Abstract
Oxime K027 is a low-toxic bisquaternary compound originally developed as a reactivator of acetylcholinesterase (AChE) inhibited by nerve agents. The reactivation potency of K027 has been tested as a potential reactivator of AChE inhibited by tabun, sarin, cyclosarin, soman, VX, Russian VX, paraoxon, methylchlorpyrifos, and DDVP. The results show that oxime K027 reactivated AChE inhibited by almost all tested inhibitors to more than 10%, which is believed to be enough for saving the lives of intoxicated organisms. In the case of cyclosarin- and soman-inhibited AChE, oxime K027 did not reach sufficient reactivation potency.
Introduction
Highly neurotoxic organophosphorus-type (OP) chemical warfare agents (“nerve agents”) and OP pesticides have been used by terrorists and during military conflicts, emphasizing the necessity for the development of potential antidotes. Numerous new oximes have been synthesized and tested in recent decades. However, an effective therapy by a single oxime to all the known nerve agents and pesticides is still lacking.
Intoxication with nerve agents or pesticides leads to inhibition of acetylcholinesterase (AChE) by phosphorylation of its active site serine residue. The subsequent accumulation of the neurotransmitter acetylcholine and overstimulation of cholinergic receptors results in a generalized cholinergic crisis, including breakdown of neuromuscular function. Generally, the reactivation of AChE, i.e. the removal of the phosphoryl or phosphonyl moiety from the active site of the enzyme, is considered to be the main mechanism of action of oximesCitation2.
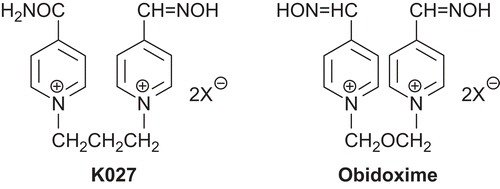
In 2003, the oxime K027 (1-(4-hydroxyiminomethyl–pyridinium)-3-(4-carbamoylpyridinium) propane dibromide) was synthesized ()Citation1. The K027 reactivator was tested in vitro for the first time as a promising oxime (with several limitations) for reactivation of nerve agent-inhibited AChECitation3–11. Then, its acute toxicity was determined, and it was found that the toxicity is very low (LD50 > 1200 mg/kg; rat)Citation12. After this finding, many in vivo studies were conducted throughout the world, and a large number of promising results were obtainedCitation2,Citation13–23. Probably the most promising finding was in the United Arab Emirates by the group of Petroianu. They found that oxime K027 can reactivate AChE inhibited by many kinds of organophosphorus pesticides in vitro. Subsequently, they confirmed their in vitro results using in vivo studies. According to this group, oxime K027 seems to be, at present, the most promising candidate to replace obidoxime () in the treatment of organophosphorus pesticide poisoningsCitation24–36.
As mentioned above, in the case of nerve-agent reactivation, the obtained results were not so promising. In particular, cyclosarin- and soman-inhibited AChE is resistant to K027 reactivation. Similar results were confirmed also using in vivo studiesCitation2,Citation17,Citation18.
Owing to the fact that all the results published on this oxime were prepared in different models (different animals, different techniques, different AChE inhibitors, etc.), in this study, the potency of this compound to reactivate a larger group of organophosphorus AChE inhibitors was tested. For this purpose, in vitro study was selected to compare only the reactivation process.
Materials and methods
All pesticides (paraoxon, dichlorvos (DDVP), methylchlorpyrifos) used in this study were obtained from Sigma-Aldrich. Nerve agents were obtained from the Military Technical Institute (Brno, Czech Republic). All inhibitors were of 95% purity and higher. The purity was evaluated by acidimetric titration prior to the experiment. Oxime K027 was synthesized at our department using a standard synthetic approachCitation1. Its purity was checked using thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) techniques prior to the experimentCitation37. All other drugs and chemicals of analytical grade were obtained commercially and used without further purification.
The reactivation efficacy of oxime K027 was assayed in vitro in a model of AChE inhibited by selected AChE inhibitors using a standard reactivation testCitation38. As a source of AChE, a homogenate from rat brains (rats of Wistar strain; individuals weighing 200–240 g without sex preference) was used. The animals were killed in ether narcosis by cutting the carotids. Narcosis did not influence the enzyme activityCitation39. The brains were removed, rinsed in saline, and then homogenized in an Ultra-Turrax homogenizer in distilled water to make a 10% homogenate. AChE homogenate (0.5 mL) was mixed with 20 µL of solution of nerve agent (in dry isopropyl alcohol) and incubated at 25°C for 30 min to reach 95% inhibition. Then, 2.5 mL of 3M NaCl was added, followed by distilled water to a final volume of 23 mL. Finally, 2 mL of 0.02 M acetylcholine iodide was added and the enzyme activity was assayed titrimetrically at pH 8.0 and 25°C using an Autotitrator RTS 822 (Radiometer, Copenhagen).
