Abstract
A series of mononuclear Ru(II) complexes of the type [Ru(S)2(K)]2+, where S = 1,10-phenanthroline/2,2′-bipyridine and K = 4-OH-btsz, 4-CH3-btsz, 3,4-di-OCH3-btsz, 4-OH-binh, 4-CH3-binh, 3,4-di-OCH3-binh, were prepared and characterized by elemental analysis, FTIR, 1H-NMR, and mass spectroscopy. The complexes displayed metal–ligand charge transfer (MLCT) transitions in the visible region. These ligands formed bidentate octahedral ruthenium complexes. The title complexes were evaluated for their in vivo anticancer activity against a transplantable murine tumor cell line, Ehrlisch’s ascites carcinoma (EAC), and in vitro cytotoxic activity against human cancer cell lines Molt 4/C8 and CEM and murine tumor cell line L1210. The ruthenium complexes showed promising biological activity especially in decreasing tumor volume and viable ascites cell counts. Treatment with these complexes prolonged the life span of mice bearing EAC tumors by 10–52%. In vitro evaluation of these ruthenium complexes revealed cytotoxic activity from 0.21 to 24 μM against Molt 4/C8, 0.16 to 19 μM aginst CEM, and 0.75 to 32 μM against L1210.
Introduction
The success of cisplatin and related platinum complexes as anticancer agents has stimulated a search for other active transition metal complexes, and ruthenium in particular has attracted researchCitation1. Metal complexes of ruthenium containing nitrogen and oxygen donor ligands are found to be effective catalysts for oxidation, reduction, hydrolysis, and other organic transformationsCitation2. The coordination environment around ruthenium plays a key role in stabilizing its different oxidation states and hence dictates the redox properties of the control atomsCitation3,Citation4.
Ruthenium compounds are regarded as promising alternatives to platinum compounds, and offer many approaches to innovative metallopharmaceuticals. The compounds are known to be stable and have predictable structures both in the solid state and in solution. The tuning of ligand affinities is accompanied by a steadily increasing knowledge of the biological effects of ruthenium compoundsCitation1,Citation5. The first systematic investigation of ruthenium compounds and their antitumor property was done at the beginning of the 1980s with the compounds fac-[RuCl3(NH3)3] and cis-[RuCl2(NH3)4]ClCitation6, preceded by the discovery in the 1970s that ruthenium red possesses antitumor propertiesCitation7,Citation8. Since then, compounds such as trans-(IndH)[Ru(ind)2Cl4] (Ind = indazole), mer-[Ru(terpy)Cl3] (terpy = 2,2′-terpyridine)Citation9–11, [Ru(dmso)4Cl2] (dmso = dimethyl sulfoxide)Citation12, ImH[Ru(im)Cl5]Citation13, ImH[Ru(im)2-Cl4]Citation14, and ImH[Ru(im)(dmso)Cl4]Citation15 (NAMI-A) (im = imidazole) have also become well-known antitumor agents.
Although the mechanism of action of ruthenium compounds is not fully understood, it is thought that, for certain species, it is similar to that of platinum drugsCitation16,Citation17. NAMI-A has high selectivity for solid tumor metastasis and low host toxicity at pharmacologically active dosesCitation18, and it was the first ruthenium compound to enter clinical trialsCitation19. It has a remarkably low general toxicityCitation20,Citation21 and shows marked efficacy against metastasesCitation22,Citation23. It does not affect primary tumor growthCitation24,Citation25 and does not exhibit cytotoxicity against tumor cells in vitro. A related ruthenium(III) compound, indazolium trans[tetrachlorobis (1H-indazole) ruthenate(III)], KP1019Citation26, has also entered clinical trials, since it was found to exhibit antiproliferative activity in vitro in human colon carcinoma cell linesCitation27.
In comparing the general toxicity of ruthenium compounds with platinum drugs, ruthenium has lower toxicity, which has been attributed to the ability of ruthenium compounds to specifically accumulate in cancer tissues. The higher specificity of these compounds for their targets may be linked to selective uptake by the tumor compared with healthy tissueCitation28,Citation29 and selective activation by reduction to cytotoxic species within the tumorCitation30.
Ruthenium compounds with bidentate ligands show intercalation properties with DNACitation31. The Ru(II) compounds are kinetically more reactive than Ru(III)Citation32. We have reported that Ru(II) compounds bearing thiosemicarbazides, 8-hydroxyquinolines, and 4-substituted thiopicolinanalides have in vivo anticancer and in vitro antibacterial activityCitation33–35. Recently, we have reported that Ru(II) compounds bearing isatin thiosemicarbazones and chloro-fluoro-phenyl imino methyl phenol have in vivo anticancer and in vitro cytotoxic activityCitation36. In this work, we describe the synthesis and characterization of some ruthenium complexes, their in vitro cytotoxic activity against human cancer cell lines Molt 4/C8 and CEM and murine tumor cell line L1210, and their in vivo anticancer activity against transplantable murine tumor cell line EAC (Ehrlisch’s ascites carcinoma).
Materials and methods
Chemistry
AR grade solvents were obtained from S.D. Fine-Chem, Mumbai, and E. Merck, Mumbai. Puriss grade reagents were obtained from Fluka and E. Merck.
