Abstract
Organophosphorus compounds pose a potential threat to both military and civilian populations. Since post-exposure therapy has its limitations, our research was focused on the possibility of improving pretreatment in order to limit the toxic effects of tabun. We determined the protective index of various combinations of atropine, oximes (K074, K048, and TMB-4), and pyridostigmine given to mice before tabun intoxication. Although the tested oximes showed very good therapeutic efficacy in tabun-poisoned mice, the given pretreatments improved therapy against tabun poisoning. These regimens ensured survival of all animals up to 25.2 LD50 of tabun. Our results indicate that even pretreatment with atropine alone is sufficiently effective in enhancing the survival of mice poisoned by multiple doses of tabun, if oxime therapy follows. K048 is our oxime of choice for future research, as it shows better protective and reactivating potency.
Introduction
The continued threat of militaryCitation1 or terroristCitation2 use of nerve agents worldwide calls for effective therapeutic preparedness. Exposure to nerve agents, the most lethal organophosphorus compounds (OPs), leads to a progression of toxic signs (hypersecretions, muscle fasciculations, tremors, convulsions, and respiratory distress) due to the inhibition of acetylcholinesterase (AChE; EC 3.1.1.7) and subsequent increase in concentration of the neurotransmitter acetylcholine (ACh), which in turn hyperstimulates the cholinergic system at central and peripheral sitesCitation3. Current standard treatment against OPs combines anticholinergics (atropine) and AChE reactivators (oximes). Atropine blocks muscarinic acetylcholine-receptors and prevents them from further stimulation by ACh, while oximes reactivate inhibited AChE by displacing the phosphoryl residue from the active site and restoring enzyme activity. Atropine has been universally adopted, but in various countries the oximes of choice differ. For example, P2S (pralidoxime mesylate) is used in the UK, obidoxime is preferred in Germany, and TMB-4 in IsraelCitation4.
One of the most hazardous OPs is the nerve agent tabun (ethyl-N,N-dimethyl phosphoramidocyanidate) because tabun-phosphorylated AChE is resistant to reactivation due to a low electrophilicity of the phosphoramidate conjugated to the AChE active siteCitation5. Although the oxime TMB-4 reactivates tabun-inhibited AChE showing beneficial effects in tabun-poisoned animalsCitation6–8, it is the most toxic of the four most investigated oximes: 2-PAM (pralidoxime chloride), HI-6, obidoxime, and TMB-4Citation9. Therefore, there is still a need to develop not only the most effective, but also the least toxic, organophosphate antidote. As post-exposure therapy has its limitationsCitation10, our research was focused on improving pretreatment in order to limit the toxic effects of tabun. Pretreatment with oximes is possible, because oximes in addition to their reactivation potency also act as protectors of AChE against phosphorylation, by reversible inhibition of the enzymeCitation11,Citation12. Based on our promising results from previous studies with K-oximesCitation13–17, we decided to test the two most potent AChE reactivators, oximes K074 and K048, in combination with atropine in the pretreatment and therapy of tabun-poisoned mice. This study was conducted to determine whether oxime and atropine in pretreatment would improve the efficacy of a standard therapy regimen. Oxime TMB-4 was included for comparison.
Current protection against nerve agent poisoning consists of pretreatment with carbamate pyridostigmineCitation18. Pyridostigmine carbamylates the active site serine of AChE and prevents phosphorylation by organophosphate. In contrast to very slow dephosphorylation, decarbamylation is faster. On the other hand, the pyridostigmine-induced increase in ACh levels can itself cause symptoms of poisoning, so it would be useful to counteract the effects of accumulated ACh with anticholinergic drugsCitation19. A potent prophylaxis, which combines pyridostigmine with benactyzine and trihexyphenidyl (PANPAL), was developed for the Czech ArmyCitation20,Citation21. With this in mind, we examined the influence of pyridostigmine alone or in combination with atropine on the resistance to tabun exposure in mice and on the therapeutic efficacy of coadministered oximes and atropine.