The activity of non-inhibited enzyme was measured in the same way (without the nerve agent or pesticide). Reactivation of the enzyme inhibited by the nerve agent was performed immediately after the inhibition. A solution of the reactivator of given concentration (1.0 mL) was added to the inhibited enzyme, and 10 min afterward, the activity of the reactivated enzyme was determined using the same method as described for the previous experiment. The percentage of reactivation was calculated from the following equation:
where ao is the activity of the intact enzyme, ai is the activity of the OP-inhibited enzyme, and ar is the activity of the OP-inhibited enzyme after incubation with the oxime reactivator.
Results and discussion
As indicated (), oxime K027 reactivated AChE inhibited by all tested nerve agents except cyclosarin and soman. In the case of pesticides, it was able to reactivate AChE inhibited by all tested members of this family. This is in very good agreement with results shown in former studiesCitation24–36. Oxime K027 was tested at two different concentrations, which had already been selected earlier in the study of Kuca and Cabal to capture the promising compoundsCitation7, 40. The higher concentraton (10−3 M) is not relevant for the majority of oximes because of their relatively high toxicity. However, for non-toxic compounds, it could be achievable. As mentioned above, oxime K027 toxicity is very low. Moreover, if considering the lower concentration (10−5 M), this is reachable also in the brain of an intoxicated organism. It is known that 1–10% of AChE reactivators, although they are quaternary compounds, can penetrate the blood–brain barrier and so act centrallyCitation24,Citation26,Citation41.
Table 1. Reactivation of nerve agent- and pesticide-inhibited AChE by oxime K027.
The minimal reactivation activity needed for saving the life of an intoxicated organism is considered to be 10%Citation41. According to this knowledge, at the lower concentration, oxime K027 was not able to reactivate AChE inhibited by the tested inhibitors except that inhibited by paraoxon and methylchlorpyrifos.
This contribution summarizes the reactivation activity of oxime K027 on several cholinesterase inhibitors in order to demonstrate the universality of this oxime. The results obtained show that oxime K027 cannot be considered a universal compound if discussing the lower concentration. To elucidate its broad-spectrum potency, further investigations should be conducted, especially on human tissue. Moreover, its potency should be compared with results obtained recently with other oximesCitation42–46.
The present results point to the development of new oximes, which could enrich the group of current commercially available reactivators.
Declaration of interest
This work was supported by a project of the Ministry of Education, Youth and Sports (Czech Republic), No. ME865.
References
- Kuca K, Bielavsky J, Cabal J, Bielavska M. Synthesis of a potential reactivator of acetylcholinesterase 1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium)-propane dibromide. Tetrahedron Lett 2003;44:3123–5.
- Kassa J, Kuca K, Bartosova L, Kunesova G. The development of new structural analogues of oximes for the antidotal treatment of poisoning by nerve agents and the comparison of their reactivating and therapeutic efficacy with currently available oximes. Curr Org Chem 2007;11:267–83.
- Kuca K, Kassa J. In vitro reactivation of acetylcholinesterase using of the oxime K027. Vet Hum Toxicol 2004;46:15–18.
- Kuca K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Hum Exp Toxicol 2004;23:167–71.
- Kuca K, Bartosova L, Kassa J, Cabal J, Bajgar J, Kunesova G, et al. Comparison of the potency of newly developed and currently available oximes to reactivate nerve agent-inhibited acetylcholinesterase in vitro and in vivo. Chem Biol Interact 2005;157–8:367–8.
- Kuca K, Cabal J, Bajgar J, Jun D. In vitro searching for a new potent reactivator of acetylcholinesterase inhibited by nerve agent VX. Lett Drug Des Discov 2005;2:23–5.
- Kuca K, Cabal J. Evaluation of newly synthesized reactivators of the brain cholinesterase inhibited by sarin-nerve agent. Toxicol Mech Methods 2005;15:247–52.
- Kuca K, Cabal J, Kassa J. A comparison of the potency of newly developed oximes (K005, K027, K033, K048) and currently used oximes (pralidoxime, obidoxime, HI-6) to reactivate sarin-inhibited rat brain acetylcholinesterase by in vitro methods. J Toxicol Environ Health A 2005;68:677–86.
- Kuca K, Cabal J, Jun D, Bajgar J, Hrabinova H. Potency of new structurally different oximes to reactivate cyclosarin inhibited-human brain acetylcholinesterases. J Enzyme Inhib Med Chem 2006;21:663–6.
