Abstract
A series of novel substituted 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine benzamides 9(a–h) were synthesized to determine their antibacterial and antifungal activities as well as possible structure–activity relationships (SARs) to improve therapeutic efficacy. The pyrazol-5-amine benzamides were screened for their antibacterial activity against standard strains of Gram-positive (Streptococcus pyogenes NCIM 2608, Staphylococcus aureus ATCC 29737, Bacillus subtilis NCIM 2010) and Gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 20852, Klebsiella pneumoniae MTCC 618) bacteria by using streptomycin as positive control. They were also tested for their antifungal activities against mycotoxic strains of Fusarium verticillioides, Aspergillus ochraceous, Aspergillus flavus, Alternaria alternata, and Penicillium chrysogenum using nystatin as positive control. Among the synthesized compounds, 9d, 9g, and 9h showed potent antimicrobial activities.
Introduction
Despite the progress made in medical sciences and health care, morbidity and mortality following nosocomial infections are still commonplace. It is important to underline the role of opportunistic pathogens, which may be part of the normal human bacterial flora and cause diseases especially when the host immunity becomes impaired. There are serious concerns that untreatable pathogens may develop at an alarming rate in the near future. Further, in recent years, increasing rates of antimicrobial resistance among community and nosocomial pathogens has severely limited the therapeutic options for treating infections caused by such organisms. In view of these considerations, further efforts are needed to develop a new group of antimicrobial compounds. In recent years, there has been an alarming increase in life-threatening microbial infections especially in immunocompromised individuals suffering from acquired immune deficiency syndrome (AIDS), cancer, etc.Citation1–4. Despite the development of several new antimicrobial agents, their clinical value is limited to treating an increasing array of life-threatening systemic infections because of their relatively high risk of toxicity, emergence of drug resistant strains, pharmacokinetic differences, and/or insufficiencies in their antimicrobial activity.
Pyrazoles and several N-substituted pyrazoles are known to possess numerous chemical, biological, medicinal, and agricultural applications because of their versatile biological activities appearing as antimicrobial activityCitation5–8, antitumor and antileukemia activityCitation9, antidepressant and anticonvulsant activitiesCitation10,Citation11, antifungal activityCitation12 and antitubercular activity against Mycobacterium tuberculosisCitation13. β-Lactamase is generally considered to be responsible for microbial resistance against a broad spectrum of β-lactam antibiotics. In 1935, Domargk showed the therapeutic value of a group of compounds known as sulfonamides. These are not specific for a special group of organisms, but are effective against a large variety of pathogenic organisms.
Bezenesulfonamide derivatives were also reported as elastase inhibitorsCitation14, carbonic anhydrase and cyclo-oxygenase-2 (COX-2) inhibitorsCitation15,Citation16, herbicides, and plant growth regulatorsCitation17. They show affinities for endothelin ETA and ETB receptors in the low nanomolar range and high functional antagonistic potency in vitroCitation18, and also exhibit a dual action to inhibit the thromboxane receptor and thromboxane synthase in cardiovascular and renal diseasesCitation19. Antiproliferative, antiviral, and antifungal activities have been similarly evaluatedCitation20. Compounds containing an amide group can alter the chemical properties, disposition, and biological activities of drugsCitation21. Amides are currently used as antidepressants, anti-inflammatory agents, antimalarial agents, antipsychotic agents, antiviral agents, steroids, and general anestheticsCitation22. Amide functional groups are also found in many antibacterial agents, as for example benzimidazole carboxamides, peptide, penicillin, cephalosporins, and thiozolidinonesCitation23.
Our earlier work on the synthesis of different heterocyclic systems containing high antimicrobial activity prompted us to synthesize a new class of heterocyclic carboxamides and sulfonamides and study their antibacterial activitiesCitation24. Recently, we reported the synthesis and antimicrobial studies of bioactive heterocyclic sulfonamides and benzamidesCitation24–26. In continuation of our research work on the synthesis of bioactive heterocycles, the activities of the synthesized compounds were tested on bacterial and fungal strains by the cup-borer method, microwell dilution assay, and turbidometric method.