Materials and methods
Chemicals
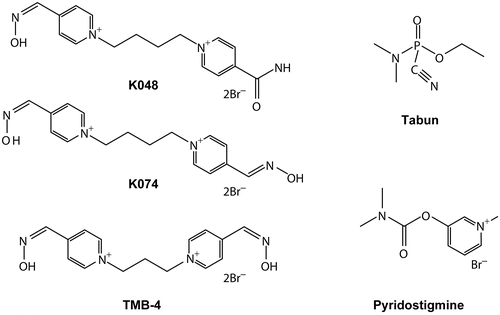
shows the structures of the studied oximes, pyridostigmine, and tabun.
Pyridostigmine bromide was purchased from Sigma-Aldrich (Steinheim, Germany). Oximes K074 (1,3-bis(4-hydroxyiminomethylpyridinium) butane dibromide) and K048 (1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium) butane dibromide) were prepared as described earlierCitation22,Citation23, while TMB-4 (1,3-bis(4-hydroxyiminomethylpyridinium) propane dibromide) was obtained from Bosnalijek, Sarajevo, Bosnia and Herzegovina. Tabun (ethyl-N,N-dimethyl phosphoramidocyanidate) was purchased from NC Laboratory, Spiez, Switzerland. Atropine sulfate was purchased from Kemika, Zagreb, Croatia.
Animals
Male BALB-C mice (purchased from the Institute of Immunology, Zagreb, Croatia) were selected by body weight (18–25 g), fed on a standard diet (PLIVA, Zagreb, Croatia), and had free access to water. Mice were kept in Macrolone cages at 21°C maintained by a thermostat, with exchanging light and dark cycles every 12 h, and were randomly distributed into groups of four animals.
Experimental design
The acute toxicity (LD50) of pyridostigmine was based on 24-h mortality rates, determined according to ThompsonCitation24 and WeilCitation25. LD50 value and the 95% confidence limits were evaluated from results obtained with 4–6 doses of pyridostigmine (dissolved in water); four animals were injected per dose. LD50 values for K074, K048, and TMB-4 were 21.4, 224.9, and 73.5 mg/kg body weight, respectively, and were determined in our earlier studiesCitation13,Citation16.
Tabun was diluted in isopropyl alcohol; further dilutions were made in water immediately before use. Mice received a subcutaneous (s.c.) dose of 1.0–50.4 multiples of the tabun LD50 (317.5 µg/kg body weight). Oximes and pyridostigmine were dissolved in water or atropine just before use and given intraperitoneally (i.p.) at doses of 5% or 25% of their LD50. Atropine was dissolved in water (5.0 mg/mL) and also administered i.p. (10.0 mg/kg body weight).
Pretreatment was given 15 min before tabun poisoning, while therapy was given 1 min after tabun poisoning. The efficacy of the antidotal regimens is expressed as a protective index (PI) with 95% confidence limits and maximal dose of poison (MDP). The PI is the ratio of the LD50 of tabun with and without treatment. The MDP is the highest multiple of the LD50 of tabun that was fully counteracted (survival of all animals) by the administered treatment.
This study was performed with the approval of the Ethics Committee of the Institute for Medical Research and Occupational Health, Zagreb, Croatia.
Results
We first administered an oxime and atropine as pretreatment or as therapy in order to test the efficiency of either combination (). With the oxime dose of 5% of its LD50, a higher therapeutic effect was obtained if the oxime was administered as therapy rather than as pretreatment. Between the three oximes at this dose, therapy with K048 ensured survival of all mice at as high a tabun dose as 10 LD50. With a higher dose of oxime (25% of the respective LD50), the highest therapeutic efficiency was achieved by pretreatment with TMB-4 and atropine. This antidotal regimen showed the lowest acute toxicity (5042.7 µg/kg) while the PI was 14.1 and the MDP was 7.9 LD50 of tabun.
Table 1. Therapeutic effect of oximes and atropine administered as pretreatment or therapy in tabun-poisoned mice.
presents the therapeutic effects of atropine and atropine plus oximes administered as pretreatment and as therapy upon tabun poisoning in mice. Mice were pretreated with either atropine alone or a combination of atropine and one of the oximes, each at two different doses. The therapy, in turn, always combined an oxime and atropine. All these antidotal regimens were potent against tabun poisoning. Particularly effective were atropine pretreatment with therapy combining K048 (25% of its LD50) and atropine, and the combination of TMB-4 (25% of its LD50) plus atropine as both pretreatment and therapy. These regimens ensured survival of all animals at 25.2 LD50 of tabun. Furthermore, at the dose of 25% of LD50, all oximes led to lower acute tabun toxicities than at the dose of 5% LD50.