Hydrated ruthenium trichloride was purchased from Loba Chemie, Mumbai, and used as received. Ultraviolet (UV)-visible spectra were recorded on a Jasco spectrophotometer. Fourier transform infrared (FTIR) spectra were recorded in KBr powder on a Jasco V410 FTIR spectrometer by the diffuse reflectance technique. Citation1H/13C-nuclear magnetic resonance (NMR) spectra were measured in CDCl3 and dimethyl sulfoxide (DMSO)-d6 on Bruker Ultraspec 500 MHz/AMX 400 MHz/300 MHz spectrometers. The reported chemical shifts were against that of tetramethylsilane (TMS). Fast atom bombardment (FAB) mass spectra were recorded on a Jeol JMS600 spectrometer with meta-nitrobenzylalcohol (mNBA) matrix. Substituted thiosemicarbazones were prepared according to the literature method.
General procedure for preparing substituted benzyl thiosemicarbazones (r-btsz)
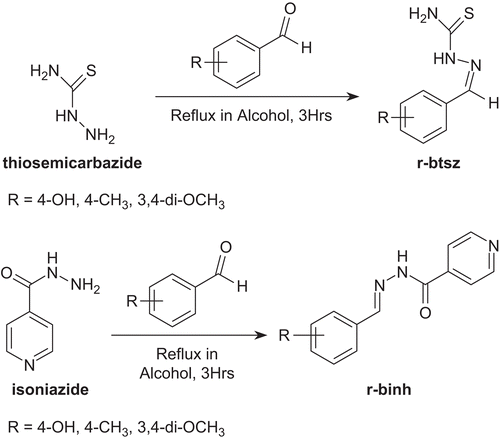
A mixture of substituted benzaldehyde (1 mmol) and thiosemicarbazide (1 mmol) in 100 mL of ethanol was refluxed for 3 h and left overnight. The solid that separated was filtered and dried. The crude solid was purified by recrystallization from alcohol to give crystals.
4-OH-btsz Yield 56%, m.p. 224–225°C (lit., 226°C). IR (KBr) cm−1: 3469–3320 (NH2 and NH), 3200–2700 (O-H), 3133 (C-H), 1610 (N-H), 1328 (C=S). Calcd. for C8H9N3OS: C, 49.21; H, 4.64; N, 21.52. Found C, 49.20; H, 4.62; N, 21.28%. λmax nm (MeOH): 242, 321, 398. 1H NMR (DMSO-d6): δ = 12.6 (1H, s), 11.24 (1H, s), 8.07 (1H, s), 7.99 (1H, s), 7.89 (1H, s, -OH), 7.73 (2H, d, J = 8.6 Hz), 6.95 (2H, d, J = 8.6 Hz).
4-CH3-btsz Yield 79%, m.p. 160–162°C (lit., 160–161°C). IR (KBr) cm−1: 3416–3321 (NH2 and NH), 3151 (C-H), 1615 (N-H), 1325 (C=S). Calcd. for C9H11N3S: C, 55.93; H, 5.74; N,21.74. Found C, 55.87; H, 5.62; N, 21.53%. λmax nm (MeOH): 234, 325, 389. 1H NMR (DMSO-d6): δ = 11.41 (1H, s), 8.10 (1H, s), 7.98 (1H, s), 7.78 (1H, s), 7.71 (2H, d, J = 8.9 Hz), 6.98 (2H, d, J = 8.9 Hz), 1.64 (3H, s, CH3).
3,4-di-OCH3-btsz Yield 56%, m.p. 194–195°C (lit., 195°C). IR (KBr) cm−1: 3406–3320 (NH2 and NH), 3133 (C-H), 1610 (N-H), 1332 (C=S). Calcd. for C10H13N3O2S: C, 50.19; H, 5.47; N,17.56. Found C, 50.21; H, 5.61; N, 17.43%. λmax nm (MeOH): 239, 331, 395. 1H NMR (DMSO-d6): δ = 11.32 (1H, s), 8.16 (1H, s), 8.02 (1H, s) 7.97 (1H, s) 7.51 (1H, d), 7.13 (1H, dd, J = 8.6 Hz), 6.94 (1H, d, J = 8.3 Hz), 3.81 (3H, s, -OCH3), 3.78 (3H, s, -OCH3).
General procedure for preparing substituted benzyl isonicotinyl hydrazones (r-binh)
A mixture of substituted benzaldehyde (1 mmol) and isoniazid (1 mmol) in 100 mL of ethanol was refluxed for 3 h and left overnight. The solid that separated was filtered and dried. The crude solid was purified by recrystallization from alcohol to give crystals.
4-OH-binh Yield 65%, m.p. 287–288°C (lit., 287°C). IR (KBr) cm−1: 3328(NH), 3180–2750 (O-H) 3148 (C-H),1683 (C=O), 1615 (N-H). Calcd. for C13H11N3O2: C, 60.22; H, 5.05; N,16.21. Found C, 60.17; H, 5.03; N, 16.07%. λmax nm (MeOH): 233, 315, 391. 1H NMR (DMSO-d6): δ = 11.52 (1H, s), 11.27 (1H, s), 8.03 (1H, s, O-H), 7.78 (2H, d, J = 8.7 Hz), 6.95 (2H, d, J = 8.7 Hz), 7.76 (2H, d, J = 8.4 Hz), 6.87 (2H, d, J = 8.4 Hz).