Materials and methods
Synthesis
Melting points were determined using a Selco-650 hot stage melting apparatus and were uncorrected. Infrared (IR) spectra were recorded using a Jasco FTIR-4100 series spectrometer. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded on Bruker, 400 MHz spectrometer using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard (chemical shift in δ ppm). Spin multiples are given as br s (broad singlet), d (doublet), t (triplet), and m (multiplet). Mass and purity were recorded on an LC/MSD-Trap-XCT apparatus. Elemental (CHN) analyses were obtained on a Vario EL III Elementar analyzer. Silica gel column chromatography was performed using Merck 7734 silica gel (60–120 mesh) and Merck thin layer chromatography (TLC) plates. 1-(4-Methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine derivatives 9(a–h) were synthesized as per the method summarized in . Initially, mono-Boc protected 1-(4-methoxybenzylidene) hydrazine (3) was synthesized by the condensation reaction of 4-methoxybenzaldehyde (1) with mono-Boc protected hydrazine (2). The subsequent double bond reduction was done using 10% Pd/C in ethanol, which yielded mono-Boc protected 1-(4-methoxybenzyl) hydrazine (4). Deprotection of the amine group was carried out using HCl in ether, which gave the free amine compound (3). Finally, the key intermediate, 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) by cyclization of 1-(4-methoxybenzyl)-2-methylhydrazine salt (5) (1.0 g, 5.36 mol) and 3-cyclopropyl-3-oxopropanenitrile (6) (0.85 g, 5.36 mol) was taken in ethanol, and then sodium ethoxide (1.09 g, 16.0 mol) was added. The reaction mixture was refluxed for 2–3 h. The progress of the reaction was monitored by TLC. When the reaction was complete, water was added to the reaction mixture and extraction was done with ethyl acetate. The organic layer was washed with 10% ammonium chloride solution followed by a water wash, and dried with anhydrous sodium sulfate. The solvent was evaporated, and the crude product obtained was purified by column chromatography over silica gel (60–120 mesh) using hexane:ethyl acetate (8:2) as eluent. The product obtained was a white crystalline solid and the yield was found to be 85%. 1H NMR (CDCl3, 400 MHz) δ: 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.95 (br s, 2H, -NH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 157.7 (C), 148.5 (C), 130.1 (2C), 128.6 (C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 243.3. IR (KBr, cm−1): 3360, 1685, 1602, 1355, 1277, 1225, 865. Anal. calcd. for C14H17N3O (in %): C-69.11; H-7.04; N-17.27. Found C-69.10; H-7.02; N-17.23.
Scheme 1. Synthesis of compounds 9(a–h). Reactions and reagent conditions: (i) EtOH/r.t, 2–3 h; (ii) 10% Pd/C/H2EtOAc, r.t, 3 h; (iii) dichloromethane, ether in HCl, 3 h; (iv) EtOH/EtCOONa, reflux 80°C, 2–3 h; (v) triethylamine, dichloromethane, 8(a–h), r.t, 4–5 h; where R-CO-Cl are: (8a) 3,5-dinitrobenzoyl chloride; (8b) 3-methoxybenzoyl chloride; (8c) 4-tert-butylbenzoyl chloride; (8d) 2,4-dichlorobenzoyl chloride; (8e) 4-chlorobenzoyl chloride; (8f) 3-bromobenzoyl chloride; (8g) 2,4-difluorobenzoyl chloride; (8h) benzoyl chloride.