- Kuca K, Jun D, Cabal J, Musilova L. The bisquaternary oximes as reactivators of tabun-inhibited human brain cholinesterases – in vitro study. Basic Clin Pharmacol Toxicol 2007;101:25–8.
- Berend S, Lucic-Vrdoljak A, Radic B, Kuca K. New bispyridinium oximes: in vitro evaluation of their biochemical parameters. Chem Biol Interact 2008;175:413–16.
- Musilek K, Jun D, Cabal J, Kassa J, Gunn-Moore F, Kuca K. Design of a potent reactivator of tabun-inhibited acetylcholinesterase – synthesis and evaluation of (E)-1-(4-carbamoylpyridinium)-4-(4-hydroxyiminomethylpyridinium)-but-2-ene dibromide (K203). J Med Chem 2007;50:5514–18.
- Kassa J. The influence of oxime and anticholinergic drug selection on the potency of antidotal treatment to counteract acute toxic effects of tabun in mice. Neurotox Res 2006;9:59–62.
- Kassa J, Kunesova G. A comparison of the potency of newly developed oximes (K027, K048) and commonly used oximes (obidoxime, HI-6) to counteract tabun-induced neurotoxicity in rats. J Appl Toxicol 2006;26:309–16.
- Kassa J, Kunesova G. The influence of antidotal treatment of low-level tabun exposure on cognitive functions in rats using a water maze. Neurotox Res 2006;9:39–45.
- Kassa J, Kuca K, Cabal J, Paar M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vitro and in vivo methods. J Toxicol Environ Health 2006;69:1875–82.
- Kassa J, Kuca K, Karasova J, Musilek K. The development of new oximes and the evaluation of their reactivating, therapeutic and neuroprotective efficacy against tabun. Mini Rev Med Chem 2008;8:1134–43.
- Calic M, Lucic-Vrdoljak A, Radic B, Jelic D, Jun D, Kuca K, et al. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology 2006;219:85–96.
- da Silva AP, Farina M, Franco JL, Dafre AL, Kassa J, Kuca K. Temporal effects of newly developed oximes (K027, K048) on malathion-induced acetylcholinesterase inhibition and lipid peroxidation in mouse prefrontal cortex. Neurotoxicology 2008;29:184–9.
- Lucic-Vrdoljak A, Calic M, Radic B, Berend S, Kuca K, Kovarik Z. Pre-treatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology 2006;228:41–50.
- Pejchal J, Osterreicher J, Kuca K, Jun D, Bajgar J, Kassa J. Possible hepatotoxicity of acetylcholinesterase reactivators – nerve agent antidotes. Basic Clin Pharmacol Toxicol 2008;103:119–23.
- Gyenge M, Kalasz H, Petroianu G, Laufer R, Kuca K, Tekes K. Measurement of K-27, an oxime-type cholinesterase reactivator by high performance liquid chromatography with electrochemical detection from different biological samples. J Chromatogr A 2007;1161:146–51.
- Tekes K, Hasan MY, Sheen R, Kuca K, Petroianu G, Ludanyi K, et al. HPLC determination of the serum concentration of K-27, a novel oxime-type cholinesterase reactivator. J Chromatogr A 2006;1122:84–7.
- Lorke DE, Kalasz H, Petroianu GA, Tekes K. Entry of oximes into the brain: a review. Curr Med Chem 2008;15:743–53.
- Lorke DE, Hasan MY, Arafat K, Kuca K, Musilek K, Schmitt A, et al. In vitro oxime protection of human red blood cell acetylcholinesterase inhibited by diisopropyl-fluorophosphate. J Appl Toxicol 2008;28:422–9.
- Lorke DE, Hasan MY, Nurulain SM, Sheen R, Kuca K, Petroianu GA. Entry of two new asymmetric bispyridinium oximes (K-27 and K-48) into the brain: comparison with obidoxime. J Appl Toxicol 2007;27:482–90.
- Lorke DE, Nurulain SM, Hasan MY, Kuca K, Musilek K, Petroianu GA. Eight new bispyridinium oximes in comparison with the conventional oximes pralidoxime and obidoxime: in vivo efficacy to protect from diisopropylfluorophosphate (DFP) toxicity. J Appl Toxicol 2008;28:920–8.
- Lorke DE, Hasan MY, Nurulain SM, Kuca K, Schmitt A, Petroianu GA. Efficacy of two new asymmetric bispyridinium oximes (K-27 and K-48) in rats exposed to diisopropylfluorophosphate: comparison with pralidoxime, obidoxime, trimedoxime, methoxime, and HI-6. Toxicol Mech Methods 2009;19:327–33.
- Nurulain SM, Lorke DE, Hasan MY, Shafiullah M, Kuca K, Musilek K, et al. Efficacy of eight experimental bispyridinium oximes against paraoxon-induced mortality: comparison with the conventional oximes pralidoxime and obidoxime. Neurotox Res 2009;16:60–7.