Preparation of cis-[bis(S)dichlororuthenium(II)] cis-[Ru(S)2Cl2]Citation37 (where S = 2,2′-bipyridine/1,10-phenanthroline)
RuCl3.H2O, 1g (2.5 mmol) and ligand S (5 mmol) were refluxed in 50 mL dimethylformamide (DMF) for 3 h under a nitrogen atmosphere. The reddish brown solution slowly turned purple and the product precipitated in the reaction mixture. The solution was cooled overnight at 0°C. A fine microcrystalline mass was filtered off. The residue was repeatedly washed with 30% LiCl solution and finally recrystallized from the same. The product was dried and stored in a vacuum desiccator over P2O5 for further use (yield 75%).
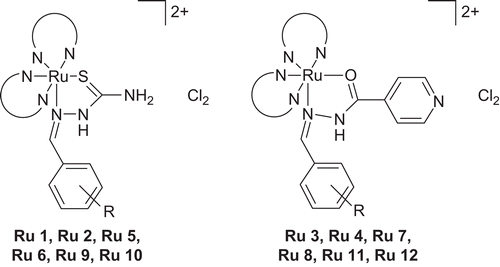
General procedure for preparing -[Ru(S)2(K)Cl2] (where S = 1,10-phenanthroline (Ru 1)/2,2′-bipyridne (Ru 2); where K = 4-OH-btsz, 4-CH3-btsz, 3,4-di-OCH3-btsz, 4-OH-binh, 4-CH3-binh, 3,4-di-OCH3-binh)
To the black microcrystalline cis-bis(S)dichlororuthenium(II) {cis-Ru(S)2Cl2} (2 mmol), excess of ligand (r-btsz and r-binh) (2.5 mmol) was added and refluxed in ethanol under a nitrogen atmosphere. The initial colored solution slowly changed to brownish orange at the end of the reaction, which was verified by TLC on silica plates. Then the excess of ethanol was distilled off and to the remaining solution was added silica gel (60–120 mesh). The product was purified by column chromatography using silica gel as the stationary phase and chloroform–methanol as the mobile phase.
Ru 1 46%, black crystals, IR (KBr) cm−1: 3402–3329 (NH2 & N-H), 3210–2700 (O-H) 3036 (C-H), 1611 (N-H), 1328 (C=S). Calcd. for C32H25Cl2N7ORuS: C, 52.81; H, 3.43; N, 13.48. Found C, 52.26; H, 3.39; N, 13.32%. 1H NMR (DMSO-d6): δ ppm: 10.02 (d, J = 5.1 Hz, 1H), 9.03 (s, 1H), 8.91 (d, J = 4.9 Hz, 1H), 8.84 (t, J = 8.6 Hz, 2H), 8.63 (d, J = 8.4 Hz, 1H), 8.49 (d, J = 8.4 Hz, 1H), 8.34–8.20 (m, 6H), 8.15–8.08 (m, 2H), 7.91 (d, J = 5.0 Hz, 1H), 7.81–7.75 (m, 2H), 7.68–7.64 (s, 1H, O-H), 7.49–7.45 (m, 1H), 6.91 (s, 2H, br, NH2), 6.73 (d, J = 14.6 Hz, 2H), 6.13 (s, 1H). FAB-MS (mNBA): 727 [Ru(phen)2 (4-OH-btsz)]2+(Cl2)−; 656 [Ru(phen)2 (4-OH-btsz)]2+; 475 [Ru(phen) (4-OH-btsz)]2+; 462 [Ru(phen)2].
Ru 2 42%, black crystals, IR (KBr) cm−1: 3401–3238 (NH2 & N-H), 3200–2700 (O-H) 3041 (C-H), 1621 (N-H), 1344 (C=S). Calcd. for C28H25Cl2N7ORuS: C, 49.48; H, 3.68; N, 14.43. Found C, 49.24; H, 3.59; N, 14.32%. 1H NMR (DMSO-d6): δ ppm: 10. (d, J = 4.9 Hz, 1H), 9.15 (s, 1H), 8.90 (d, J = 5.0 Hz, 1H), 8.72–8.42 (m, 5H), 8.12–7.98 (m, 2H), 7.82–7.53 (m, 3H), 7.45–7.32 (m, 2H), 7.22–7.16 (m, 1H), 7.09–6.99 (m, 2H), 6.92–6.72 (m, 3H), 6.61 (s, 2H, br, NH2), 6.34–6.13 (m, 2H). FAB-MS (mNBA): 679 [Ru(bpy)2 (4-OH-btsz)]2+(Cl2)−; 608 [Ru(bpy)2 (4-OH-btsz)]2+; 452 [Ru(bpy) (4-OH-btsz)]2+; 413 [Ru(bpy)2].
Ru 3 44%, black crystals, IR (KBr) cm−1: 3318 (N-H), 3200–2700 (O-H), 3041 (C-H), 1601 (N-H), 1681 (C=O). Calcd. for C37H27Cl2N7O2Ru: C, 57.43; H, 3.49; N, 12.67. Found C, 57.26; H, 3.34; N, 12.32%. 1H NMR (DMSO-d6): δ ppm: 10.01 (d, J = 5.1 Hz, 1H), 9.02 (s, 1H), 8.87 (d, J = 5.6 Hz, 1H),8.64 (d, J = 8.3 Hz, 1H), 8.46 (d, J = 8.6 Hz, 1H), 8.37–8.19 (m, 6H), 8.13–8.07 (m, 2H), 7.93 (d, J = 5.1 Hz, 2H), 7.84–7.78 (m, 2H), 7.64–7.60 (s, 1H, O-H), 7.46–7.43 (m, 2H), 7.38–7.32 (m, 2H), 6.93 (s, 2H, br, NH2), 6.77 (d, J = 15.2 Hz, 2H), 6.11 (s, 1H). FAB-MS (mNBA): 773 [Ru(phen)2 (4-OH-binh)]2+(Cl2)−; 702 [Ru(phen)2 (4-OH-binh)]2+; 521 [Ru(phen) (4-OH-binh)]2+; 462 [Ru(phen)2].