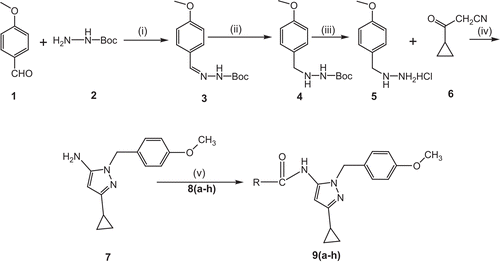
The nucleophilic substitution reactions of 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) with different substituted aromatic acid chlorides (R-CO-Cl) (8) were carried out in the presence of triethylamine and dichloromethane (DCM) as solvents. The presence of an N-H proton peak at δ = 10.51 ppm in carboxamide 9(a-h) in proton NMR confirmed our products. They were also confirmed by IR data for the carboxamide series 9(a–h), which showed an asymmetric stretching frequency of R-CO-Cl in the range 1650–1710 cm−1 and symmetric stretching frequency at 1640–1720 cm−1. All the compounds obtained were in good yield with high purity. The structures and physical data of the synthesized molecules are tabulated in .
Table 1. Chemical structures and physical data of the synthesized compounds 9(a–h).
General procedure for the synthesis of 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine derivatives 9(a–h)
A solution of 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (1.0 eq) in dry dichloromethane was taken and cooled to 0–5°C in an ice bath. Triethylamine (3.0 eq) was added to the cold reaction mixture and stirred for 10 min, and different acid chlorides (1.0 eq) were added; the mixture was stirred at room temperature for 4–5 h and progress of the reaction was monitored by TLC. When the reaction was complete, water was added to the reaction mixture and extraction was done with ethyl acetate. The organic layer was washed with 10% ammonium chloride solution followed by a water wash, and dried with anhydrous sodium sulfate. The solvent was evaporated, and the crude product obtained was purified by column chromatography over silica gel (60–120 mesh) using hexane:ethyl acetate (8:2) as eluent. The product obtained was a white solid with good yield and high purity.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-3,5-dinitrobenzamide (9a) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 3,5-dinitrobenzoyl chloride (8a) (0.28 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 11.37 (s, 1H, Ar-H), 11.27 (s, 2H, Ar-H), 10.51 (s, 1H, -NH), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.8 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 149.4 (2C), 139.2 (C), 136.0 (C), 130.1 (2C), 128.6 (C), 128.5 (2C), 122.1 (C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 437.41. IR (KBr, cm−1): 3250, 1683, 1604, 1353, 1274, 1222, 863. Anal. calcd. For C21H19N5O6 (in %): C-57.66; H-4.38; N-16.01. Found C-57.64; H-4.32; N-16.00.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-3-methoxybenzamide (9b) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 3-methoxybenzoyl chloride (8b) (0.21 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.53 (s, 2H, Ar-H), 7.46 (s, 2H, Ar-H), 7.33 (d, 1H, J = 7.4 Hz, Ar-H), 7.02 (d, 1H, J = 7.6 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 160.8 (C), 157.7 (C), 139.2 (C), 135.2 (C), 130.1 (2C), 129.9 (C), 128.6 (C), 119.8 (C), 117.7 (2C), 114.2 (2C), 89.3 (C), 55.9 (2C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 377.44. IR (KBr, cm−1): 3290, 1681, 1601, 1353, 1274, 1223, 864. Anal. calcd. for C22H23N3O3 (in %): C-70.01; H-6.14; N-11.13. Found C-69.01; H-6.04; N-11.10.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-4-tert-butylbenzamide (9c) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 4-tert-butylbenzoyl chloride (8c) (0.24 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.87 (d, 2H, J = 7.9 Hz, Ar-H), 7.47 (s, 1H, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 1.36 (s, 3H, -CH3), 1.34 (s, 3H, -CH3), 1.32 (s, 3H, -CH3), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 153.4 (C), 139.2 (C), 131.1 (C), 130.1 (2C), 128.6 (C), 127.1 (2C), 125.2 (2C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 40.7 (C), 31.4 (3C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 403.52. IR (KBr, cm−1): 3350, 1683, 1601, 1353, 1274, 1223, 863. Anal. calcd. For C25H29N3O2 (in %): C-74.41; H-7.24; N-10.41. Found C-74.35; H-7.20; N-10.35.