Table 2. Therapeutic effect of atropine and atropine plus oximes administered as pretreatment and therapy in tabun-poisoned mice.
shows the protective efficacy of pretreatment with pyridostigmine alone or in combination with atropine. Pyridostigmine alone, regardless of the dose (5% or 25% of its LD50), did not decrease the tabun-induced acute toxicity in mice. However, the protective efficacy was improved when pyridostigmine was applied as pretreatment in combination with atropine.
Table 3. Therapeutic effect of pyridostigmine or pyridostigmine plus atropine administered as pretreatment in tabun-poisoned mice.
shows the therapeutic effect of pyridostigmine plus atropine pretreatment followed by therapy with oximes and atropine. Lower acute toxicities were obtained with higher antidote doses. K048 distinguished itself in therapeutic potency at both applied doses.
Table 4. Therapeutic effect of pretreatment with pyridostigmine and atropine followed by therapy with oximes and atropine in tabun-poisoned mice.
Discussion
Organophosphorus compounds pose a threat to both military and civilian populations, as evidenced by recent terrorist attacksCitation26. As insecticides, they are an occupational hazard as well. As the first reaction of the OPs is interaction with cholinesterases, pretreatment is focused on protecting AChE against inhibition or on decreasing the concentrations of OPs. Pyridostigmine, an AChE carbamylating compound that has been used as prophylaxis, calls for a replacement that will provide better protectionCitation21,Citation27. In addition to carbamylation, the AChE catalytic site can also be protected by ligands that reversibly bind to AChE, such as huperzine A, SAD, and decamethonium, due to direct competition between the ligand and the phosphorylating agentCitation28–31. A new approach to reduce the in vivo toxicity of chemical warfare nerve agents is the use of bioscavengers—enzymes that react with a nerve agent before it inhibits AChE at physiologically important target sites (i.e. brain)Citation32,Citation33. Among the enzymes examined as potential stoichiometric or catalytic scavengers, significant progress has been made with human butyrylcholinesterase (BChE; EC 3.1.1.8) administered as prophylaxisCitation34,Citation35. Another approach to prevent the effects of OPs is based on the use of current antidotes (anticholinergics, reactivators, and others)Citation21. Pyridinium compounds reversibly bind to the AChE active site and interfere with the binding of phosphorylating agentsCitation11. In the case of imminent threat of tabun exposure, pretreament should have an important role, because tabun-induced deleterious effects are extraordinarily difficult to counteract with currently used oximesCitation8,Citation9,Citation20,Citation36.
In order to improve the efficacy of standard therapy, we determined the protective index of various combinations of atropine, oximes, and pyridostigmine given to mice before tabun poisoning. Despite pretreatment, the animals showed severe signs of toxicity. Muscle fasciculations and tremor generally occurred within 1–2 min after poisoning. Convulsions appeared with 3–4 min of latency. During the acute phase all animals exhibited cyanosis, dyspnea, and respiratory disorders. Within 24 h, animals that survived remained active, but tremor could be provoked. Although the intensity and duration of symptoms differed between pretreated and non-pretreated animals, the pattern described above was the same.