Ru 4 44%, black crystals, IR (KBr) cm−1: 3312 (N-H), 3200–2700 (O-H), 3041 (C-H), 1615 (N-H), 1675 (C=O). Calcd. for C33H27Cl2N7O2Ru: C, 54.62; H, 3.72; N, 13.52. Found C, 53.89; H, 3.55; N, 13.28%. 1H NMR (DMSO-d6): δ ppm: 9.98. (d, J = 4.9 Hz, 1H), 9.18 (s, 1H), 8.91 (d, J = 5.3 Hz, 1H), 8.74–8.44 (m, 5H), 8.11–7.97 (m, 2H), 7.93–7.89 (m, 2H), 7.80–7.51 (m, 3H), 7.46–7.22 (m, 2H), 7.21–7.15 (s,1H, O-H), 7.10–7.01 (m, 2H), 6.94–6.72 (m, 3H), 6.63 (s, 2H, br, NH2), 6.36–6.15 (m, 2H). FAB-MS (mNBA): 725 [Ru(bpy)2 (4-OH-binh)]2+(Cl2)−; 654 [Ru(bpy)2 (4-OH-binh)]2+; 498 [Ru(bpy) (4-OH-binh)]2+; 413 [Ru(bpy)2].
Ru 5 44%, black crystals, IR (KBr) cm−1: 3414–3224 (NH2 & N-H), 3032 (C-H), 1632 (N-H), 1331 (C=S). Calcd. for C33H27Cl2N7RuS: C, 54.62; H, 3.72; N, 13.52. Found C, 53.26; H, 3.72, N, 14.47%. 1H NMR (DMSO-d6): δ ppm: 10.15–10.04 (m, 2H), 9.41 (s, 1H), 8.87–8.83 (m, 2H), 8.71 (s, 1H, br), 8.53–8.51 (m, 1H), 8.46–8.34 (d, J = 5.7 Hz, 3H), 8.31–8.24 (m, 4H), 8.01 (s, 2H, br, NH2), 7.91–7.85 (m, 4H), 7.59–7.45 (dd, 1H, J = 8.2, 8.1 Hz), 7.51–7.42 (m, 2H), 7.23 (d, J = 8.3 Hz, 2H, br), 6.95 (d, 1H, J = 8.5 Hz), 6.13 (s, 1H). FAB-MS (mNBA): 725 [Ru(phen)2 (4-CH3-btsz)]2+(Cl2)−; 654 [Ru(phen)2 (4-CH3-btsz)]2+; 474 [Ru(phen) (4-CH3-btsz)]2+; 462 [Ru(phen)2].
Ru 6 44%, black crystals, IR (KBr) cm−1: 3409–3219 (NH2 & N-H), 3035 (C-H), 1615 (N-H), 1327 (C=S). Calcd. for C29H27Cl2N7RuS: C, 51.41; H, 3.98; N, 14.47. Found C, 50.98; H, 3.79; N, 14.35%. 1H NMR (DMSO-d6): δ ppm: 10.01 (m, 1H), 8.82–8.76 (m, 2H), 8.70 (d, 1H, J = 5.6 Hz), 8.61 (d, 1H, J = 8.0 Hz), 8.43 (d, 1H, J = 8.0 Hz), 8.06–8.00 (m, 3H, 7.79–7.73 (m, 2H), 7.65–7.59 (m, 2H), 7.46 (d, 1H, J = 5.6 Hz), 7.31–7.22 (m, 3H), 7.19–7.16 (mt, 3H, J = 12.0 Hz ), 6.97 (d, 2H, J = 12.0 Hz ), 6.22 (s, 2H, br, NH2), 1.61(s, 3H, -CH3) FAB-MS (mNBA): 677 [Ru(bpy)2 (4-CH3-btsz)]2+(Cl2)−; 606 [Ru(bpy)2 (4-CH3-btsz)]2+; 452 [Ru(bpy) (4-CH3-btsz)]2+; 413 [Ru(bpy)2].
Ru 9 46%, black crystals, IR (KBr) cm−1: 3418–3226 (NH2 & N-H), 3042 (C-H), 1608 (N-H), 1339 (C=S). Calcd. for C34H29Cl2N7O2RuS: C, 52.91; H, 3.76; N, 12.71. Found C, 52.87; H, 3.68; N, 12.42%. 1H NMR (DMSO-d6): δ ppm: 10.09 (d, J = 5.2 Hz, 1H), 8.98 (d, J = 5.6 Hz, 1H), 8.80 (t, J = 8.8 Hz, 2H), 8.68 (d, J = 8.6 Hz, 1H), 8.51 (d, J = 8.6 Hz, 1H), 8.40–8.20 (m, 6H), 8.11–8.03 (m, 2H), 7.88 (d, J = 5.0 Hz, 1H), 7.83–7.77 (m, 2H), 7.67–7.63 (m, 1H), 7.46–7.42 (m, 1H), 6.98 (s, 2H, br, NH2), 6.75 (d, J = 14.9 Hz, 2H), 3.68 (s, 3H, -OCH3), 3.62 (s, 3H, -OCH3), FAB-MS (mNBA): 771 [Ru(phen)2 (3,4-di-OCH3-btsz)]2+(Cl2)−; 700 [Ru(phen)2 (3,4-di-OCH3-btsz)]2+; 521 [Ru(phen) (3,4-di-OCH3-btsz)]2+; 461 [Ru(phen)2].