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-2,4-dichlorobenzamide (9d) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 2,4-dichlorobenzoyl chloride (8d) (0.25 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.83 (s, 1H, Ar-H), 7.46 (s, 1H, Ar-H), 7.33 (t, 1H, J = 7.4 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 139.2 (2C), 133.7 (C), 130.5 (2C), 129.3 (3C), 128.6 (C), 127.1 (C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 416.3. IR (KBr, cm−1): 3320, 1683, 1601, 1353, 1272, 1223, 864. Anal. calcd. For C21H19Cl2N3O2 (in %): C-60.59; H-4.60; N-10.09. Found C-60.49; H-4.50; N-10.06.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-4-chlorobenzamide (9e) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 4-chlorobenzoyl chloride (8e) (0.21 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.89 (d, 2H, J = 8.2 Hz, Ar-H), 7.45 (d, 2H, J = 7.6 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 139.2 (C), 137.7 (C), 132.3 (C), 130.1 (2C), 129.4 (2C), 128.9 (2C), 127.6 (C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 381.86. IR (KBr, cm−1): 3364, 1683, 1600, 1353, 1273, 1222, 862. Anal. calcd. For C21H20ClN3O2 (in %): C-66.05; H-5.28; N-11.00. found C-66.02; H-5.26; N-11.03.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-3-bromobenzamide (9f) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 3-bromobenzoyl chloride (8f) (0.27 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.58 (s, 1H, Ar-H), 10.51 (s, 1H, -NH), 7.89 (d, 1H, J = 8.2 Hz, Ar-H), 7.68 (d, 1H, J = 7.52 Hz, Ar-H), 7.33(t, 1H, J = 7.3 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 139.2 (C), 136.4 (C), 135.1 (C), 131.1 (2C), 130.1 (2C), 128.6 (C), 126.5 (C), 123.2 (C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 426.31. IR (KBr, cm−1): 3260, 1684, 1603, 1354, 1276, 1224, 864. Anal. calcd. for C21H20BrN3O2 (in %): C-59.15; H-4.73; N-9.86. Found C-59.17; H-4.70; N-9.83.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl)-2,4-difluorobenzamide (9g) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), 2-fluorobenzoyl chloride (8g) (0.19 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.93 (t, 1H, J = 7.58 Hz, Ar-H), 7.49 (t, 1H, J = 7.42 Hz, Ar-H), 7.21 (t, 1H, J = 7.12 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 167.9 (C), 165.4 (C), 164.8 (C), 159.6 (C), 157.7 (C), 139.2 (C), 130.1 (3C), 128.6 (C), 120.7 (C), 114.2 (2C), 111.2 (C), 104.8 (C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 365.4. IR (KBr, cm−1): 3260, 1685, 1602, 1355, 1277, 1225, 865. Anal. calcd. for C21H19F2N3O2 (in %): C-69.03; H-5.52; N-11.50. Found C-69.01; H-5.50; N-11.50.
Synthesis of N-(1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-yl) benzamide (9h) The product as a yellow colored solid was obtained from 1-(4-methoxybenzyl)-3-cyclopropyl-1H-pyrazol-5-amine (7) (0.25 g, 1.13 mmol), benzoyl chloride (8h) (0.17 g, 1.13 mmol), and triethylamine (0.46 g, 3.39 mmol). 1H NMR (CDCl3, 400 MHz) δ: 10.51 (s, 1H, -NH), 7.95 (d, 2H, J = 7.62 Hz, Ar-H), 7.51 (t, 1H, J = 7.86 Hz, Ar-H), 7.44 (d, 2H, J = 7.2 Hz, Ar-H), 6.95 (d, 2H, J = 6.52 Hz, Ar-H), 6.92 (d, 2H, J = 6.2 Hz, Ar-H), 5.18 (s, 1H, Ar-H), 5.02 (s, 2H, -CH2), 3.85 (s, 3H, -OCH3), 1.85 (m, 1H, -CH), 0.92 (m, 2H, -CH2), 0.71 (m, 2H, -CH2). Citation13C NMR (CDCl3, 400 MHz) δ: 165.4 (C), 164.8 (C), 157.7 (C), 139.2 (C), 134.2 (C), 132.2 (C), 130.1 (2C), 129.4 (C), 128.6 (2C), 127.5 (2C), 114.2 (2C), 89.3 (C), 55.9 (C), 51.5 (C), 9.3 (C), 8.2 (2C). MS (ESI) m/z: 347.41. IR (KBr, cm−1): 3328, 1682, 1601, 1352, 1274, 1223, 864. Anal. calcd. for C21H21N3O2 (in %): C-72.60; H-6.09; N-12.10. Found C-72.58; H-6.05; N-12.07.