- Petroianu GA, Arafat K, Nurulain SM, Kuca K, Kassa J. In vitro oxime reactivation of red blood cell acetylcholinesterase inhibitied by methyl-paraoxon. J Appl Toxicol 2007;27:168–75.
- Petroianu GA, Lorke DE. Pyridinium oxime reactivators of cholinesterase inhibited by diisopropyl-fluorophosphate (DFP): predictive value of in-vitro testing for in-vivo efficacy. Mini Rev Med Chem 2008;8:1328–42.
- Petroianu GA, Arafat K, Kuca K, Kassa J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: in vitro reactivation of red blood cell acetylcholinesterase inhibitied by paraoxon. J Appl Toxicol 2006;26:64–71.
- Petroianu GA, Hasan MY, Nurulain SM, Nagelkerke N, Kassa J, Kuca K. New K-oximes (K-27and K-48) in comparison with obidoxime (LuH-6), HI-6, trimedoxime (TMB-4) and pralidoxime (2-PAM): survival in rats exposed ip to the organophosphate paraoxon. Toxicol Mech Methods 2007;17:401–8.
- Petroianu GA, Nurulain SM, Nagelkerke N, Al-Sultan MAH, Kuca K, Kassa J. Five oximes (K-27, K-33, K-48, BI-6 and methoxime) in comparison with pralidoxime: survival in rats exposed to the organophosphate paraoxon. J Appl Toxicol 2006;26:262–8.
- Petroianu GA, Nurulain SM, Nagelkerke N, Shafiullah M, Kassa J, Kuca K. Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with pralidoxime: survival in rats exposed to methyl-paraoxon. J Appl Toxicol 2007;27:453–7.
- Park NJ, Jung YS, Musilek K, Jun D, Kuca K. Potency of several structurally different acetylcholinesterase reactivators to reactivate house fly and bovine acetylcholinesterase inhibited by paraoxon and DFP. Bull Korean Chem Soc 2006;27:1401–9.
- Jun D, Stodulka P, Kuca K, Koleckar V, Dolezal B, Simon P, et al. TLC analysis of intermediates arising during the preparation of oxime HI-6 dimethanesulfonate. J Chromatogr Sci 2008;46:316–19.
- Musilek K, Lipka L, Racakova V, Kuca K, Jun D, Dohnal V, et al. New methods in synthesis of acetylcholinesterase reactivators and evaluation of their potency to reactivate cyclosarin-inhibited AChE. Chem Pap 2006;60:48–51.
- Novotny L, Karasova J, Kuca K, Bajgar J, Misik J. Influence of different ways of euthanasisa on the activity of cholinesterases in the rat. J Appl Biomed 2009;7:115–18.
- Kuca K, Musilek K, Jun D, Pohanka M, Zdarova Karasova J, Novotny L, Musilova L. Could oxime HI-6 really be considered as “broad-spectrum” antidote?. J Appl Biomed 2009;7,143–149.
- Bajgar J, Fusek J, Kuca K, Bartosova L, Jun D. Treatment of organophosphate intoxication using cholinesterase reactivators: facts and fiction. Mini Rev Med Chem 2007;7:461–6.
- Musilek K, Holas O, Kuca K, Jun D, Dohnal V, Opletalova V, et al. Synthesis of monooxime-monocarbamoyl bispyridinium compounds bearing (E)-but-2-ene linker and evaluation of their reactivation activity against tabun- and paraoxon-inhibited acetylcholinesterase. J Enzyme Inhib Med Chem 2007;23:70–6.
- Pohanka M, Jun D, Kuca K. Photometric microplate assay for estimation of the efficacy of paraoxon-inhibited acetylcholinesterase reactivation. J Enzyme Inhib Med Chem 2008;23:781–4.
- Pohanka M, Jun D, Kuca K. In vitro reactivation of trichlorfon-inhibited butyrylcholinesterase using HI-6, obidoxime, pralidoxime and K048. J Enzyme Inhib Med Chem 2009;24:680–3.
- Kassa K, Karasova J, Bajgar J, Kuca K, Musilek K. A comparison of the therapeutic and reactivating efficacy of newly developed bispyridinium compounds (K206, K269) with currently available oximes against tabun in rats and mice. J Enzyme Inhib Med Chem 2008;23:776–80.
- Kassa J, Karasova J, Bajgar J, Kuca K, Musilek K, Kopelikova I. A comparison of the reactivating and therapeutic efficacy of newly developed bispyridinium oximes (K250, K251) with commonly used oximes against tabun in rats and mice. J Enzyme Inhib Med Chem 2009;24:1040–4.