Ru 10 43%, black crystals, IR (KBr) cm−1: 3406–3217 (NH2 & N-H), 3025 (C-H), 1612 (N-H), 1322 (C=S). Calcd. for C30H29Cl2N7O2RuS: C, 49.79; H, 4.01; N, 13.55. Found C, 49.56; H, 3.95; N, 13.42%. 1H NMR (DMSO-d6): δ ppm: 10.02 (d, J = 5.0 Hz, 1H), 8.73–8.72 (d, J = 5.4 Hz, 1H,), 8.63–8.41 (m, 5H), 8.10–8.03 (m, 3H), 7.88–7.70 (m, 6H), 7.46 (d, J = 4.9 Hz, 2H), 7.39–7.12 (m, 3H), 6.94 (s, 2H, br, NH2), 3.76 (s, 3H, -OCH3), 3.69 (s, 3H, -OCH3), FAB-MS (mNBA): 723 [Ru(bpy)2 (3,4-di-OCH3-btsz)]2+(Cl2)−; 652 [Ru(bpy)2 (3,4-di-OCH3-btsz)]2+; 496 [Ru(bpy) (3,4-di-OCH3-btsz)]2+; 413[Ru(bpy)2].
Antineoplastic activity
Albino swiss mice (18–20 g body weight) were maintained in identical laboratory conditions and given standard food pellets (Hindustan Lever Ltd, Bombay, India) and water ad libitum. LD50 values of the synthesized compounds were determined according to the literatureCitation38. All compounds were dissolved in 10% DMSO solution. The animals were divided into 15 groups each containing 12 mice. Group I was the vehicle control group (5mL/kg body weight, i.p.) and group II was the EAC control group (2 × 106 EAC cells/mouse, i.p.). Group III were treated with the standard drug cisplatin (2 mg/kg body weight). All the compounds were administered (i.p.) at a dose of 2 mg/kg body weight in groups IV–XV, respectively. Mice were treated with the compounds and cisplatin daily for 9 days starting 24 h after tumor transplantation. Six animals from each group were sacrificed 18 h after the last dose. Ascitic fluid volume and Ascitic cell count parameters were noted. Mean survival time (MST) for the remaining six mice of each group was noted.
Tumor volume and viable cell count
Ascites volume was noted by taking it in a graduated centrifuge tube, and packed cell volume determined by centrifuging at 1000g for 5 min. The viability of ascitic cells was checked by Trypan blue (0.4% in normal saline) dye exclusion test and the count was taken in a Neubauer counting chamber. The effect of the ruthenium complexes on tumor growth was monitored by recording the mortality daily, and percentage increase in life span (ILS%) was calculated by the following formula:
ILS (%) = [(mean survival of treated group)/(mean survival of control group) − 1] × 100
Cytotoxic evaluation
The compounds prepared in the laboratory were evaluated against Molt 4/C8, CEM, and L1210 cells by a literature procedureCitation39.
Results and discussion
Chemistry
Ligands type r-binh (r-binh = substituted benzyl isonicotinyl hydrazones) were prepared by reacting substituted benzaldehydes with isoniazid in alcohol at 1:1 molar ratio (), and r-btsz (r-btsz = substituted benzyl thiosemicarbazones) were prepared by reacting substituted benzaldehydes with thiosemicarbazide in alcohol at 1:1 molar ratio (). All ligands were confirmed for their purity by their melting point, elemental analysis, and other spectral studies. Details of the strategy adopted for the synthesis of these ruthenium homoleptic compounds are as follows. The starting material for synthesis of the compounds was cis-bis(1,10-phenanthroline) dichlororuthenium(II)/cis-bis(2,2′-bipyridine) dichlororuthenium(II). Ruthenium trichloride was refluxed in DMF in the presence of 1,10-phenanthroline/2,2′-bipyridine and in excess of the stoichiometric amount, which afforded the final product cis-bis(1,10-phenanthroline) dichlororuthenium(II)/cis-bis(2,2′-bipyridine)dichlororuthenium (II)Citation37 (). The third ligand was introduced in alcohol in the presence of a nitrogen atmosphere ().
The structures of the ligands, especially r-inh and r-btsz, were capable of exhibiting bidentate behavior. There are very few cases in which the thiosemicarbazide acts as a monodentate ligand, binding to the metal center through the sulfur atomCitation40,Citation41. In the case of r-btsz ligands the chelating mode was via the sulfur atom and imine nitrogen by a coordination covalent bond. In the case of r-binh ligands a covalent bond was formed between the metal ion and oxygen atom and a coordinate covalent bond with the imine nitrogen.
The infrared spectra of all ligands and their ruthenium(II) compounds were recorded in KBr powder by the diffuse reflectance technique, and are reported in their respective titles by tentative assignments. The r-btsz ligands showed vibrational frequency from 3400 to 3200 cm−1 for NH2 and N-H stretching, and from 1335 to 1325 cm−1for C=S stretching. The r-binh ligands showed vibrational frequency from 3320 to 3200 cm−1 for N-H stretching and from 1690 to 1670 cm−1 for C=O stretching.