Antibacterial activity
Microorganisms used were Gram-positive (Streptococcus pyogenes NCIM 2608, Staphylococcus aureus ATCC 29737, Bacillus subtilis NCIM 2010) and Gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 20852, Klebsiella pneumoniae MTCC 618) bacteria. A single colony of each of the cultures was inoculated in 5 mL of Luria–Bertani (LB) broth and incubated under shaking conditions overnight at 37°C. Each of the overnight cultures (500 μL) was inoculated into 25 mL of LB broth and incubated at 37°C at 0.2 optical density (OD; λmax = 600 nm). Each of these cultures (500 μL) was inoculated into a conical flask containing 30 mL of nutrient broth. The contents of the flasks were poured into petri plates and allowed to solidify. The plates were appropriately labeled. On solidifying, 10 wells were made on each plate using a cork-borer first for the negative control (dimethylsulfoxide, DMSO), second for the positive control (streptomycin), and the remaining third and fourth wells for the compounds. The plates were kept at room temperature for 30 min and then at 37°C for 24 h. After 24 h, the zone of inhibition was measured and recorded. The minimum inhibitory concentration (MIC) values were also determined for the microorganisms that were determined as sensitive to the compound disk diffusion assay. The inocula of microorganisms were prepared from 12 h broth cultures and suspensions were adjusted to 0.5 McFarland standard turbidity and the compounds were dissolved in DMSO. MIC values of compounds against each bacterial strain were determined based on the microwell dilution method with some modification. The 96-well plates were prepared by dispensing 95 μL of nutrient broth and 5 μL of the inoculum into each well. Initially, prepared compound (100 μL) at the concentration of 600 μg/mL was added into the first well. Then, 100 μL from the serial dilution was transferred into six consecutive wells. The last well containing 195 μL of nutrient broth without compound and 5 μL of inoculum on each strip was used as the negative control. The final volume in each well was 200 μL. Streptomycin and DMSO at the concentration of 100 μL were prepared in nutrient broth and used as standard drugs for the positive and negative controls, respectively. The plates were covered with a sterile plate sealer. The contents of each well were mixed on a plate shaker at 300 rpm for 30 min and then incubated at appropriate temperatures for 24 h. Microbial growth was determined by the absorbance at 610 nm using an ELx800 universal microplate reader (Bio-Tek Instruments Inc., Highland Park, Vermont, USA) and confirmed by plating 5 μL samples from clear wells on nutrient agar medium. The compounds used in this study were screened twice against each organism. The MIC was defined as the lowest concentration of each compound required to inhibit the growth of a particular organism.
