision of spectroscopic and bioassay facilities.
References
- Zaika LL. Spices and herbs: their antimicrobial activity and its determination. J Food Safet 1975;9:97–118.
- Leon LL, Machado GMC, Carvalho-Paes LE, Grimaldi JG. Antigenic differences of Leishmania amazonensis isolate causing diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 1990;84:678–80.
- Korolkovas A, Burckhalter JH.Química Farmacêutica. Rio de Janeiro: Guanabara, 1988:39.
- Frezard F, Michalick MSM, Soares CF, Demicheli C. Novel methods for encapsulation of meglumine antimoniate into liposomes. Braz J Med Biol Res 2000;33:841–6.
- Ji-Feng L, Lee J, Dalton AM, Grace B, Libing Y, Baldino CM, et al. Microwave-assisted one-pot synthesis of 2,3-disubstituted 3H-quinazolin-4-ones. Tetrahedron Lett 2005;46:1241–4.
- Seleh MA, Abde-Mageed MF, Abdo MA, Abdel-Basset MS. Synthesis of novel 3H-quinazolin-4-ones containing pyrazolinone, pyrazole and pyrimidinone moieties. Molecules 2003;8:363–73.
- Edward C, Lawson WA, Kinney MJ, Costanzo WJ, Hoekstra JA, Kauffman DK, et al. Structure-function study of quinazolinone-based vitronectin receptor (αVβ3) antagonists: computer-assisted analysis of ligand-receptor interactions. Lett Drug Des Discov 2004;1:14–18.
- Haruhiko F, Kobayashi T, Tokitoh T, Ytorii Y, Natsugari H. Synthetic studies on 3-arylquinazolin-4-ones: intramolecular nucleophilic aromatic substitution reaction of 2-carboxamido-3-arylquinazolin-4-ones and its application to the synthesis of secondary aryl amines. Tetrahedron 2005;61:4297–312.
- Wolf JF, Rathnam TL, Sleevi MC, Campbell JA, Greenwood TD. Synthesis and anticonvulsant activity of some new 2-substituted 3-aryl-4(3H)-quinazolinones. J Med Chem 1990;33:161–6.
- Mhaske SB, Argade NP. The chemistry of recently isolated naturally quinazolinone alkaloids. Tetrahedron 2006;62:9787–826.
- Connolly DJ, Cusack D, O’Sullivan TP, Guiry PJ. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005;61:10153–202.
- Nosova EA, Lipunova GN, Charushin VN. Cyclization of 2-halobenzoylchlorides with dinucleophiles as a versatile approach to fused heterocycles. Russ Chem Bull Int Ed 2004;53:1137.
- Ji-Feng Liu. Rapid synthesis of biologically active qunazolinone – natural products using microwave technology. Curr Organic Synth 2007;4:223–37.
- Witt A, Bergman J. Synthesis and reactions of some 2-vinyl-3H-quinazolin-4-ones. Tetrahedron 2000;56:7245–53.
- Arfan M, Khan R, Imran M, Khan H, Mehmood J. One-pot synthesis and antimicrobial activities of some 2-aryl/alkyl, 3-aminoquinazolin-4(3H)-ones. J Chem Soc Pak 2008;30:299–305.
- Arfan M, Khan R, Anjum S, Ahmad S, Choudhary MI. Water promoted, microwave assisted oxidative novel deamination on N-aminoquinazolone. Chin Chem Lett 2008;19:161–5.
- National Committee for Clinical Laboratory Standards. Method for broth dilution antifungal susceptibility testing yeast; approved standard, M27-A, 15, 10. Tuscon: NCCLS, VA Medical Center, 1996.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard. NCCLS document M100-S12. Wayne, PA: NCCLS, 2002.
- Carron RA, Marran JM, Montero-Fernandozlago L, and Dominguez AA. Antimicrobial properties of some extracts obtained from some mediterranean plants of medicinal value. Plantes Medicinales ET Phytotherapie 1987;21:195–202.
- Atta-ur-rehman Choudhary, MI, Thomsen WJ. Manual of Bioassay Techniques for Natural Products Research. Amsterdam: Harvard Academic Press, 1999:12.
- Evan DA, ed. Hand Book on Isolation, Characterization and Cryopreservation of Leishmania. Geneva: WHO, 1989;7:10–12, 28.
- Ben Salah A, Zakraoui H, Zaatour A, Ftaiti A, Aafouri B, Garraoui A, et al. A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am J Trop Med Hyg 1995;53:162–6.
- Shariat M, Abdollahi S. Synthesis of benzoxazinone derivatives: a new route to 2-(N-phthaloylmethyl)-4H-3,1-benzoxazin-4-one. Molecules 2004;9:705–12.
- Pei-Wen H, Tsong-Long H, Chin-Chung W, Fang-Rong C, Tsai-Wei W, Yang-Chang W. The evaluation of 2,8-disubstituted benzoxazinone derivatives as anti-inflammatory and anti-platelet aggregation agents. Bioorg Med Chem Lett 2005;15:2786–9.
- Narasimhulu M, Mahesh KC, Reddy TS, Rajesh K, Venkateswarlu Y. Lanthanum(III) nitrate hexahydrate or p-toluenesulfonic acid catalyzed one-pot synthesis of 4(3H)-quinazolinones under solvent-free conditions. Tetrahedron Lett 2006;47:4381–3.
- Paxton JD.Assay for antifungal activity. In:Hostemann K, Dey PM, Harborne JB, eds. Methods in Plant Biochemistry. London: Academic Press, 1991;6:33.