A comparison of IR spectra of r-btsz ligands and ruthenium complexes confirmed coordination to the metal center by the sulfur atom and imine nitrogen. Comparing the IR spectra of r-binh ligands and ruthenium compounds confirmed coordination to the metal center by an oxygen atom and imine nitrogen. In complexes such as Ru 1–Ru 2, Ru 5–Ru 6, Ru 9–Ru 10, coordination occurred via the sulfur and imine nitrogen but not with the terminal amine group; this was confirmed by the spectra, which indicated no change in vibrational frequency of the NH2 group between 3400 and 3300 cm−1.
Coordination of ligands (K = r-binh, r-btsz) to ruthenium resulted in compounds such as [Ru(S)2(K)]2+Cl2(Ru 1–Ru 12), respectively. These compounds did not possess any C2 axes of symmetry. Such a loss of C2 axis of symmetry was seen for [Ru(L)2(R)]Citation33–35 (where L = 2,2′-bipyridine/1,10-phenanthroline and R = acetazolamide, 7-iodo-8-hydroxy-quinoline, 4-substituted thiopicolinanalide, etc.). All compounds had well-resolved resonance peaks, which corresponded to four different aromatic ring protons of the two 2,2′-bipyridine/1,10-phenanthroline ligands and the third ligand.
These compounds showed broad and intense visible bands between 340 and 510 nm due to a metal–ligand charge transfer transition (MLCT). In the UV region the bands at 280 and 310 nm were assigned to 2,2′-bipyridine/1,10-phenanthroline ligand p–p* charge transfer transitions. The same transition was found in free 2,2′-bipyridine/1,10-phenanthroline at 270 nm, so that coordination of the ligand resulted in a red shift in the transition energy. There were also two shoulders at 380 and 500 nm, which were, tentatively, attributed to metal–ligand charge transfer transitions involving 2,2′-bipyridine, 1,10-phenanthroline, and the third ligand.
In the Citation1H-NMR spectra of the complexes, there were resolved resonance peaks at low field at δ 10.02 (s, br, NH), 7.68 (s, 1H, O-H). Thus, in the case of Ru 1, there were 25 resonance peaks (δ 10.03–6.13), and 25 well-resolved peaks (δ 10.00–6.34) for Ru 2.
The mass spectra of the complexes confirmed the formulae suggested by their molecular ion peaks. The spectrum showed numerous peaks representing successive degradation of the molecule. FAB mass spectroscopic data clearly suggested that mononuclear complexes had been formed in each case, the first fragment being due to the [Ru(S)2(K)]2+–Cl2− ion pair. The complex also showed a peak due to the complex cation [Ru(S)2(K)]2+ and others due to [Ru(S)(K)]2+ and [Ru(S)2]2+ respectively (where S = 1,10-phenanthroline/2,2′-bipyridine and K = r-binh, r-btsz). This type of fragmentation has been reported for [Ru(phen)2(nmit)]Cl2 and [Ru(bpy)2(ihqs)]Cl2 (where phen = 1,10-phenanthroline, bpy = 2,2′-bipyridine, nmit = N-methyl isatin thiosemicarbazone, ihqs = 7-iodo-8-hydroxyquinoline-5-sulfonicacid)Citation33. In all cases, the loss of chlorine ions was detected where S = 2,2′-bipyridine/1,10-phenanthroline and K = r-binh, r-btsz. Thus, based on the above observations, it is tentatively suggested that Ru(II) complexes show an octahedral geometry ().
Biological activity and discussion
Results are summarized in and and the pharmacological data were analyzed statistically by ANOVA (analysis of variance). Statistical significance was considered only when p < 0.05 and F > Fcritical. All the complexes were tested for their anticancer activity in mice bearing EAC tumors. Ru 6 was found to increase the life span of the tumor hosts by 52%, while the remaining ruthenium complexes were able to increase the life span in the tumor hosts by 10–38% only. The results of the present study clearly demonstrated the tumor inhibitory activity of the ruthenium complexes against the transplantable murine tumor cell line ().
Table 1. Antineoplastic activity of ruthenium complexes against EAC bearing mice.
The in vitro cytotoxic activity was evaluated for all the synthesized ligands and the ruthenium complexes against human Molt 4/C8 and CEM T-lymphocytes as well as murine L1210 cells, and the results are summarized in . The relative potencies between ligands and their ruthenium complexes revealed the importance of ruthenium metal using the 4/C8 and CEM assays and murine L1210 assay. These determinations showed that in comparison to the ligands, the ruthenium complexes were more potent.
Table 2. Cytotoxic studies of ligands and ruthenium compounds.
The cytotoxicity data in revealed that most ruthenium complexes had significant cytotoxic potencies (IC50 values in the range 0.21–3.1 for Molt 4/C8, and 0.75–5.9 μM for L1210). On the other hand, for the ligands, the IC50 values were in excess (84–223 μM against CEM, 96–328 μM for Molt 4/C8, and 64–244 μM for L1210). Of the tested ligands and ruthenium complexes, Ru 3 showed cytotoxicity against all three cell lines tested in the region of 0.21, 0.24, and 0.78 μM for Molt 4/C8, CEM, and L1210, respectively. Another complex, Ru 5, showed cytotoxicity against the cell lines tested at 0.39 μM for Molt 4/C8, 0.48 for CEM, and 0.82 for L1210. Yet another complex, Ru 7, showed cytotoxicity against the cell lines tested at 0.29 μM for Molt 4/C8, 0.16 for CEM, and 0.75 for L1210. The remaining ruthenium complexes showed low-μM values for Molt 4/C8 and CEM and higher-μM values for L1210. In comparison with the ruthenium complexes, the ligands displayed cytotoxicty at higher-μM concentration.