Antifungal activity
Synthesized compounds were screened in vitro for their antifungal activities against mycotoxigenic strains of Fusarium verticillioides, Aspergillus ochraceous, Aspergillus flavus, Alternaria alternata, and Penicillium chrysogenum, which are capable of producing toxins that have proved to be toxic to both animals and plants. Potato dextrose agar (PDA) medium was used as the growth medium for F. verticillioides and A. alternata, and Czapek–Dox agar (CDA) medium for Aspergillus and Penicillium spp. A spore suspension of pathogen was prepared in 50 mL sterile distilled water. The spore concentration was adjusted to 1 × 10Citation6 spores/mL using a hemocytometer. A suspension of 2 mL was spread on PDA and CDA plates uniformly. After solidifying, four wells were bored in each petri plate using a cork borer (0.5 cm diameter). DMSO (negative control) in the first well, nystatin (positive control) in the second well, and synthesized compounds in the remaining two wells were taken in each plate. The plates were incubated at 23 ± 2°C under alternate cycles of 12/12 h NUV (near-ultraviolet light) and darkness. After 4 days of incubation, plates were evaluated based on the zone of inhibition caused by the compounds. Compounds were screened for their antifungal activity at different concentrations, using nystatin as positive control and DMSO as negative control. To the culture tubes containing 1.9 mL of sterile medium, 0.1 mL of test compound was added under sterile conditions. Fresh inoculum was added to all the tubes including standard and controls, with a spore concentration adjusted to 1 × 10Citation6 spores/mL. After incubating all tubes at 37°C for 48 h, the absorbance was recorded at 610 nm. Percentage inhibition was calculated according to the formula:
% Inhibition = 100(P − Q)/P
where P is absorbance without test sample and Q is absorbance with test sample. The MIC was recorded in μg/mL. All determinant tests were performed in duplicate and the results are reported as the mean of these values.
Results and discussion
The synthesized compounds showed significant and moderate inhibition against the bacterial strains. Among the compounds 9(a–h), 9d, 9g, and 9h showed significant inhibitory activity (zones of inhibition in the order 9d > 9g > 9h, 4.9–8.0 mm, 5.1–7.3 mm, and 5.8–7.2 mm, respectively) against bacterial strains. The remaining compounds showed moderate inhibitory activity compared to the standard, streptomycin. Minimum inhibitory concentration was 06 μg/mL against K. pneumoniae MTCC 618, 07 μg/mL against B. subtilis NCIM 2010, and 09 μg/mL against P. aeruginosa ATCC 20852. The antibacterial activities of the tested compounds are shown in and . As for the antifungal activity of compounds against the fungal pathogens, among the series 9(a–h), compounds 9d, 9g, and 9h showed significant inhibitory activity in the order of 9d > 9g > 9h (zones of inhibition 2.3–4.4 mm, 2.3–3.5 mm, and 2.1–3.2 mm, respectively). The other compounds showed moderate inhibitory activity compared to the standard drug, nystatin. Minimun inhibitory concentration for antifungal activity was 09 μg/mL against Aspergillus ochraceous, 11 μg/mL against A. flavus, and 12 μg/mL against A. alternata, respectively. The antifungal activities of the tested compounds are shown in and . From the results obtained, it is evident that the presence of two chloro groups at the 2nd and 4th positions in 9d, the presence of two fluorine atoms at the 2nd and 4th positions in 9g, and the presence of a benzoyl group in 9h might be the reason for the significant inhibitory activity. These results confirm our previous reportsCitation27,Citation28 which indicated that chlorine and fluorine atoms possess potent inhibitory activity. In this connection, different electron withdrawing groups attached to the phenyl ring as substituents linked to the benzoyl group were studied for antimicrobial efficacy. On the other hand, compound 9g showed higher activity against bacterial strains, with an electron withdrawing fluorine atom at the 2nd and 4th positions. A further structure-activity relationship (SAR) study revealed that compound 9h, without a substituent on the phenyl ring, also showed relatively significant inhibitory activity. We have briefly investigated the different SARs of the carboxamide 9(a-h) functionalized derivatives with different groups added on the phenyl ring. These modifications change the potency of the antibacterial and antifungal activity profile of the synthesized compounds.
Table 2. Inhibition zone (diameter) in mm of synthesized compounds tested against bacterial strains.
Table 3. Minimum inhibitory concentration (MIC) in μg/mL of synthesized compounds tested against bacterial strains.
Table 4. Inhibition zone (diameter) in mm of synthesized compounds tested against fungal pathogens.