The first step was to evaluate the efficiency of the adopted regimen of therapy (oxime plus atropine) using two newer reactivators (K074 and K048) and conventional TMB-4 (). In previous studies, K048 and K074 showed very good therapeutic efficacy in tabun-poisoned miceCitation13,Citation15. Applied at the dose of 25% of their LD50 immediately after tabun poisoning, both K048 and K074 ensured survival of all animals at 5.0 LD50 of tabunCitation13,Citation16. Results in show that both of these oximes are equally or even more effective at the lower dose applied in therapy. Combined atropine plus K048 therapy at 5% of K048 LD50 provided survival of all experimental animals after the administration of 10.0 LD50 of tabun. Thereby, oxime K048 appears to be superior to the currently used TMB-4, which applied in therapy regardless of the dose, did not show such a high antidotal potential. However, given as pretreatment at the dose of 25% its LD50, TMB-4 plus atropine showed a high protective index. Although in our previous in vitro experiments TMB-4 showed a relatively weak protective potentialCitation13,Citation15, it is known that this oxime possesses curare- and atropine-like effects essential for the survival of tabun-poisoned miceCitation37. This raises the question of how much atropine contributes to protection against tabun poisoning in mice. Therefore, for pretreatment, we used atropine alone or in combination with the tested oximes. Therapy that followed the poisoning combined one of the oximes and atropine (). In animal studies, the anticonvulsant effect of atropine has been documented to help against the side effects of nerve agentsCitation38,Citation39, and even though alternative agents have been investigatedCitation40, atropine remains the most important drug against organophosphate poisoningCitation41. In our experiments, pretreatment with atropine alone, followed by combined therapy with oximes (25% of their LD50) and atropine (), ensured survival at 3–5 times higher doses of tabun than therapy alone (). Pretreatment combining atropine and the oximes did not achieve any higher survival. Our results show that the oximes exerted dose-dependent effects against tabun toxicity. This is in accordance with our previous resultsCitation14 and also with suggestions by other authors that the culprit for the ineffectiveness of oximes could be inadequate dosageCitation42. When atropine alone was used in pretreatment and was followed by combined therapy with the lower dose of the oximes, the protective index (PI) ranged from 6.0 to 16.8, while with the higher dose it ranged from 15.9 to 35.6. Although 25% LD50 is a relatively high dose of antidote, it is applicable for counteracting the lethal effects of organophosphorus compounds.
Atropine was also effective in protecting the mice when it was applied with pyridostigmine 15 min before tabun poisoning (). Pyridostigmine is a “pretreatment adjunct”—a drug that must be taken before exposure to be effective, but only confers benefit if post-exposure therapy is given as wellCitation43. Therefore, we investigated the influence of pretreatment consisting of pyridostigmine and atropine on the potency of administered therapy in tabun-poisoned mice (). With that kind of treatment regimen, a good therapeutic efficacy of oxime K048 was confirmed once again. In combined therapy with atropine, K048 applied at either dose was highly effective against tabun poisoning thanks to combined pretreatment with pyridostigmine and atropine. On the other hand, pyridostigmine plus atropine as pretreatment only in the case of TMB-4 did not cause a striking improvement of therapy. Actually, better results were achieved by applying oxime TMB-4 plus atropine as pretreatment (). A reasonable explanation for that could lie in the high acute toxicities of pyridostigmine and TMB-4. Also, according to some authors, pyridostigmine may reduce the efficacy of oxime–atropine therapy and therefore should be used with cautionCitation7,Citation44.
Conclusion
Herein we showed that the combined pretreatment of atropine, oximes, and pyridostigmine in general improves the overall therapy. The main principle—protection of AChE against phosphorylation by tabun was reached using AChE inhibitors: oximes or pyridostigmine. In this way the enzyme is resistant to inhibition by tabun. Also, as atropine is required to compete with the excess of ACh at synapses, ample protection against intoxication by organophosphates and also elimination of side effects can be ensured by adding atropine to pretreatment regimens. Moreover, pretreatment with atropine alone is sufficient to enhance the survival of mice poisoned by multiple lethal doses of tabun if followed by oxime therapy. Between K074 and K048, K048 is our oxime of choice for future research, as it shows better protective and reactivating potency.
Acknowledgements
We wish to thank Jasna Mileković and Marija Kramarić for technical assistance, and Dado Čakalo for helpful language advice.
Declaration of interest
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (Grant No. 022-0222148-2139 and No. 022-0222148-2889) and by NATO (CBP.EAP.CLG 983024).
References
- Macilwain C. Study proves Iraq used nerve gas. Nature 1993;363:3.
- Nagao M, Takatori T, Matsuda Y, Nakajima M, Iwase H, Iwadate K. Definitive evidence for the acute sarin poisoning diagnosis in the Tokyo subway. Toxicol Appl Pharmacol 1997;144:198–203.
- Taylor P. Anticholinesterase agents. In: Hardman LELJG, Gilman AG, eds. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 2001:175–97.
- Moore DH, Saunders-Price BB. Pharmaceutical countermeasures to chemical warfare agents. ASA Newslett 2, 2005;1:27–9; http://www.asanltr.com/newsletter/05-2/articles/052c.htm.