From the results presented in , it is clear that several ruthenium complexes exhibited a marked inhibitory effect on the proliferation of tumor cells, with IC50 values from as low as 0.21 μM for Molt 4/C8, 0.16 μM for CEM, and 0.75 μM for L1210. Thus, the ruthenium complexes proved inhibitory to tumor growth at submicromolar concentration. Their ligands, however, were not antitumorally active.
Declaration of interest
The authors declare no conflict of interest. The authors alone are responsible for the writing and content of this paper.
Acknowledgement
The authors are thankful to the Principal, S.R. College of Pharmacy, Hanamkonda, for providing the chemicals for carrying out this research.
References
- Clarke MJ, Zhu F, Frasca DR. Non-platinum chemotherapeutic metallo pharmaceuticals. Chem Rev 1999;99:2511–34.
- Kureshy RI, Khan NH. Mononuclear chiral ruthenium(II) Schiff base complexes; synthesis, physicochemical studies and reactivity with π-acceptor ligands. Polyhedron 1999;12:195–201.
- Chakravarty J, Bhattacharya S. Ruthenium phenolates, synthesis, characterization and electron-transfer properties of some salylaldiminato and 2-(arylazo) phenolates complexes of ruthenium. Polyhedron 1996;15:1047–55.
- Baitalik S, Adhikary B. Heterochelates of ruthenium(II): electrochemistry, absorption spectra, and luminescence properties. Polyhedron 1997;16:4073–80.
- Clarke MJ. Ruthenium metallo pharmaceuticals. Coord Chem Rev 2003;236:209–33.
- Clarke MJ. Oncological implications of the chemistry of ruthenium. Met Ions Biol Syst 1980;11:231–83.
- Rudolph R. Electron microscopy demonstration of sulphurated acid monopolysaccarides in canine mast cell tumours, using barium chloride and ruthenium red, together with comments on the classification and differentiation of tumor cells. Arch Exp Veterinarmed 1971;25:925–35.
- Anghileri LJ, Krebsforsch Z. The in vivo inhibition of tumor growth by ruthenium red: its relationship with the metabolism of calcium in the tumor. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol 1975;83:213–17.
- Keppler BK, Henn M, Juhl UM, Berger MR, Niebi R, Wagner FE. New ruthenium complexes for the treatment of cancer. Prog Clin Biochem Med 1989;10:41–69.
- Novakova O, Kasparkova J, Vrana O, Van Vilet PM, Reedijk J, Brabec V. Correlation between cytotoxicty and DNA binding polypyridyl ruthenium complexes. Biochemistry 1995;34:12369–78.
- Vilaplana RA, Gonazalez-Vichez F, Gutierrez-Puebla E, Ruiz-Valero C. The first isolated antineoplatic Ru(IV) complex; synthesis and structure of [Cl2 [1,2-cyclohexane diaminotetra acetate) Ru] 2H2O. Inorg Chim Acta 1994;224:15–18.
- Sava G, Bergamo A, Zorzet S, Gava B, Casarsa C. Influence of chemical stability on the activity of the antimetastasis ruthenium compound NAMI-A. Eur J Cancer 2002;38:427–35.
- Keppler BK, Wehe D, Enders H, Rupp W. Syntheisis, antitumor activity, and X-ray structure of bis (imidazolium) imidazole-pentachloro ruthenate (III), (ImH)2 (RuImCl5). Inorg Chem 1987;26:844–6.
- Keppler BK, Rupp W, Juhl UM, Endres H, Nieu R, Blazer WS. Synthesis, molecular structure and tumor-inhibiting property of imidazolium-trans-bis(imidazole) tetra chlororuthenate (III) and its methyl substituted derivatives. Inorg Chem 1987;26:4366–70.
- Sava G, Gangliyardi R, Bergamo A, Alessio E, Mestroni G. Treatment of metastases of solid mouse tumors by NAMI-A; comparison with cisplatin, cyclophosphamide and decarbazine. Anticancer Res 1999;19:969–72.
- Velders AH, Pazderski L, Ugozzoli F, Biagini-Cingi M, Reedijk J. Synthesis, characterization and crystal structure of trans-aquatrichlorobis (5,7-dimethyl 1,2,4 triazolo 1,5-a pyrimidine-N3) ruthenium (III) monohydrate. Inorg Chim Acta 1998;273:259–65.
- Velders AH, Ugozzoli F, Biagini-Cingi M, Reedijk JJ. A unique fourfold intramolecular hydrogen bonding stabilizes the structure of trans-bis (2-amino-5,7-dimethyl [1,2,4] triazolo [1,5-a] pyrimidine-N3) aquatrichloro ruthenium (III) monohydrate. Eur J Inorg Chem 1999;273:213–15.
- Sava G, Bergamo A. Ruthenium-based compounds and tumor growth control. Int J Oncol 2000;17:353–65.
- Rademaker-Lakhai JM, Bongard DV, Pluim D, Beijnen JH. A phase I and pharmacological study with imidazolium-trans-DMSO-imidazole-tetrachlororuthenate, a novel ruthenium anticancer agent. Clin Cancer Res 2004;10:3717–27.
- Gagliardi R, Sava G, Pacor S, Mestroni G, Alessio E. Antimetastatic action and toxicity on healthy tissues of Na [trans-RuCl4 (DMSO) Im] in the mouse. Clin Exp Metastasis 1994;12:93–100.