Table 5. Minimum inhibitory concentration (MIC) in µg/mL of synthesized compounds tested against fungal pathogens.
Declaration of interest
One of the authors H. Raju is grateful to the UGC Research Fellowships in Sciences for Meritorious Students Scheme (RFSMS), New Delhi, for financial support under RFSMS-JRF order No. DV5/373[13]/RFSMS/2008-09. The CHN, IR and other data obtained from instruments purchased under DST-FIST and UGC-SAP (phase I) Programs are gratefully acknowledged.
References
- Vanden Bossche H, Dromer F, Isham N. Onychomycosis: strategies to improve efficacy and reduce recurrence. Med Mycol 1998;36(Suppl. 1):119–28.
- Andriole VT. Current and future therapy of invasive fungal infections. Curr Clin Top Infect Dis 1998;18:19–36.
- Bouruah CR, Skibo EB. A comparison of the cytotoxic and physical properties of aziridinyl quinone derivatives based on the pyrrolo[1,2-a]benzimidazole and pyrrolo[1,2-a]indole ring systems. J Med Chem 1994;37:1625–8.
- Ghannooun MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999;12:501–17.
- Akbas E, Berber I. Antibacterial and antifungal activities of new pyrazolo [3, 4-d] pyridazine derivatives. Eur J Med Chem 2005;40:401–8.
- Abunada NM, Hassaneen HM, Kandile NG, Miqdad OA. Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo[3,4-d]-pyrimidine and pyrazolo[4,3-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives. Molecules 2008;13:1501–17.
- Sivaprasad G, Paramasivan TP, Vaiyapuri RP, Narayanasamy M. Synthesis and anti-microbial activity of pyrazolylbisindoles—promising anti-fungal compounds. Bioorg Med Chem Let 2006;16:6302–12.
- Sridhar R, Paramasivam TP, Sundaresan E, Guruswamy S, Mondikalipudur NP, Vaiyapuri RP, et al. Design, synthesis and anti-microbial activity of 1H-pyrazole carboxylates. Bioorg Med Chem Lett 2004;14:6035–48.
- Shealy YF, O’Dell CA. Synthesis, antileukemic activity, and stability of 3-(substituted-triazeno)pyrazole-4-carboxylic acid esters and 3-(substituted-triazeno)pyrazole-4-carboxamides. J Pharm Sci 1970;60:554–60.
- Ozdemir Z, Kandilici HB, Gumusel B, Calis U, Bilgin A. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 2007;42:373–9.
- Rajendra PY, Lakshmana RA, Prasoona L, Murali K, Ravi KP. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(200-hydroxy naphthalen-100-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett 2005;15:5030–4.
- Xinhua L, Jing Z, Chunxiu P, Bao’an S, Bo L. Synthesis and fungicidal activity of novel 3,5-diarylpyrazole derivatives. Front Chem China 2008;3:418–21.
- Castagnolo D, De Logu A, Radi M, Bechi B, Manetti F, Magnani M, et al. Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis. Bioorg Med Chem 2008;16:8587–91.
- Finney N, Ghosh S, Hirst J, Norman P, Rotella DP. Paper alert. Curr Opin Drug Discov Dev 1999;2:175–82.
- Padmakar VK, Sharma V, Karmarkar S, Claudiu TS. QSAR studies on benzene sulfonamide carbonic anhydrase inhibitors: need of hydrophobic parameter for topological modeling of binding constants of sulfonamides to human CA-II. Bioorg Med Chem Lett 2005;15:923–7.
- Singh SK, Reddy PG, Rao KS, Lohray BB, Misra P, Rajjak SA, et al. Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors. Bioorg Med Chem Lett 2004;14:499–506.
- Akira K, Masao Y, Masuo M. Effect of chlorsulfuron on shoot sprouting from the stem segment of Torenia fournieri and Nicotiana tabacum. Weed Biol Manag 2002;2:213–18.