- Eto M. Organophosphorus Pesticides: Organic and Biological Chemistry. Cleveland, OH: CRC Press, 1976:142.
- Maksimović M, Bošković B, Radović L, Tadić V, Deljac V, Binenfeld Z. Antidotal effects of bis-pyridinium-2-monooxime carbonyl derivatives in intoxications with highly toxic organophosphorus compounds. Acta Pharm Jugoslav 1980;30:151–60.
- Dawson RM. Review of oximes available for the treatment of nerve agent poisoning. J Appl Toxicol 1994;14:317–31.
- Jokanović M, Maksimović M, Kilibarda V, Jovanović D, Savić D. Oxime-induced reactivation of acetylcholinesterase inhibited by phosphoramidates. Toxicol Lett 1996;85:35–9.
- Clement JG, Shiloff JD, Gennings C. Efficacy of a combination of acetylcholinesterase reactivators, HI-6 and obidoxime against tabun and soman poisoning of mice. Arch Toxicol 1987;61:70–5.
- Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology 2007;233:145–54.
- Reiner E. Inhibition of acetylcholinesterase by 4,4′-bipyridine and its effect upon phosphylation of the enzyme. Croat Chem Acta 1986;59:925–31.
- Simeon-Rudolf V, Reiner E, Škrinjarić-Špoljar M, Radić B, Lucić A, Primožič I, et al. Quinuclidinium-imidazolium compounds: synthesis, mode of interaction with acetylcholinesterase and effect upon soman intoxicated mice. Arch Toxicol 1998;72:289–95.
- Čalić M, Lucić Vrdoljak A, Radić B, Jelić D, Jun D, Kuča K, et al. In vitro and in vivo evaluation of pyridinium oximes: mode of interaction with acetylcholinesterase, effect on tabun- and soman-poisoned mice and their cytotoxicity. Toxicology 2006;219:85–96.
- Lucić Vrdoljak A, Čalić M, Radić B, Berend S, Jun D, Kuča K, et al. Pretreatment with pyridinium oximes improves antidotal therapy against tabun poisoning. Toxicology 2006;228:41–50.
- Berend S, Lucić Vrdoljak A, Radić B, Kuča K. New bispyridinium oximes: in vitro and in vivo evaluation of their biological efficency in soman and tabun poisoning. Chem Biol Interact 2008;175:413–16.
- Kovarik Z, Čalić M, Šinko G, Bosak A, Berend S, Lucić Vrdoljak A, et al. Oximes: reactivators of phosphorylated acetylcholinesterase and antidotes in therapy against tabun poisoning. Chem Biol Interact 2008;175:173–9.
- Kovarik Z, Lucić Vrdoljak A, Berend S, Čalić M, Kuča K, Musilek K, et al. Evaluation of oxime K203 as antidote in tabun poisoning. Arh Hig Rada Toksikol 2009; 60:19–25.
- Anderson DR, Harris LW, Woodard CL, Lennox WI. The effect of pyridostigmine pretreatment on oxime efficacy against intoxication by soman or VX in rats. Drug Chem Toxicol 1992;15:285–92.
- Kassa J, Vachek J. A comparison of the efficacy of pyridostigmine alone and the combination of pyridostigmine with anticholinergic drugs as pharmacological pretreatment of tabun-poisoned rats and mice. Toxicology 2002;177:179–85.
- Vachek J, Kassa J, Fusek J, Bajgar J. The present possibilities of the treatment of poisonings with organophosphates. Sbor věd Prací VLA JEP Hradec Králové 1993;116:67–95.
- Bajgar J. Prophylaxis against organophosphorus poisoning. J Med Chem Def 2003;1:1–19.
- Kuča K, Bielavsky J, Cabal J, Kassa J. Synthesis of a new reactivator of tabun-inhibited acetylcholinesterase. Bioorg Med Chem Lett 2003;13:3545–7.
- Kuča K, Cabal J, Musilek K, Jun D, Bajgar J. Effective bisquaternary reactivators of tabun-inhibited AChE. J Appl Toxicol 2005;25;491–5.
- Thompson WR. Use of moving averages and interpolation to estimate median-effective dose. Bacteriol Rev 1947;11:115–45.