- Maganarin M, Bergamo A, Carotenuto ME, Zorzet S, Sava G. Increase of tumor infiltrating lymphocytes in mice treated with antimetastatic doses of NAMI-A. Anticancer Res 2000;20:2939–44.
- Cocchietto M, Sava G. Blood concentration and toxicity of the antimetastasis agent NAMI-A following repeated intravenous treatment in mice. Pharmacol Toxicol 2000;87:193–7.
- Zorzet S, Sorc A, Casarsa C, Cocchietto M, Sava G. Pharmacological effects of the ruthenium complex NAMI-A given orally to CBA mice with MCa mammary carcinoma. Met Based Drugs 2001;8:1–7.
- Sava G, Clerici K, Capozzi I, Cocchietto M, Gagliardi R, Alessio. E, et al. Reduction of lung metastasis by ImH (trans-RuCl4 (DMSO) Im: mechanism of the selective action investigated on mouse tumors. Anticancer Drugs 1999;10:129–38.
- Sava G, Gagliardi R, Cocchietto M, Clerici K, Capozzi I, Marella M, et al. Comparison of the effects of the antimetastatic compound ImH (trans-RuCl4 (DMSO) (Im)] (NAMI-A) on the arthritic rat and on MCa mammary carcinoma in mice. Pathol Oncol Res 1998;4:30–6.
- Keppler BK, Henn M, Juhl UM, Berger MR, Niebel R, Wagner FE. New ruthenium complexes for the treatment of cancer. Prog Clin Biochem Med 1989;10:41–69.
- Kreuser ED, Keppler BK, Berdel WE, Piest A, Thiel E. Synergistic antitumor interactions between newly synthesized ruthenium complexes and cytokines in human colon carcinoma cell lines. Semin Oncol 1992;19:73–81.
- Sava G, Pacor S, Zorzet S, Alessio E, Mestroni G. Antitumor properties of dimethyl sulphoxide ruthenium (II) complexes in the Lewis lung carcinoma system. Pharmacol Res 1989;21:617–28.
- Zorzet S, Bergamo A, Cocchietto M, Sorc A, Gava B, Alessio E, et al. Lack of in vitro cytotoxicity, associated to increased G2-M cell fraction and inhibition of matrigel invasion, may predict in vivo selective antimetastasis activity of ruthenium complexes. Pharmacol Exp Ther 2000;295:927–33.
- Clarke MJ, Galang RD, Roudriguez VM, Kumar R, Pell S, Bryan DM. Chemical considerations in the design of ruthenium anticancer agents. In: Nicolini M, ed. Platinum and Other Metal Coordination Compounds in Cancer Chemotherapy. Boston, MA: Martinus Nijhoff, 1988:582–601.
- Tysoe SA, Morgan RJ, Baker D. Spectroscopic investigation of differential binding modes of δ- and λ- Ru (bpy)2 (ppz)2+ with calf thymus DNA. J Phys Chem 1993;97:1707–11.
- Clairs SA, Paul JD. Ruthenium in medicine: current clinical uses and future prospects. Platinum Met Rev 2001;45:62–9.
- Mazumder UK, Gupta M., Bera A, Bhattacharya S, Karki S, Manikadan L, et al. Synthesis, antitumor and antibacterial activity of some Ru(bpy)22+/4-substituted thiosemicarbazide complexes. Indian J Chem 2003;42A:313–17.
- Mazumder UK, Gupta M, Karki S, Bhattacharya S, Suresh R, Sivakumar T. Synthesis, and pharmacological activities of some mononuclear Ru(II) complexes. Bioorg Med Chem 2005;13:5766–73.
- Suresh R, Karki SS, Bhattacharya S, Manikadan L, Prabhakaran SG, Mazumder UK, et al. Synthesis, and anticancer activity of certain mononuclear Ru(II) complexes. J Enzyme Inhib Med Chem 2006;21:501–7.
- Karki SS, Sreekanth T, Balzarini J, Clercq DE. Synthesis, anticancer and cytotoxic activities of some mononuclear Ru(II) compounds. Bioorg Med Chem 2007;15:6632–41.
- Giordano PJ, Bock CR, Wrighton MS. Excited state proton transfer of ruthenium(II) complexes of 4, 7-dihydroxy-1,10-phenanthroline. Increased acidity in the excited state. J Am Chem Soc 1978;100:6960–6.
- Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949;96:99–113.
- Balzarini J, De Clercq E, Mertes MP, Shugar D, Torrence PF. 5-Substituted 2′-deoxy uridines: correlation between inhibition of tumor cell growth and inhibition of thymidine kinase and thymidylate synthetase. Biochem Pharmacol 1982;31:3673–82.
- Nardelli M, Gasparri GF, Battislini GG, Musatti A. Configuration of thiosemicarbazide molecules in monochloro monothiosemicarbazide silver. Chem Commun 1965:187–188.
- Gasparri GF, Mangia A, Musatti A, Nardelli M. The crystal and molecular structure of monothiosemicarbazide silver(I) chloride. Acta Crystallogr 1968;24:367–74.

![Scheme 2. Preparation of cis-[Ru(S)2Cl2].](/cms/asset/24822bb4-8352-4743-b7e3-7f0704412f87/ienz_a_435935_f0002_b.gif)
![Scheme 3. Preparation of tris chelates from cis-[Ru(S)2Cl2].](/cms/asset/2bb78ed3-4ada-49cc-96f1-15fc2cb13107/ienz_a_435935_f0003_b.gif)