- Andrea S, Fabrizio B, Marc AI, Calaudin TS. Protease inhibitors – Part 5. Alkyl/arylsulfonyl- and arylsulfonylureido-/arylureido-glycine hydroxamate inhibitors of Clostridium histolyticum collagenase. J Med Chem 2000;43:292–6.
- Medina JC, Shan B, Beckmann H, Farrell RP, Clark DL, Learned RM, et al. Novel antineoplastic agents with efficacy against multidrug resistant tumor cells. Bioorg Med Chem Lett 1998;8:2653–63.
- Kirk KL, Filler R. In: Biomedical Frontiers of Fluorine Chemistry, Symposium Series. Washington, DC: American Chemical Society, 1996;1:639.
- GeldersYG Heylen, SLE, Vander Bussche G, Reyntjens AJM, Janssen PAJ. Pilot clinical investigation of risperidone in the treatment of psychotic patients. Pharmacopsychiatry 1990;23:206–12.
- Dollery C. Therapeutic Drugs. Edinburgh: Churchill Livingstone, 1999;3:326.
- Nanjunda Swamy S, Basappa Sarala, G, Priya BS, Gaonkar SL, Shashidhara Prasad J, et al. Microwave assisted synthesis of N-alkylated benzotriazole derivatives: antimicrobial studies. Bioorg Med Chem Lett 2006;16:999–1004.
- Ananda Kumar CS, Vinaya K, Narendra Sharath Chandra J, Thimmegowda NR, Benaka Prasad SB, Sadashiva CT, et al. Synthesis and antimicrobial studies of novel 1-benzhydryl-piperazine sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 2008;23:462–9.
- Priya BS, Basappa Nanjunda, Swamy S, Rangappa KS. Synthesis and characterization of novel 6-fluoro-4-piperidinyl- 1,2-benzisoxazole amides and 6-fluoro-chroman-2-carbaxamides: antimicrobial studies. Bioorg Med Chem 2005;13:2623–8.
- Sadashiva MP, Mallesha H, Karunakara Murthy, Rangappa KS. Enhancement in antimicrobial activity of 2-(phenyl)-3-(2-butyl-4-chloro-1H-imidazolyl)-5-butylate isoxazolidine. Bioorg Med Chem Lett 2006;15:1811–14.
- Vinaya K, Raja Naika, Ananda Kumar CS, Benaka Prasad SB, Chandrappa S, Ranganatha SR, et al. Synthesis of medicinally important N-trimethylene dipiperidine sulfonamides and carboxamides containing a substituted benzophenone moiety—an antibacterial agents. Lett Drug Des Discov 2008;5:250–60.
- Vinaya K, Kavitha CV, Ananda Kumar CS, Benaka Prasad SB, Chandrappa S, Deepak SA, et al. Synthesis and antimicrobial activity of 1-benzhydryl-sulfonyl-4-(3-(piperidin-4-yl) propyl) piperidine derivatives against pathogens of Lycopersicon esculatum: a structure-activity evaluation study. Arch Pharm Res 2009;32:33–41.
- (a) Elhance, D.N. Fundamentals of Statistics, 30th ed., 1984. (b) Colton, T., Statistics in medicine, 1st ed.; 1974.
- Priya BS, Basappa Nanjunda Swamy Sand Rangappa KS. Synthesis and characterization of novel 6-fluoro-4-piperidinyl- 1, 2-benzisoxazole amides and 6-fluoro-chroman-2-carbaxamides: Antimicrobial studies. Bioorg Med Chem 2005;13:2623–2628.
- Vinaya K, Kavitha CV, Ananda Kumar CS, Benaka Prasad SB, Chandrappa S, Deepak SA, Nanjundaswamy S, Umesha S, Rangappa KS. Synthesis and Antimicrobial Activity of 1-Benzhydryl-sulfonyl-4-(3-(piperidin-4-yl) propyl) piperidine Derivatives against Pathogens of Lycopersicon esculatum: A Structure-activity Evaluation Study. Arch Pharm Res 2009;32(1): 33–41.