- Weil CS. Tables for convenient calculation of median-effective dose (LD50 or ED50) and instruction in their use. Biometrics 1952;8:249–63.
- Ohtomi S, Takase S, Kumagai F. Sarin poisoning in Japan. A clinical experience in Japan Self Defense Force (JSDF) Central Hospital. Int Rev Armed Serv 1996;69:97–102.
- Harris LW, McDonough JH Jr, Stitcher DL, Lennox WJ. Protection against both lethal and behavioural effects of soman. Drug Chem Toxicol 1984;7:605–24.
- Harris LW, Heyl WC, Stitcher DL, Broomfield CA. Effects of 1,1′-oxydimethylene bis-(4-tert-butylpyridinium chloride) (SAD-128) and decamethonium on reactivation of soman- and sarin-inhibited cholinesterase by oximes. Biochem Pharmacol 1978;27:757–61.
- Škrinjarić-Špoljar M, Burger N, Lovrić J. Inhibition of acetylcholinesterase by three new pyridinium compounds and their effect on phosphonylation of the enzyme. J Enzym Inhib 1999;14:331–41.
- Lallement G, Baille V, Baubichon D, Carpentier P, Collombet JM, Filliat P, et al. Review of the value of huperzine as pretreatment of organophosphate poisoning. Neurotoxicology 2002;23:1–5.
- Eckert S, Eyer P, Muckter H, Worek F. Kinetic analysis of the protection afforded by reversible inhibitors against irreversible inhibition of acetylcholinesterase by highly toxic organophosphorus compounds. Biochem Pharmacol 2006;72:344–57.
- Dunn MA, Hackley BE, Sidell FR. Pretreatment for nerve agent exposure. In: Sidell FR, Takafuji ET, Franz DR, eds. Medical Aspects of Chemical Biological Warfare. Washington, DC: Walter Reed Army Medical Center, 1997:181–96.
- Lenz DE, Broomfield CA, Maxwell DM, Cerasoli DM. Nerve agent bioscavengers: protection against high- and low-dose organophosphorus exposure. In: Somani SM, Romano JA Jr, eds. Chemical Warfare Agents: Toxicity at Low Levels. Boca Raton, FL: CRC Press, 2001:215–43.
- Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem Biol Interact 2005;157–8:167–71.
- Saxena A, Sun W, Luo C, Doctor BP. Human serum butyrylcholinesterase: in vitro and in vivo stability, pharmacokinetics, and safety in mice. Chem Biol Interact 2005;157–8:199–203.
- Puu G, Artursson E, Bucht G. Reactivation of nerve agent inhibited acetylcholinesterase by HI-6 and obidoxime. Biochem Pharmacol 1986;35:1505–10.
- Antonijević B, Bokonjić D, Stojiljković MP, Kilibarda V, Milovanović ZA, Nedeljković M, et al. Efficacy of trimedoxime in mice poisoned with dichlorvos, heptenophos or monocrotophos. Basic Clin Pharmacol Toxicol 2005;96:111–17.
- Shih TM, Koviak TA, Capacio BR. Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev 1991;15:349–62.
- Shih TM, McDonough JH, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci 1999;6:86–96.
- Petroianu GA, Hasan MY, Nurulain SM, Shafiullah M, Sheen R, Nagelkerke N. Ranitidine in acute high-dose organophosphate exposure in rats: effect of the time-point of administration and comparison with pyridostigmine. Basic Clin Pharmacol Toxicol 2006;99:312–16.
- Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology 2007;233:145–54.
- Kuča K, Bartošová L, Jun D, Patočka J, Cabal J, Kassa J, et al. New quaternary pyridine aldoximes as causal antidotes against nerve agents intoxications. Biomed Papers 2005;149:75–82.
- Golomb BA, Anthony CR. A review of the scientific literature as it pertains to Gulf War ilness: pyridostigmine bromide. RAND Health Testimony. Santa Monica, CA: RAND Health, 1999.
- Koplovitz I, Harris LW, Anderson DR, Lennox WJ, Stewart JR. Reduction by pyridostigmine pretreatment of the efficacy of atropine and 2-PAM treatment of sarin and VX poisoning in rodents. Fundam Appl Toxicol 1992;18:102–6.